Subclavian steal syndrome is characterized by reversed vertebral artery flow due to proximal subclavian artery stenosis or occlusion. Treatment options include percutaneous transluminal angioplasty with or without stenting and surgical bypass. This review evaluates the effectiveness, safety, and long-term outcomes of these interventions.
Material and methodsA systematic scoping review was conducted using Embase and PubMed databases. Article screening was performed independently by multiple reviewers to ensure the inclusion of relevant studies. Based on predefined inclusion and exclusion criteria, 16 articles published between 2000 and 2023 were ultimately included. The primary outcomes assessed were technical success, symptom resolution, complication rates, and long-term patency.
ResultsSurgical bypass showed higher technical success and better long-term patency compared to endovascular approaches. Primary stenting was superior to balloon angioplasty alone. Initial symptom resolution was similar between treatments. Surgical bypass had higher perioperative complications, including cranial nerve injuries, while endovascular procedures had fewer complications but included risks of embolic events and stroke. Endovascular treatment was associated with shorter hospital stays. Both surgical and endovascular treatments are effective for subclavian steal syndrome.
ConclusionEndovascular approaches are preferred as first-line treatment in high-risk patients due to lower perioperative morbidity and shorter hospitalization. Surgical bypass is favored for younger patients, total occlusions, or when endovascular treatment fails, given its superior long-term outcomes. Treatment choice should be individualized based on lesion, patient comorbidities, and expertise.
El síndrome de robo de la subclavia se caracteriza por el flujo reverso a través de la arteria vertebral secundario a una estenosis u oclusión de la arteria subclavia. Las opciones terapéuticas incluyen la angioplastia transluminal percutánea con o sin posicionamiento de stent o la cirugía de bypass. Esta revisión evalúa la efectividad, la seguridad y los desenlaces a largo plazo de estas intervenciones.
Materiales y métodosSe realizó un scoping review mediante el uso de las bases de datos Embase® y PubMed®. Los artículos fueron evaluados de forma independiente por múltiples revisores. En función de los criterios de inclusión y exclusión, se incluyeron 16 artículos publicados entre los años 2000 y 2023. Los principales desenlaces evaluados fueron el éxito de la intervención, la resolución sintomática, la tasa de complicaciones y la permeabilidad a largo plazo.
ResultadosLa cirugía de bypass mostró mejores tasas de éxito y mayor permeabilidad a largo plazo en comparación con las intervenciones endovasculares. La resolución inicial de los síntomas fue similar entre las 2 modalidades de tratamiento. La cirugía de bypass presentó una mayor tasa de complicaciones perioperatorias, incluyendo lesiones nerviosas, mientras que los procedimientos endovasculares tuvieron menores complicaciones, pero mayor riesgo de eventos embólicos. El tratamiento endovascular se asoció a estancias hospitalarias más cortas. Tanto el tratamiento quirúrgico como el tratamiento endovascular son efectivos.
ConclusionesLas aproximaciones endovasculares se prefieren como primera línea de manejo en los pacientes de alto riesgo por una menor morbilidad perioperatoria y menor duración de la hospitalización. La cirugía de bypass se prefiere para pacientes jóvenes, oclusiones totales o como segunda línea después del tratamiento endovascular. La elección del tratamiento debe ser individualizada en función de la lesión, de las comorbilidades del paciente y de la experticia del cirujano.
Subclavian steal syndrome (SSS), first described in 1961, is a hemodynamic phenomenon characterized by the retrograde flow through the vertebral artery in response to an injury, stenosis, or occlusion of the ipsilateral subclavian artery.1 Its prevalence ranges from 0.6% to 6.5% in the general population, with a higher incidence among males, primarily due to subclavian artery stenosis caused by atherosclerosis.2 Among the most significant risk factors associated with this syndrome are atherosclerosis, Takayasu arteritis (primarily affecting women), compression of the subclavian artery in the thoracic operculum (often observed in athletes), and patients who have undergone surgical repair for aortic coarctation.3,4
SSS leads to reduced perfusion in the ipsilateral upper limb, diverting blood flow from the vertebral artery to the subclavian artery, ultimately affecting cerebral perfusion.3 Consequently, it induces transient neurological symptoms of vertebrobasilar insufficiency in approximately 5% of cases, including manifestations such as dizziness, diplopia, nystagmus, tinnitus, and sensorineural hearing loss.5
The diagnosis of SSS begins with clinical suspicion. Physical examination findings typically reveal a systolic arterial blood pressure difference of more than 15mmHg between the upper limbs, diminished pulses on the affected side, and the detection of a murmur in the supraclavicular fossa.6 The definitive diagnosis is established through the use of Doppler ultrasonography and computed tomography angiography to assess subclavian artery stenosis.7 Furthermore, in the presence of neurological symptoms, it is imperative to perform transcranial Doppler examinations.8
Patients exhibiting mild to moderate symptoms typically undergo medical and expectant management. Conversely, those with severe symptoms are deemed eligible for surgical intervention, which may entail percutaneous angioplasty or bypass surgery, with the choice dependent on the location of the defect.9 Nevertheless, considerable uncertainties persist regarding the most efficacious and secure approach for SSS treatment, particularly in light of initial treatment failures, the necessity for re-intervention, and the substantial cardiovascular morbidity and mortality rates ranging from 40% to 57%.10
The objective of this review is to compare angioplasty and surgical bypass, considering their efficacy and pertinent surgical outcomes during both the immediate and long-term postoperative periods through a narrative review of literature. This analysis aims to assist surgeons in making more informed decisions regarding the approach to managing patients with SSS.
Material and methodsSearch strategyA systematic scoping review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate the management outcomes of SSS, focusing on two treatment modalities: surgical bypass and percutaneous angioplasty. Study selection was guided by a structured PICO framework: population (patients with SSS); intervention (endovascular percutaneous angioplasty); comparator (surgical bypass), and outcomes (procedural success rates, postoperative complications, symptom improvement, restenosis rates, and symptom recurrence).
A structured search strategy was applied using the Embase and PubMed databases, employing the following search algorithm: (‘Subclavian’ AND ‘Steal’ AND ‘Syndrome’) AND (‘treatment’ OR ‘management’ OR ‘surgical’ OR ‘endovascular’ OR ‘angioplasty’ OR ‘bypass’) AND (‘outcomes’ OR ‘effectiveness’). Eligible study types included cohort studies, randomized controlled trials, prospective studies, systematic reviews, narrative reviews, and meta-analyses. Articles published in English and Spanish up to January 2024 were considered, as these are the languages proficiently understood by the reviewers.
Article selectionFollowing the initial database search, articles were screened based on titles, keywords, and abstracts using the Rayyan bibliographic manager to streamline the selection process. The following inclusion and exclusion criteria were applied. Inclusion criteria were: (1) studies involving the target population—patients with SSS; (2) studies evaluating endovascular or surgical bypass approaches for management; and (3) studies reporting relevant outcomes, including procedural success rates, postoperative complications, symptom improvement, restenosis rates, and symptom recurrence. Exclusion criteria included studies that did not report these outcomes; studies involving pediatric or very elderly populations with SSS; and studies focused on other conditions, such as coronary SSS oncological diagnosis, or immunosuppressive states.
Article screening was performed by four independent reviewers based on the predefined inclusion and exclusion criteria, initially evaluating titles and abstracts. Full-text reviews were conducted for studies that met the screening criteria. Articles were included upon consensus among the reviewers. In cases of disagreement, conflicts were resolved by a fifth reviewer.
Data extractionSubsequently, selected articles underwent data extraction, capturing the following variables: author(s), year of publication, study design, patient characteristics, intervention details, and reported outcomes. When specific data were not provided within a study, they were recorded as “Not Available” (NA). Quality assessment was performed using appropriate methodological criteria relevant to each study type. Data synthesis was conducted using narrative synthesis techniques, given the heterogeneity of study designs and outcome measures.
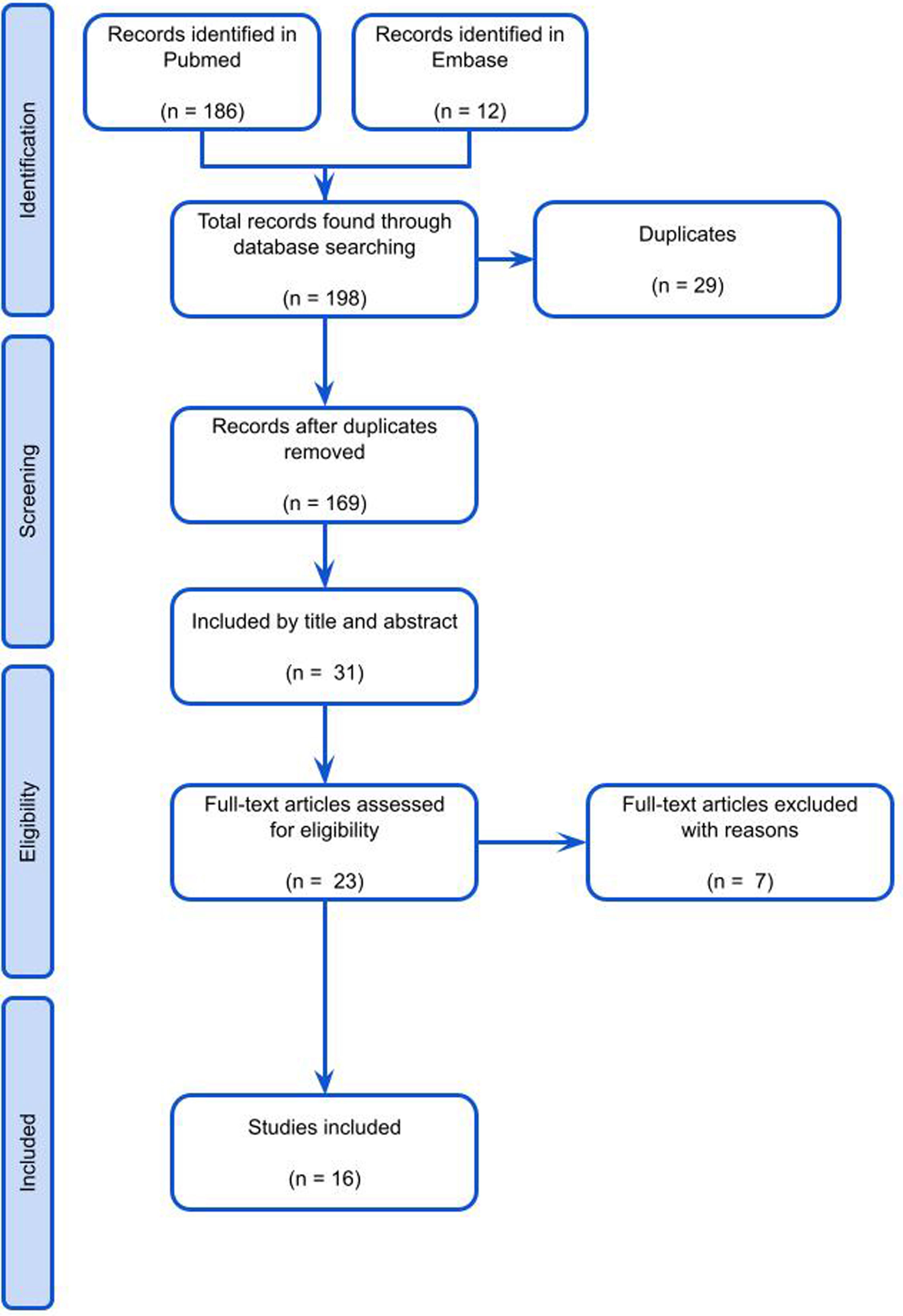
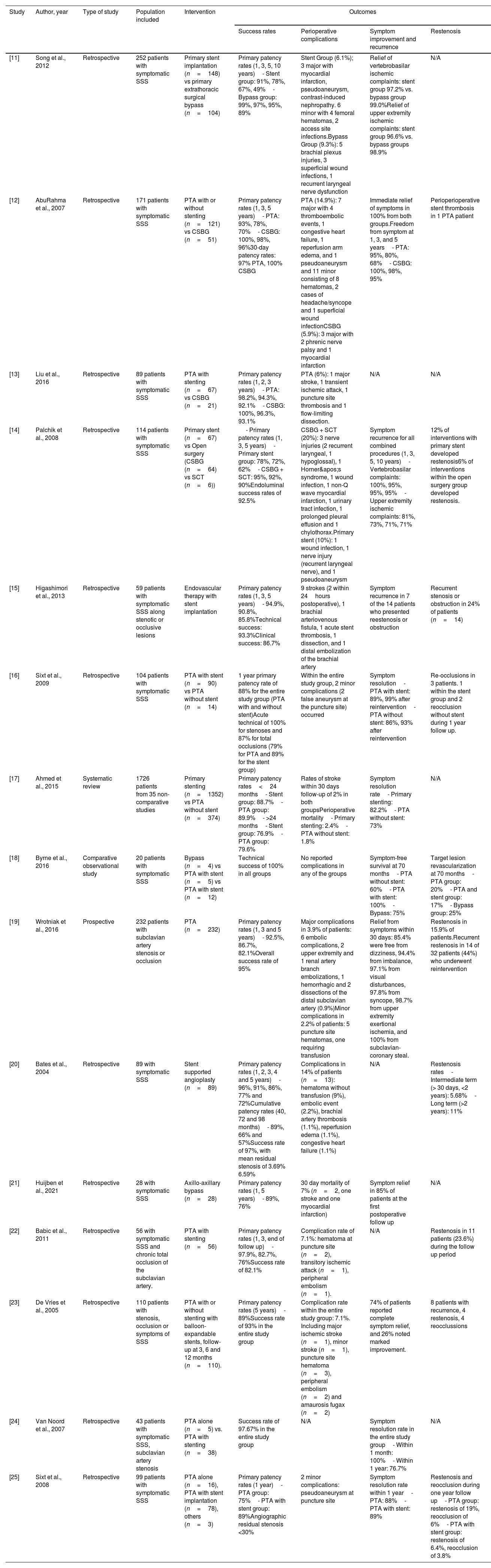
ResultsFollowing the application of inclusion and exclusion criteria, a total of 198 articles were identified through the search strategy outlined in Fig. 1. Subsequently, refinement resulted in the final selection of 23 articles, which were subjected to detailed analysis. The gathered information was structured to align with the format of a comprehensive review article, presenting a synthesized overview of the current literature regarding the management outcomes of SSS with a focus on surgical bypass and percutaneous angioplasty. Finally 16 articles were studied to conduct a narrative review of literature. Table 1 includes study results and characteristics of included papers.
Summary characteristics of included papers.
| Study | Author, year | Type of study | Population included | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Success rates | Perioperative complications | Symptom improvement and recurrence | Restenosis | |||||
| [11] | Song et al., 2012 | Retrospective | 252 patients with symptomatic SSS | Primary stent implantation (n=148) vs primary extrathoracic surgical bypass (n=104) | Primary patency rates (1, 3, 5, 10 years)- Stent group: 91%, 78%, 67%, 49%- Bypass group: 99%, 97%, 95%, 89% | Stent Group (6.1%); 3 major with myocardial infarction, pseudoaneurysm, contrast-induced nephropathy. 6 minor with 4 femoral hematomas, 2 access site infections.Bypass Group (9.3%): 5 brachial plexus injuries, 3 superficial wound infections, 1 recurrent laryngeal nerve dysfunction | Relief of vertebrobasilar ischemic complaints: stent group 97.2% vs. bypass group 99.0%Relief of upper extremity ischemic complaints: stent group 96.6% vs. bypass groups 98.9% | N/A |
| [12] | AbuRahma et al., 2007 | Retrospective | 171 patients with symptomatic SSS | PTA with or without stenting (n=121) vs CSBG (n=51) | Primary patency rates (1, 3, 5 years)- PTA: 93%, 78%, 70%- CSBG: 100%, 98%, 96%30-day patency rates: 97% PTA, 100% CSBG | PTA (14.9%): 7 major with 4 thromboembolic events, 1 congestive heart failure, 1 reperfusion arm edema, and 1 pseudoaneurysm and 11 minor consisting of 8 hematomas, 2 cases of headache/syncope and 1 superficial wound infectionCSBG (5.9%): 3 major with 2 phrenic nerve palsy and 1 myocardial infarction | Immediate relief of symptoms in 100% from both groups.Freedom from symptom at 1, 3, and 5 years- PTA: 95%, 80%, 68%- CSBG: 100%, 98%, 95% | Perioperioperative stent thrombosis in 1 PTA patient |
| [13] | Liu et al., 2016 | Retrospective | 89 patients with symptomatic SSS | PTA with stenting (n=67) vs CSBG (n=21) | Primary patency rates (1, 2, 3 years)- PTA: 98.2%, 94.3%, 92.1%- CSBG: 100%, 96.3%, 93.1% | PTA (6%): 1 major stroke, 1 transient ischemic attack, 1 puncture site thrombosis and 1 flow-limiting dissection. | N/A | N/A |
| [14] | Palchik et al., 2008 | Retrospective | 114 patients with symptomatic SSS | Primary stent (n=67) vs Open surgery (CSBG (n=64) vs SCT (n=6)) | - Primary patency rates (1, 3, 5 years)- Primary stent group: 78%, 72%, 62%- CSBG + SCT: 95%, 92%, 90%Endoluminal success rates of 92.5% | CSBG + SCT (20%): 3 nerve injuries (2 recurrent laryngeal, 1 hypoglossal), 1 Horner's syndrome, 1 wound infection, 1 non-Q wave myocardial infarction, 1 urinary tract infection, 1 prolonged pleural effusion and 1 chylothorax.Primary stent (10%): 1 wound infection, 1 nerve injury (recurrent laryngeal nerve), and 1 pseudoaneurysm | Symptom recurrence for all combined procedures (1, 3, 5, 10 years)- Vertebrobasilar complaints: 100%, 95%, 95%, 95%- Upper extremity ischemic complaints: 81%, 73%, 71%, 71% | 12% of interventions with primary stent developed restenosis6% of interventions within the open surgery group developed restenosis. |
| [15] | Higashimori et al., 2013 | Retrospective | 59 patients with symptomatic SSS along stenotic or occlusive lesions | Endovascular therapy with stent implantation | Primary patency rates (1, 3, 5 years)- 94.9%, 90.8%, 85.8%Technical success: 93.3%Clinical success: 86.7% | 9 strokes (2 within 24hours postoperative), 1 brachial arteriovenous fistula, 1 acute stent thrombosis, 1 dissection, and 1 distal embolization of the brachial artery | Symptom recurrence in 7 of the 14 patients who presented reestenosis or obstruction | Recurrent stenosis or obstruction in 24% of patients (n=14) |
| [16] | Sixt et al., 2009 | Retrospective | 104 patients with symptomatic SSS | PTA with stent (n=90) vs PTA without stent (n=14) | 1 year primary patency rate of 88% for the entire study group (PTA with and without stent)Acute technical of 100% for stenoses and 87% for total occlusions (79% for PTA and 89% for the stent group) | Within the entire study group, 2 minor complications (2 false aneurysm at the puncture site) occurred | Symptom resolution- PTA with stent: 89%, 99% after reintervention- PTA without stent: 86%, 93% after reintervention | Re-occlusions in 3 patients. 1 within the stent group and 2 reocclusion without stent during 1 year follow up. |
| [17] | Ahmed et al., 2015 | Systematic review | 1726 patients from 35 non-comparative studies | Primary stenting (n=1352) vs PTA without stent (n=374) | Primary patency rates<24 months- Stent group: 88.7%- PTA group: 89.9%- >24 months- Stent group: 76.9%- PTA group: 79.6% | Rates of stroke within 30 days follow-up of 2% in both groupsPerioperative mortality- Primary stenting: 2.4%- PTA without stent: 1.8% | Symptom resolution rate- Primary stenting: 82.2%- PTA without stent: 73% | N/A |
| [18] | Byrne et al., 2016 | Comparative observational study | 20 patients with symptomatic SSS | Bypass (n=4) vs PTA with stent (n=5) vs PTA with stent (n=12) | Technical success of 100% in all groups | No reported complications in any of the groups | Symptom-free survival at 70 months- PTA without stent: 60%- PTA with stent: 100%- Bypass: 75% | Target lesion revascularization at 70 months- PTA group: 20%- PTA and stent group: 17%- Bypass group: 25% |
| [19] | Wrotniak et al., 2016 | Prospective | 232 patients with subclavian artery stenosis or occlusion | PTA (n=232) | Primary patency rates (1, 3 and 5 years)- 92.5%, 86.7%, 82.1%Overall success rate of 95% | Major complications in 3.9% of patients: 6 embolic complications, 2 upper extremity and 1 renal artery branch embolizations, 1 hemorrhagic and 2 dissections of the distal subclavian artery (0.9%)Minor complications in 2.2% of patients: 5 puncture site hematomas, one requiring transfusion | Relief from symptoms within 30 days: 85.4% were free from dizziness, 94.4% from imbalance, 97.1% from visual disturbances, 97.8% from syncope, 98.7% from upper extremity exertional ischemia, and 100% from subclavian-coronary steal. | Restenosis in 15.9% of patients.Recurrent restenosis in 14 of 32 patients (44%) who underwent reintervention |
| [20] | Bates et al., 2004 | Retrospective | 89 with symptomatic SSS | Stent supported angioplasty (n=89) | Primary patency rates (1, 2, 3, 4 and 5 years)- 96%, 91%, 86%, 77% and 72%Cumulative patency rates (40, 72 and 98 months)- 89%, 66% and 57%Success rate of 97%, with mean residual stenosis of 3.69% 6.59% | Complications in 14% of patients (n=13): hematoma without transfusion (9%), embolic event (2.2%), brachial artery thrombosis (1.1%), reperfusion edema (1.1%), congestive heart failure (1.1%) | N/A | Restenosis rates- Intermediate term (> 30 days, <2 years): 5.68%- Long term (>2 years): 11% |
| [21] | Huijben et al., 2021 | Retrospective | 28 with symptomatic SSS | Axillo-axillary bypass (n=28) | Primary patency rates (1, 5 years)- 89%, 76% | 30 day mortality of 7% (n=2, one stroke and one myocardial infarction) | Symptom relief in 85% of patients at the first postoperative follow up | N/A |
| [22] | Babic et al., 2011 | Retrospective | 56 with symptomatic SSS and chronic total occlusion of the subclavian artery. | PTA with stenting (n=56) | Primary patency rates (1, 3, end of follow up)- 97.9%, 82.7%, 76%Success rate of 82.1% | Complication rate of 7.1%: hematoma at puncture site (n=2), transitory ischemic attack (n=1), peripheral embolism (n=1). | N/A | Restenosis in 11 patients (23.6%) during the follow up period |
| [23] | De Vries et al., 2005 | Retrospective | 110 patients with stenosis, occlusion or symptoms of SSS | PTA with or without stenting with balloon-expandable stents, follow-up at 3, 6 and 12 months (n=110). | Primary patency rates (5 years)- 89%Success rate of 93% in the entire study group | Complication rate within the entire study group: 7.1%. Including major ischemic stroke (n=1), minor stroke (n=1), puncture site hematoma (n=3), peripheral embolism (n=2) and amaurosis fugax (n=2) | 74% of patients reported complete symptom relief, and 26% noted marked improvement. | 8 patients with recurrence, 4 restenosis, 4 reocclussions |
| [24] | Van Noord et al., 2007 | Retrospective | 43 patients with symptomatic SSS, subclavian artery stenosis | PTA alone (n=5) vs. PTA with stenting (n=38) | Success rate of 97.67% in the entire study group | N/A | Symptom resolution rate in the entire study group- Within 1 month: 100%- Within 1 year: 76.7% | N/A |
| [25] | Sixt et al., 2008 | Retrospective | 99 patients with symptomatic SSS | PTA alone (n=16), PTA with stent implantation (n=78), others (n=3) | Primary patency rates (1 year)- PTA group: 75%- PTA with stent group: 89%Angiographic residual stenosis <30% | 2 minor complications: pseudoaneurysm at puncture site | Symptom resolution rate within 1 year- PTA: 88%- PTA with stent: 89% | Restenosis and reocclusion during one year follow up- PTA group: restenosis of 19%, reocclusion of 6%- PTA with stent group: restenosis of 6.4%, reocclusion of 3.8% |
CSBG: carotid-subclavian bypass grafts, N/A: Non-Applicable, PTA: Percutaneous Transluminal Angioplasty, SCT: Subclavian-Carotid Transpositions, SSS: Subclavian Steal Syndrome.
The short term success rate of procedures for the management of subclavian steal syndrome, has been evaluated based on residual stenosis, patency, and the reduction of the difference in systolic blood pressure between the upper extremities following intervention.
In a retrospective study, Song et al. reported a short-term success rate of 97.3% in patients undergoing percutaneous transluminal angioplasty, compared to 99% in those treated with surgical bypass, as well as a reduction in the blood pressure gradient between both upper extremities without a significant difference among the studied groups.11 Similarly, AbuRahma et al. reported a 30-day success rate of 98% for patients treated with PTA and stenting and 100% for the bypass group.12 Likewise, Liu et al. reported a success rate of 77.6% in the endovascular group versus 100% in the surgical group, with higher primary patency rates at 12, 24, and 36 months in the surgical group (100%, 96.3%, and 93.1%, respectively) compared to the endovascular group (98.2%, 94.3%, and 92.1%, respectively).13 Palchik et al. documented five failures in the primary stenting group, which subsequently required surgical bypass. The primary patency rate was 78±6% in the stenting group, compared to 95±3% in the group initially treated with bypass.14 The main limitation in the endovascular group was the difficulty in crossing the occlusive lesion.
Higashimori et al. reported that the immediate success rate of endovascular treatment was higher in cases of stenosis compared to occlusion (100% vs. 71.4%),15 with similar findings by Sixt et al. (100% vs. 87%), who also reported a one-year patency rate of 88% across the entire cohort.16 Both studies documented a statistically significant reduction in systolic blood pressure gradients post-intervention (p<0.001).15,16
Regarding the effectiveness of different endovascular modalities, a systematic review by Ahmed et al. revealed a significantly higher success rate in the stenting group compared to PTA alone (92.8% vs. 86.9%), although no significant difference was observed in short-term patency (88.7% vs. 89.9%).17 In a parallel observational study evaluating postoperative outcomes in 20 patients treated for SSS, 5 with PTA and 12 with PTA and stenting, Byrne et al. reported a 100% immediate success rate.18
Postoperative complicationsThe comparison between endovascular interventions (angioplasty with or without stent placement) and surgical revascularization (primarily carotid-subclavian or axillo-axillary bypass) for the treatment of SSS reveals significant differences in postoperative complication profiles, both in frequency and severity.
Overall, endovascular procedures are associated with lower rates of major complications and reduced perioperative mortality compared to open surgery. Multiple studies have consistently reported a low incidence of serious adverse events following PTA and subclavian artery stenting. Most reported complications were minor, including access site hematomas, pseudoaneurysms successfully managed with compression, and transient neurological symptoms without permanent sequelae.15,16,18–20 Major complication rates ranged from 3.9% to 10%,15,19 involving distal embolization, subclavian artery dissection, ischemic stroke, and intracranial hemorrhage. Procedure-related mortality was exceptionally low and observed only in isolated cases.12,20
In contrast, surgical approaches, despite demonstrating near-100% technical success and excellent long-term primary and assisted patency rates, are consistently associated with higher perioperative risk. Reported complications include nerve injuries (e.g., phrenic, recurrent laryngeal, or brachial plexus palsies), surgical site infections, chylothorax, pleural effusions, and major cardiovascular events such as myocardial infarction or stroke.11,14,21 Thirty-day postoperative mortality reached up to 7% in certain series.21 Although surgical reintervention rates and long-term restenosis were lower (5% at 5 years) compared to the endovascular group (up to 24%),14 this advantage came at the cost of increased perioperative morbidity and longer hospital stays.
Notably, even in technically demanding settings such as chronic total occlusions, endovascular therapy has demonstrated a favorable safety profile, with technical success rates between 77% and 93% and complication rates comparable to or lower than those of surgical management, even in ostial or heavily calcified lesions.22,23 Appropriate patient selection, use of combined femoral-brachial approaches, and distal embolic protection—particularly in right-sided lesions—have been shown to mitigate neurological risk.13
Symptom improvement and recurrenceRegarding symptom improvement, both endovascular stenting and surgical bypass demonstrate high initial success rates in relieving vertebrobasilar symptoms (97.2% vs. 99.0%) and upper extremity ischemic complaints (96.6% vs. 98.9%), as reported by Song et al.11 These findings are consistent with those of AbuRahma et al., who documented a 100% success rate at 30 days in the surgical bypass group compared to 98% in the percutaneous transluminal angioplasty with stenting group regarding overall symptom resolution.12 Similarly, the meta-analysis by Ahmed et al. found no significant difference in symptom resolution between stenting (82.2%) and percutaneous transluminal angioplasty alone (73%).17 Furthermore, Wrotniak et al. detailed that, 30 days after percutaneous transluminal angioplasty, a significant majority of patients experienced resolution of specific symptoms such as dizziness (85.4%), imbalance (94.4%), and those related to upper extremity ischemia (98.7%).19 Byrne et al. also reported a 100% success rate in symptom relief in both percutaneous transluminal angioplasty and percutaneous transluminal angioplasty with stenting groups in their observational study.18
However, long-term symptom recurrence remains a significant concern, particularly with endovascular approaches. Song et al. observed a high incidence of in-stent occlusion and stenosis leading to symptom recurrence and the requirement of revascularization over a 67-month follow-up period in the stent group.11 AbuRahma et al. reported higher symptom recurrence rates in the percutaneous transluminal angioplasty with stenting group compared to the carotid-subclavian bypass graft group at 3 and 5 years (80% and 68% vs. 98% and 95%, respectively).12 Wrotniak et al. also found that recurrence of symptoms such as dizziness, imbalance, and symptoms related to upper extremity ischemia were significantly more frequent in cases of restenosis.19 Similarly, Van Noord et al. reported a symptomatic recurrence rate of 23.3% at 12 months following percutaneous transluminal angioplasty with stenting, with a higher rate among patients who underwent the procedure prior to coronary artery bypass grafting.24
In contrast, surgical bypass often provides more sustained symptom relief. AbuRahma et al. reported a 95% symptom-free rate at 5 years in the carotid-subclavian bypass graft group, compared to 68% in the percutaneous transluminal angioplasty with the stenting group.12 Byrne et al., demonstrated a 75% symptom-free survival at 70 months in the surgical bypass group, which was lower than the 100% observed in the angioplasty with the stenting group but higher than the 60% in the angioplasty-alone group.18 Palchik et al. also reported a high rate of symptom resolution in the surgical bypass group at 1 year (100% for vertebrobasilar symptoms and 81% for arm ischemia).14
Interestingly, the study rates of symptom recurrence after endovascular therapy in subclavian artery stenosis and prevalence of subclavian artery stenosis prior to coronary artery bypass grafting suggests a higher incidence of symptom recurrence in patients with a history of prior coronary intervention following endovascular therapy for subclavian artery stenosis. This highlights the potential influence of overall atherosclerotic burden on long-term outcomes. Although Higashimori et al. reported favorable long-term symptom relief with primary stenting, the occurrence of new cerebrovascular events in some patients during follow-up warrants consideration.15 Likewise, De Vries et al. demonstrated a high rate of primary clinical patency after percutaneous transluminal angioplasty at 5 years, suggesting durable symptom relief in most cases.23
Restenosis ratesSong et al. demonstrated that patients who underwent bypass surgery exhibited superior long-term graft patency rates compared to those treated with stenting. At 10 years, the secondary graft patency rate was 94% in the bypass group versus 69% in the stent group.11 However, no significant differences were observed in all-cause mortality between the two groups (p=0.527).11 These findings are reflected in the 10-year target vessel revascularization rate of 46.6%. Similarly, Huijben et al. reported a 5-year secondary patency rate of 87% and a 12-month occlusion rate of 14%.21
Furthermore, a meta-analysis by Ahmed et al. found that patients undergoing bypass for the treatment of subclavian steal syndrome achieved better outcomes in both primary graft patency (92%; 95% CI: 86–94%) and secondary graft patency (79%; 95% CI: 74–85%) compared to those treated with stent placement. No significant differences in long-term mortality were noted between the groups.17
The combination of therapies has also shown favorable results. Sixt et al. reported a 1-year graft patency rate of 87% in patients treated with angioplasty and stent placement, with 4% experiencing reocclusion and 8% developing significant restenosis.25 Byrne et al. found symptom-free survival at 70 months to be 60% in the angioplasty group, 100% in the angioplasty plus stent group, and 75% in the bypass group.18 However, further high-quality research is needed to robustly compare clinical outcomes following combination revascularization strategies for subclavian steal syndrome.
SSS and its implication in coronary-subclavian steal syndromeOne uncommon but lethal entity related to SSS is coronary-subclavian steal syndrome (CSSS). It is defined as an acquired, functional graft failure due to an otherwise undetected, hemodynamically significant subclavian stenosis proximal to left internal mammary artery (LIMA) origin, and it represents a fatal complication in patients undergoing coronary artery bypass graft (CABG).26 Due to the low frequency of subclavian stenosis among patients referred for CABG, with a documented prevalence between 0.2% and 6.8%, CSSS becomes an overlooked and underestimated entity.27,28 It develops when an occlusion of the subclavian artery produces reversal blood flow through the mammary graft causing reduction in myocardial perfusion and increasing the risk for myocardial ischemia, acute myocardial infarction, new onset heart failure, ventricular arrhythmias and sudden cardiac death.26,29
Several case reports have been published, where the clinical presentation, findings and treatment options are thoroughly described. In 2022, Monteagudo-Vela et al. reported a case of cardiogenic shock on the seventh day after CABG in a 62-year-old male due to CSSS as a consequence of chronic total occlusion of the left subclavian artery, which required a new paradigm in management with percutaneous coronary intervention and coil embolization of the LIMA graft when subclavian artery revascularization was not feasible.29
The clinical and prognostic implications of CSSS elucidate the importance of preoperative screening for SSS among CABG candidates with LIMA grafts, especially among patients with specific risk factors such as peripheral artery disease.30 These screening strategies include bilateral blood pressure measurement and further evaluation with image studies if significant blood pressure difference is detected. If CSSS develops, endovascular stenting of the subclavian artery is the first line of treatment. In severe chronic total occlusions or complex cardiogenic shock, where percutaneous subclavian revascularization is not feasible, other interventions such as carotid-subclavian bypass or LIMA graft mobilization may become unavoidable.29
Despite CSSS being a rare complication related with SSS, its potentially catastrophic nature and the excellent results possible with an early intervention, illustrate the importance of increasing awareness among cardiac surgeons, interventional cardiologists, and vascular specialists. Further research is still needed to ensure maximization of results following coronary revascularization with IMA grafts.
ConclusionSSS can lead to vertebrobasilar insufficiency or upper limb ischemia. While surgical revascularization, including percutaneous angioplasty and bypass, are established treatments, determining the optimal approach necessitates evaluating short, medium, and long-term outcomes. Analysis indicates both techniques achieve high immediate technical success, with a slight advantage for bypass and primary stenting superior to angioplasty alone, particularly in severe stenosis. However, endovascular procedures struggle with total occlusions, where bypass maintains an advantage. Both methods offer near-total initial symptom resolution, though surgical bypass demonstrates superior long-term symptom-free survival.
Surgical bypass carries higher perioperative complication rates, including nerve injuries, while endovascular techniques have lower overall immediate complications but include risks of embolization and stroke. Endovascular procedures offer shorter hospital stays. Long-term, surgical bypass exhibits superior primary patency and lower restenosis rates compared to angioplasty/stenting, reducing the need for reinterventions. Treatment selection should be individualized based on lesion characteristics, patient comorbidities, and institutional expertise. While angioplasty/stenting is less invasive and suitable for proximal stenotic lesions, bypass may be preferred for total occlusions, failed endovascular treatment, younger patients, or when greater durability is required. Further prospective randomized trials are needed to establish definitive recommendations.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Author contributionsAll authors were involved in article screening, inclusion of articles, writing of the manuscript and final article revision.
Ethical considerationsAs this scoping review used only publicly available data from previously published studies, no ethical approval was required. No new data involving human participants or animals were collected.
Use of artificial intelligenceDuring the preparation of this work the authors used ChatGPT as an aid for the translation of the manuscript.
FundingNone.
Conflict of interestNone were declared by the authors.