Cardiac surgery with cardiopulmonary bypass (CPB) improves survival in patients with cardiovascular diseases, but postoperative complications like postcardiotomy low cardiac output syndrome, vasoplegic syndrome and excessive bleeding contribute to high morbidity and mortality. This study examines the relationship between these complications and adverse in-hospital outcomes.
MethodsThis retrospective study included 555 adult patients who underwent cardiac surgery with CPB. Postoperative complications were assessed, and their associations with outcomes like prolonged ICU stays, morbidity, and in-hospital mortality were analyzed. Logistic regression was used to identify predictors of adverse outcomes.
ResultsPatients were grouped based on the number of postoperative syndromes experienced. Group 4 (three syndromes) had the highest rates of complications, including delirium, cerebrovascular events, and in-hospital mortality. Logistic regression showed that two or more postoperative syndromes were significant predictors of severe complications and mortality.
ConclusionsMultiple postoperative syndromes significantly increase the risk of adverse outcomes after cardiac surgery with CPB. Early identification and targeted management of these patients can reduce ICU time, improve recovery, and enhance long-term survival.
La cirugía cardiaca con circulación extracorpórea (CEC) mejora la supervivencia en pacientes con enfermedades cardiovasculares, pero las complicaciones postoperatorias como el síndrome de bajo gasto cardiaco poscardiotomía, el síndrome vasoplégico y el sangrado excesivo están asociadas a alta morbilidad y mortalidad. Este estudio analiza la relación entre estas complicaciones y los resultados adversos intrahospitalarios.
MétodosEste estudio retrospectivo incluyó a 555 pacientes adultos que se sometieron a cirugía cardiaca con CEC. Se evaluaron las complicaciones postoperatorias y sus asociaciones con resultados como estancias prolongadas en la UCI, morbilidad y mortalidad intrahospitalaria. Se utilizó regresión logística para identificar los predictores de resultados adversos.
ResultadosLos pacientes fueron agrupados según el número de síndromes postoperatorios. El grupo 4 (3 síndromes) presentó las mayores tasas de complicaciones, incluyendo delirium, eventos cerebrovasculares y mortalidad intrahospitalaria. La regresión logística reveló que la presencia de 2 o más síndromes postoperatorios es un predictor significativo de complicaciones graves y mortalidad.
ConclusionesLa presencia de múltiples síndromes postoperatorios aumenta significativamente el riesgo de resultados adversos después de cirugía cardiaca con CEC. La identificación temprana y un manejo adecuado de estos pacientes pueden reducir el tiempo en la UCI, mejorar la recuperación y aumentar la supervivencia a largo plazo.
Cardiac surgery, especially when performed with cardiopulmonary bypass (CPB), is a critical procedure designed to improve the quality of life and survival for patients with various cardiovascular diseases. Despite significant advancements in surgical techniques, postoperative complications remain a primary concern, contributing to high morbidity and mortality rates within this patient group. Common postoperative complications include postcardiotomy low cardiac output syndrome (LCOS), vasoplegic syndrome (VS) and excessive bleeding, each presenting unique challenges in terms of diagnosis and management. LCOS, which is characterized by an insufficient cardiac output to meet the metabolic demands of tissues, often results from left or right ventricular dysfunction and is closely associated with poor clinical outcomes. By definition, this entity excludes primary causes of reduced cardiac output, such as hypovolemia, obstructive shock (e.g., tamponade, pulmonary embolism), and other causes of myocardial dysfunction (e.g., myocardial infarction).1,2 On the other hand, VS is marked by profound vasodilation leading to hypotension, despite adequate or elevated cardiac output, and is often refractory to conventional vasopressor therapies; may be associated in up to 50% of all LCOS patients, usually with decreased or normal (at the lower limit) systemic vascular resistance, in association with a reduced cardiac index and elevated filling pressures.3,4 Excessive bleeding, although a common occurrence after surgery, becomes problematic when it exceeds specific thresholds, complicating recovery.5–8
The pathophysiological mechanisms underlying these complications are multifactorial, involving ischemia–reperfusion injury, inflammatory responses triggered by CPB, endothelial dysfunction, and other systemic disturbances. A deeper understanding of these conditions, their incidence, and their predictors is essential for improving postoperative care and optimizing management strategies, ultimately reducing morbidity and mortality rates in patients undergoing cardiac surgery.9 Therefore, the identification of patients at high risk for developing these complications and the implementation of targeted therapeutic strategies are vital to enhancing recovery.
ImportanceThe incidence of postoperative complications following cardiac surgery with CPB can lead to prolonged stays in the intensive care unit (ICU), the need for additional interventions such as reoperation, and higher rates of in-hospital mortality.10 Early identification and prompt treatment of complications like LCOS, VS, and excessive bleeding are crucial for improving outcomes in this vulnerable patient population. This study is significant as it focuses on the relationship between postoperative syndromes and adverse in-hospital outcomes, which can guide clinical decision-making, reduce ICU time, and improve patient prognosis. The prevalence and impact of these complications warrant careful investigation to refine perioperative management protocols and ultimately improve the long-term survival and quality of life for cardiac surgery patients.2,10
Goals of investigationThis study aims to assess the prevalence of postoperative complications, specifically LCOS, VS and excessive bleeding in patients undergoing cardiac surgery with CPB. Additionally, the study seeks to determine the association between these complications and adverse intrahospital outcomes, including in-hospital mortality.
MethodsStudy designThis is a retrospective, observational, and analytical study designed to examine the prevalence and impact of postoperative complications following cardiac surgery with CPB. The study will identify the presence of LCOS, VS and excessive bleeding in patients and analyze their association with adverse clinical outcomes. The study is observational in nature, as it relies on existing data from clinical records, and it is longitudinal, capturing data during the first 24h post-surgery. It is also analytical in that it explores the relationships between the identified complications and specific outcomes.
Study populationThe study population includes all adult patients (aged >18 years) who underwent cardiac surgery with CPB and were subsequently admitted to the cardiovascular intensive care unit (ICU) in a tertiary cardiovascular center in Mexico City. This institution is a specialized center that provides comprehensive care for patients undergoing cardiac surgery, making it an appropriate setting for this investigation.
Sample sizeThe sample consists of all patients who underwent cardiac surgery with CPB and were admitted to the ICU during the study period, from June 2022 to December 2023. Given the retrospective nature of the study, there was no predefined sample size; all eligible patients were included for data collection.
Inclusion criteria- •
Adults aged 18 years or older.
- •
Patients who underwent cardiac surgery with CPB.
- •
Patients who were admitted to the cardiovascular ICU post-surgery.
- •
Patients who died during the surgical procedure.
- •
Patients who died during the first 12h in the ICU after surgery.
- •
Patients with incomplete or missing medical records, which may lead to inaccurate or incomplete data analysis.
Data were gathered from the hospital's patient database, which contains clinical information for all patients undergoing cardiac surgery with CPB. Data collection included preoperative, intraoperative, and postoperative variables such as patient demographics, surgical details (type of surgery, duration of CPB), hemodynamic parameters (e.g., blood pressure, cardiac output), and outcomes (e.g., bleeding, need for inotropes, use of vasopressors, reoperation, ICU stay). Information was extracted from both electronic and physical medical records to ensure comprehensive data capture.
Definition of postoperative clinical syndromes, diagnosed upon the patient's arrival to intensive careLCOS- •
Cardiac index <2.2L/min/m2, with systolic blood pressure <90mmHg and pulmonary artery occlusion pressure (PAOP) ≥16mmHg and/or central venous pressure (CVP) ≥12mmHg.
- •
Urine output <0.5mL/kg/h, central venous saturation <60%, lactate >3mmol/L.
- •
Postoperative use of inotropes and/or ventricular assist devices for at least 12h.
- •
Real or relative hypovolemia has been ruled out or managed, without predictors of fluid responsiveness.
- •
Hypotension:
- ∘
Systolic blood pressure <80mmHg.
- ∘
Mean arterial pressure <50mmHg.
- ∘
- •
Vasodilation:
- ∘
Systemic vascular resistance <800dyn/s/cm−5.
- ∘
CVP >5mmHg.
- ∘
PAOP >10mmHg.
- ∘
- •
Cardiac index >2.2L/min/m2.
- •
Use of vasopressors:
- ∘
Norepinephrine >0.3mcg/kg/min.
- ∘
- •
Real or relative hypovolemia has been ruled out or managed, without predictors of fluid responsiveness.
- •
300mL in the first hour after surgery.
- •
200mL per hour for more than 2h.
- •
100mL per hour for more than 3h.
Continuous variables were assessed for normality using the Shapiro–Wilk test. For normally distributed data, means with standard deviations were calculated, while non-normally distributed variables were presented as medians and interquartile ranges. Group comparisons for continuous variables were performed using the Mann–Whitney U test. Categorical variables were summarized as frequencies and percentages, and group differences were analyzed using the chi-square test or Fisher's exact test, depending on the distribution of data. Logistic regression analysis was conducted to identify independent predictors of adverse in-hospital outcomes, adjusting for potential confounders such as age and sex. A p-value of <0.05 was considered statistically significant. All statistical analyses were conducted using STATA version 14 software.
They were categorized into four groups based on the number of postoperative syndromes they experienced: Group 1 (no postoperative syndromes, n=399, 71.9%), Group 2 (one postoperative syndrome, n=131, 23.6%), Group 3 (two postoperative syndromes, n=21, 3.8%), and Group 4 (three postoperative syndromes, n=4, 0.72%).
Ethical considerationsThis study was approved by the ethics committee at the Instituto Nacional de Cardiología Ignacio Chávez. As a retrospective, non-interventional study, it adhered to ethical standards for research involving human data, with patient confidentiality maintained throughout the process. All data used in the analysis were anonymized to protect patient privacy. The study was deemed of minimal risk by the institutional review board, as it involved only the review of existing clinical records without any experimental intervention.
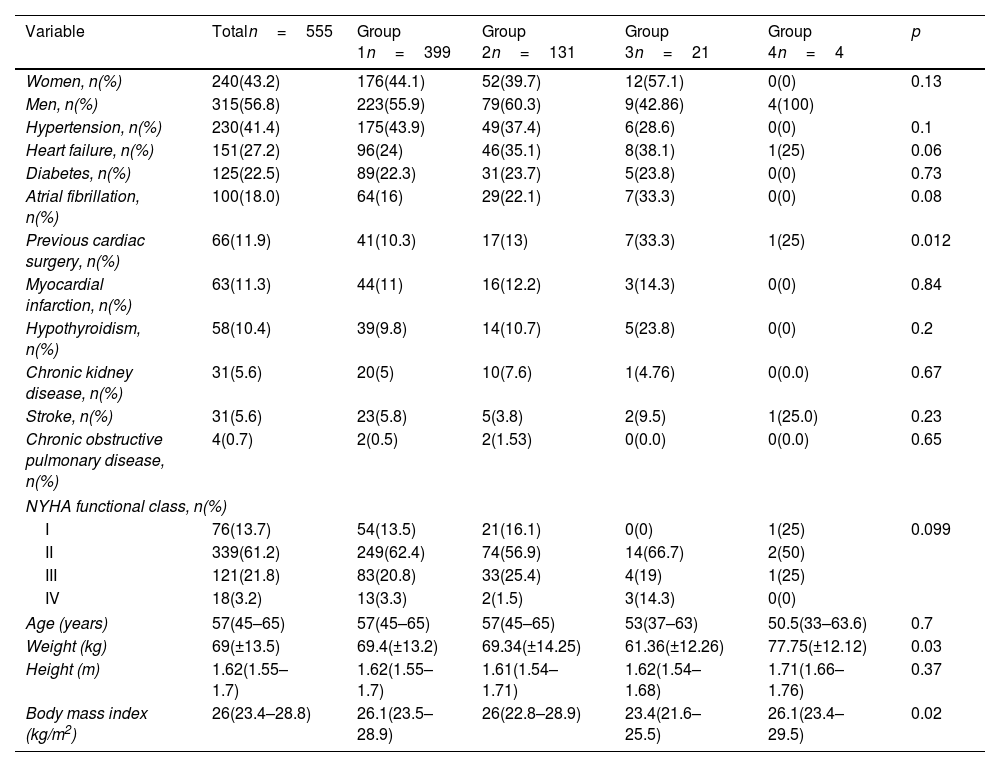
ResultsDemographic characteristics of the study populationA total of 555 patients were included in the study. During the study period, 33 patients were excluded (26 due to incomplete or missing medical records, 4 who died during the first 12h in the ICU after surgery and 3 who died during the surgical procedure). The cohort's average age was 57 years, with 43.2% of patients being female. The most common comorbidities observed were hypertension (41.4%), heart failure (27.2%), type 2 diabetes mellitus (22.5%), and atrial fibrillation (18%). A history of prior cardiac surgery was noted in 11.9% of patients, and 61.2% were classified as NYHA functional class II. Significant clinical and statistical differences were noted between groups regarding prior cardiac surgery (Table 1).
Demographic description of the population.
| Variable | Totaln=555 | Group 1n=399 | Group 2n=131 | Group 3n=21 | Group 4n=4 | p |
|---|---|---|---|---|---|---|
| Women, n(%) | 240(43.2) | 176(44.1) | 52(39.7) | 12(57.1) | 0(0) | 0.13 |
| Men, n(%) | 315(56.8) | 223(55.9) | 79(60.3) | 9(42.86) | 4(100) | |
| Hypertension, n(%) | 230(41.4) | 175(43.9) | 49(37.4) | 6(28.6) | 0(0) | 0.1 |
| Heart failure, n(%) | 151(27.2) | 96(24) | 46(35.1) | 8(38.1) | 1(25) | 0.06 |
| Diabetes, n(%) | 125(22.5) | 89(22.3) | 31(23.7) | 5(23.8) | 0(0) | 0.73 |
| Atrial fibrillation, n(%) | 100(18.0) | 64(16) | 29(22.1) | 7(33.3) | 0(0) | 0.08 |
| Previous cardiac surgery, n(%) | 66(11.9) | 41(10.3) | 17(13) | 7(33.3) | 1(25) | 0.012 |
| Myocardial infarction, n(%) | 63(11.3) | 44(11) | 16(12.2) | 3(14.3) | 0(0) | 0.84 |
| Hypothyroidism, n(%) | 58(10.4) | 39(9.8) | 14(10.7) | 5(23.8) | 0(0) | 0.2 |
| Chronic kidney disease, n(%) | 31(5.6) | 20(5) | 10(7.6) | 1(4.76) | 0(0.0) | 0.67 |
| Stroke, n(%) | 31(5.6) | 23(5.8) | 5(3.8) | 2(9.5) | 1(25.0) | 0.23 |
| Chronic obstructive pulmonary disease, n(%) | 4(0.7) | 2(0.5) | 2(1.53) | 0(0.0) | 0(0.0) | 0.65 |
| NYHA functional class, n(%) | ||||||
| I | 76(13.7) | 54(13.5) | 21(16.1) | 0(0) | 1(25) | 0.099 |
| II | 339(61.2) | 249(62.4) | 74(56.9) | 14(66.7) | 2(50) | |
| III | 121(21.8) | 83(20.8) | 33(25.4) | 4(19) | 1(25) | |
| IV | 18(3.2) | 13(3.3) | 2(1.5) | 3(14.3) | 0(0) | |
| Age (years) | 57(45–65) | 57(45–65) | 57(45–65) | 53(37–63) | 50.5(33–63.6) | 0.7 |
| Weight (kg) | 69(±13.5) | 69.4(±13.2) | 69.34(±14.25) | 61.36(±12.26) | 77.75(±12.12) | 0.03 |
| Height (m) | 1.62(1.55–1.7) | 1.62(1.55–1.7) | 1.61(1.54–1.71) | 1.62(1.54–1.68) | 1.71(1.66–1.76) | 0.37 |
| Body mass index (kg/m2) | 26(23.4–28.8) | 26.1(23.5–28.9) | 26(22.8–28.9) | 23.4(21.6–25.5) | 26.1(23.4–29.5) | 0.02 |
NYHA: New York Heart Association.
Median (interquartil range); mean±standard deviation.
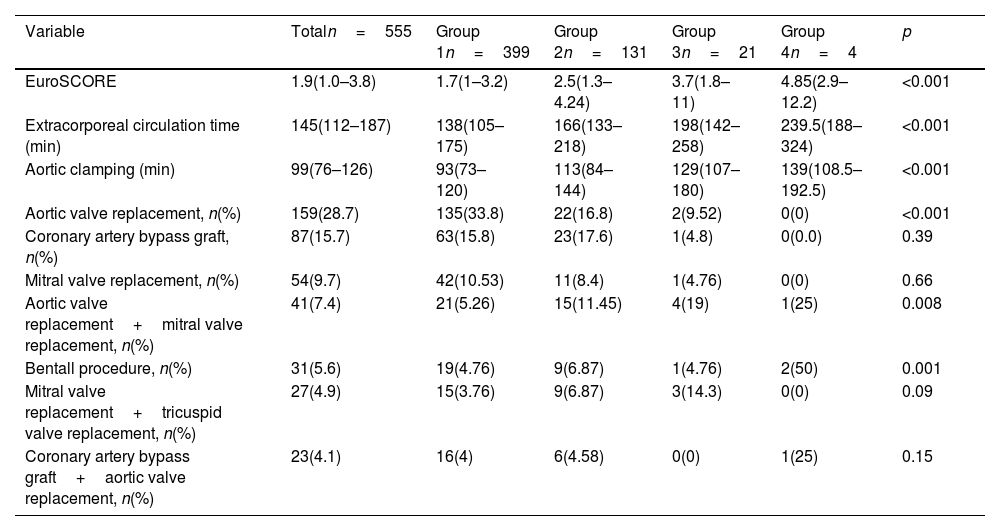
The most common surgical procedures performed were aortic valve replacement (28.7%), coronary revascularization (15.7%), and mitral valve replacement (9.7%). The median duration of extracorporeal circulation (ECC) was 145min, while the median time for aortic clamping was 99min. Patients in Groups 3 and 4 had the highest EuroSCOREs, underwent more complex surgeries involving double valve replacements (mitral and aortic), and experienced longer ECC and aortic clamping times. These differences were both clinically and statistically significant (Table 2).
Surgical characteristics.
| Variable | Totaln=555 | Group 1n=399 | Group 2n=131 | Group 3n=21 | Group 4n=4 | p |
|---|---|---|---|---|---|---|
| EuroSCORE | 1.9(1.0–3.8) | 1.7(1–3.2) | 2.5(1.3–4.24) | 3.7(1.8–11) | 4.85(2.9–12.2) | <0.001 |
| Extracorporeal circulation time (min) | 145(112–187) | 138(105–175) | 166(133–218) | 198(142–258) | 239.5(188–324) | <0.001 |
| Aortic clamping (min) | 99(76–126) | 93(73–120) | 113(84–144) | 129(107–180) | 139(108.5–192.5) | <0.001 |
| Aortic valve replacement, n(%) | 159(28.7) | 135(33.8) | 22(16.8) | 2(9.52) | 0(0) | <0.001 |
| Coronary artery bypass graft, n(%) | 87(15.7) | 63(15.8) | 23(17.6) | 1(4.8) | 0(0.0) | 0.39 |
| Mitral valve replacement, n(%) | 54(9.7) | 42(10.53) | 11(8.4) | 1(4.76) | 0(0) | 0.66 |
| Aortic valve replacement+mitral valve replacement, n(%) | 41(7.4) | 21(5.26) | 15(11.45) | 4(19) | 1(25) | 0.008 |
| Bentall procedure, n(%) | 31(5.6) | 19(4.76) | 9(6.87) | 1(4.76) | 2(50) | 0.001 |
| Mitral valve replacement+tricuspid valve replacement, n(%) | 27(4.9) | 15(3.76) | 9(6.87) | 3(14.3) | 0(0) | 0.09 |
| Coronary artery bypass graft+aortic valve replacement, n(%) | 23(4.1) | 16(4) | 6(4.58) | 0(0) | 1(25) | 0.15 |
Median (interquartil range); mean±standard deviation.
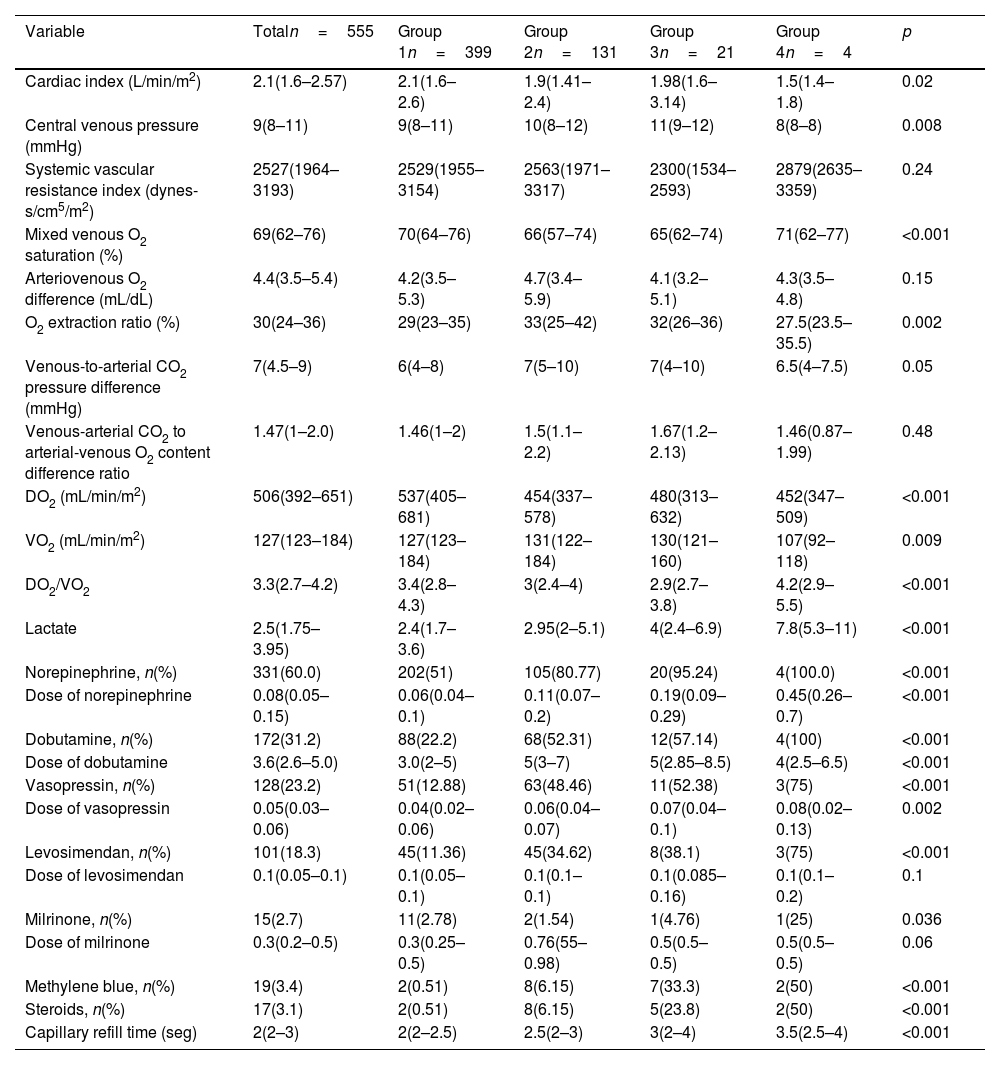
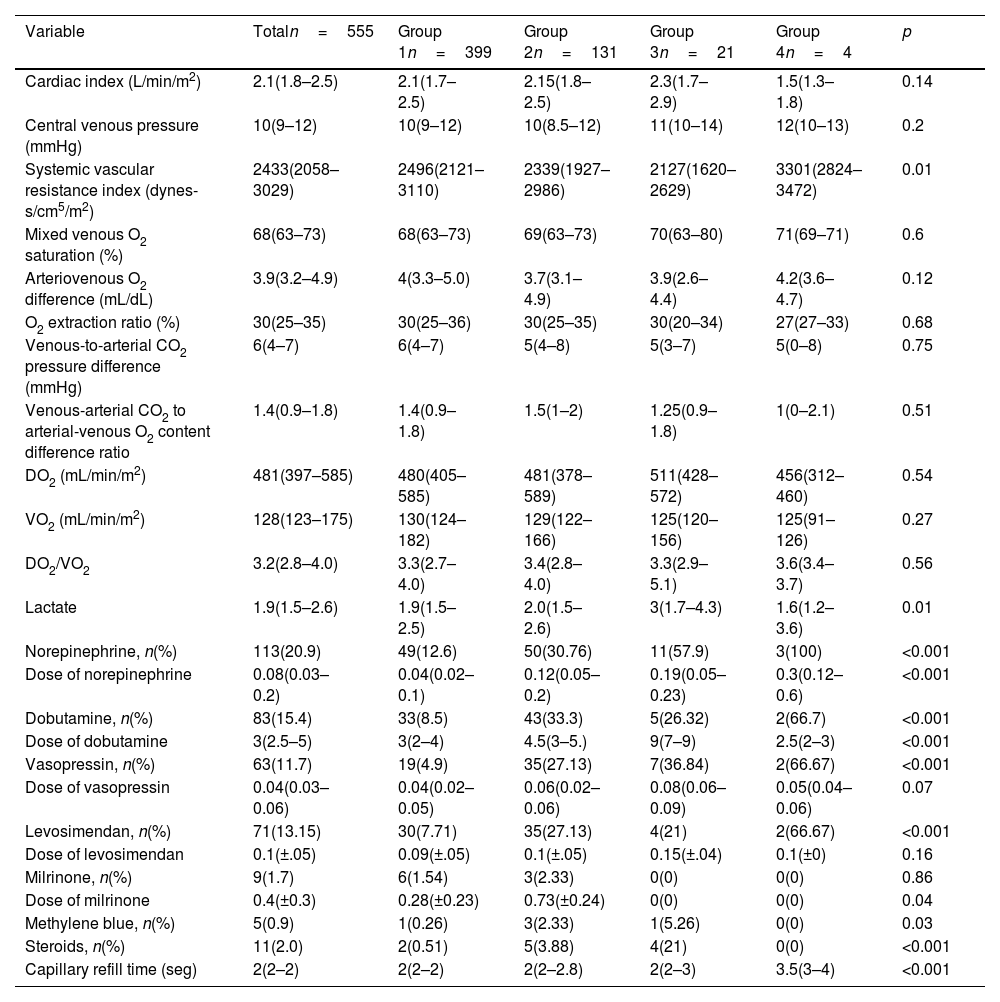
At 6h post-surgery, patients in Groups 3 and 4 showed elevated lactate and prolonged capillary refill times. Additionally, they exhibited a higher need for norepinephrine, dobutamine, vasopressin, levosimendan, methylene blue, and steroids, with a tendency toward requiring higher doses of these medications, especially norepinephrine and dobutamine. By 24h, Group 4 patients had lower cardiac indexes, higher systemic vascular resistance, and continued elevated demands for vasoactive agents, alongside persistent prolonged capillary refill times (Tables 3 and 4).
Hemodynamic variables (6h).
| Variable | Totaln=555 | Group 1n=399 | Group 2n=131 | Group 3n=21 | Group 4n=4 | p |
|---|---|---|---|---|---|---|
| Cardiac index (L/min/m2) | 2.1(1.6–2.57) | 2.1(1.6–2.6) | 1.9(1.41–2.4) | 1.98(1.6–3.14) | 1.5(1.4–1.8) | 0.02 |
| Central venous pressure (mmHg) | 9(8–11) | 9(8–11) | 10(8–12) | 11(9–12) | 8(8–8) | 0.008 |
| Systemic vascular resistance index (dynes-s/cm5/m2) | 2527(1964–3193) | 2529(1955–3154) | 2563(1971–3317) | 2300(1534–2593) | 2879(2635–3359) | 0.24 |
| Mixed venous O2 saturation (%) | 69(62–76) | 70(64–76) | 66(57–74) | 65(62–74) | 71(62–77) | <0.001 |
| Arteriovenous O2 difference (mL/dL) | 4.4(3.5–5.4) | 4.2(3.5–5.3) | 4.7(3.4–5.9) | 4.1(3.2–5.1) | 4.3(3.5–4.8) | 0.15 |
| O2 extraction ratio (%) | 30(24–36) | 29(23–35) | 33(25–42) | 32(26–36) | 27.5(23.5–35.5) | 0.002 |
| Venous-to-arterial CO2 pressure difference (mmHg) | 7(4.5–9) | 6(4–8) | 7(5–10) | 7(4–10) | 6.5(4–7.5) | 0.05 |
| Venous-arterial CO2 to arterial-venous O2 content difference ratio | 1.47(1–2.0) | 1.46(1–2) | 1.5(1.1–2.2) | 1.67(1.2–2.13) | 1.46(0.87–1.99) | 0.48 |
| DO2 (mL/min/m2) | 506(392–651) | 537(405–681) | 454(337–578) | 480(313–632) | 452(347–509) | <0.001 |
| VO2 (mL/min/m2) | 127(123–184) | 127(123–184) | 131(122–184) | 130(121–160) | 107(92–118) | 0.009 |
| DO2/VO2 | 3.3(2.7–4.2) | 3.4(2.8–4.3) | 3(2.4–4) | 2.9(2.7–3.8) | 4.2(2.9–5.5) | <0.001 |
| Lactate | 2.5(1.75–3.95) | 2.4(1.7–3.6) | 2.95(2–5.1) | 4(2.4–6.9) | 7.8(5.3–11) | <0.001 |
| Norepinephrine, n(%) | 331(60.0) | 202(51) | 105(80.77) | 20(95.24) | 4(100.0) | <0.001 |
| Dose of norepinephrine | 0.08(0.05–0.15) | 0.06(0.04–0.1) | 0.11(0.07–0.2) | 0.19(0.09–0.29) | 0.45(0.26–0.7) | <0.001 |
| Dobutamine, n(%) | 172(31.2) | 88(22.2) | 68(52.31) | 12(57.14) | 4(100) | <0.001 |
| Dose of dobutamine | 3.6(2.6–5.0) | 3.0(2–5) | 5(3–7) | 5(2.85–8.5) | 4(2.5–6.5) | <0.001 |
| Vasopressin, n(%) | 128(23.2) | 51(12.88) | 63(48.46) | 11(52.38) | 3(75) | <0.001 |
| Dose of vasopressin | 0.05(0.03–0.06) | 0.04(0.02–0.06) | 0.06(0.04–0.07) | 0.07(0.04–0.1) | 0.08(0.02–0.13) | 0.002 |
| Levosimendan, n(%) | 101(18.3) | 45(11.36) | 45(34.62) | 8(38.1) | 3(75) | <0.001 |
| Dose of levosimendan | 0.1(0.05–0.1) | 0.1(0.05–0.1) | 0.1(0.1–0.1) | 0.1(0.085–0.16) | 0.1(0.1–0.2) | 0.1 |
| Milrinone, n(%) | 15(2.7) | 11(2.78) | 2(1.54) | 1(4.76) | 1(25) | 0.036 |
| Dose of milrinone | 0.3(0.2–0.5) | 0.3(0.25–0.5) | 0.76(55–0.98) | 0.5(0.5–0.5) | 0.5(0.5–0.5) | 0.06 |
| Methylene blue, n(%) | 19(3.4) | 2(0.51) | 8(6.15) | 7(33.3) | 2(50) | <0.001 |
| Steroids, n(%) | 17(3.1) | 2(0.51) | 8(6.15) | 5(23.8) | 2(50) | <0.001 |
| Capillary refill time (seg) | 2(2–3) | 2(2–2.5) | 2.5(2–3) | 3(2–4) | 3.5(2.5–4) | <0.001 |
Median (interquartil range); mean±standard deviation.
Hemodynamic variables (24h).
| Variable | Totaln=555 | Group 1n=399 | Group 2n=131 | Group 3n=21 | Group 4n=4 | p |
|---|---|---|---|---|---|---|
| Cardiac index (L/min/m2) | 2.1(1.8–2.5) | 2.1(1.7–2.5) | 2.15(1.8–2.5) | 2.3(1.7–2.9) | 1.5(1.3–1.8) | 0.14 |
| Central venous pressure (mmHg) | 10(9–12) | 10(9–12) | 10(8.5–12) | 11(10–14) | 12(10–13) | 0.2 |
| Systemic vascular resistance index (dynes-s/cm5/m2) | 2433(2058–3029) | 2496(2121–3110) | 2339(1927–2986) | 2127(1620–2629) | 3301(2824–3472) | 0.01 |
| Mixed venous O2 saturation (%) | 68(63–73) | 68(63–73) | 69(63–73) | 70(63–80) | 71(69–71) | 0.6 |
| Arteriovenous O2 difference (mL/dL) | 3.9(3.2–4.9) | 4(3.3–5.0) | 3.7(3.1–4.9) | 3.9(2.6–4.4) | 4.2(3.6–4.7) | 0.12 |
| O2 extraction ratio (%) | 30(25–35) | 30(25–36) | 30(25–35) | 30(20–34) | 27(27–33) | 0.68 |
| Venous-to-arterial CO2 pressure difference (mmHg) | 6(4–7) | 6(4–7) | 5(4–8) | 5(3–7) | 5(0–8) | 0.75 |
| Venous-arterial CO2 to arterial-venous O2 content difference ratio | 1.4(0.9–1.8) | 1.4(0.9–1.8) | 1.5(1–2) | 1.25(0.9–1.8) | 1(0–2.1) | 0.51 |
| DO2 (mL/min/m2) | 481(397–585) | 480(405–585) | 481(378–589) | 511(428–572) | 456(312–460) | 0.54 |
| VO2 (mL/min/m2) | 128(123–175) | 130(124–182) | 129(122–166) | 125(120–156) | 125(91–126) | 0.27 |
| DO2/VO2 | 3.2(2.8–4.0) | 3.3(2.7–4.0) | 3.4(2.8–4.0) | 3.3(2.9–5.1) | 3.6(3.4–3.7) | 0.56 |
| Lactate | 1.9(1.5–2.6) | 1.9(1.5–2.5) | 2.0(1.5–2.6) | 3(1.7–4.3) | 1.6(1.2–3.6) | 0.01 |
| Norepinephrine, n(%) | 113(20.9) | 49(12.6) | 50(30.76) | 11(57.9) | 3(100) | <0.001 |
| Dose of norepinephrine | 0.08(0.03–0.2) | 0.04(0.02–0.1) | 0.12(0.05–0.2) | 0.19(0.05–0.23) | 0.3(0.12–0.6) | <0.001 |
| Dobutamine, n(%) | 83(15.4) | 33(8.5) | 43(33.3) | 5(26.32) | 2(66.7) | <0.001 |
| Dose of dobutamine | 3(2.5–5) | 3(2–4) | 4.5(3–5.) | 9(7–9) | 2.5(2–3) | <0.001 |
| Vasopressin, n(%) | 63(11.7) | 19(4.9) | 35(27.13) | 7(36.84) | 2(66.67) | <0.001 |
| Dose of vasopressin | 0.04(0.03–0.06) | 0.04(0.02–0.05) | 0.06(0.02–0.06) | 0.08(0.06–0.09) | 0.05(0.04–0.06) | 0.07 |
| Levosimendan, n(%) | 71(13.15) | 30(7.71) | 35(27.13) | 4(21) | 2(66.67) | <0.001 |
| Dose of levosimendan | 0.1(±.05) | 0.09(±.05) | 0.1(±.05) | 0.15(±.04) | 0.1(±0) | 0.16 |
| Milrinone, n(%) | 9(1.7) | 6(1.54) | 3(2.33) | 0(0) | 0(0) | 0.86 |
| Dose of milrinone | 0.4(±0.3) | 0.28(±0.23) | 0.73(±0.24) | 0(0) | 0(0) | 0.04 |
| Methylene blue, n(%) | 5(0.9) | 1(0.26) | 3(2.33) | 1(5.26) | 0(0) | 0.03 |
| Steroids, n(%) | 11(2.0) | 2(0.51) | 5(3.88) | 4(21) | 0(0) | <0.001 |
| Capillary refill time (seg) | 2(2–2) | 2(2–2) | 2(2–2.8) | 2(2–3) | 3.5(3–4) | <0.001 |
Median (interquartil range); mean±standard deviation.
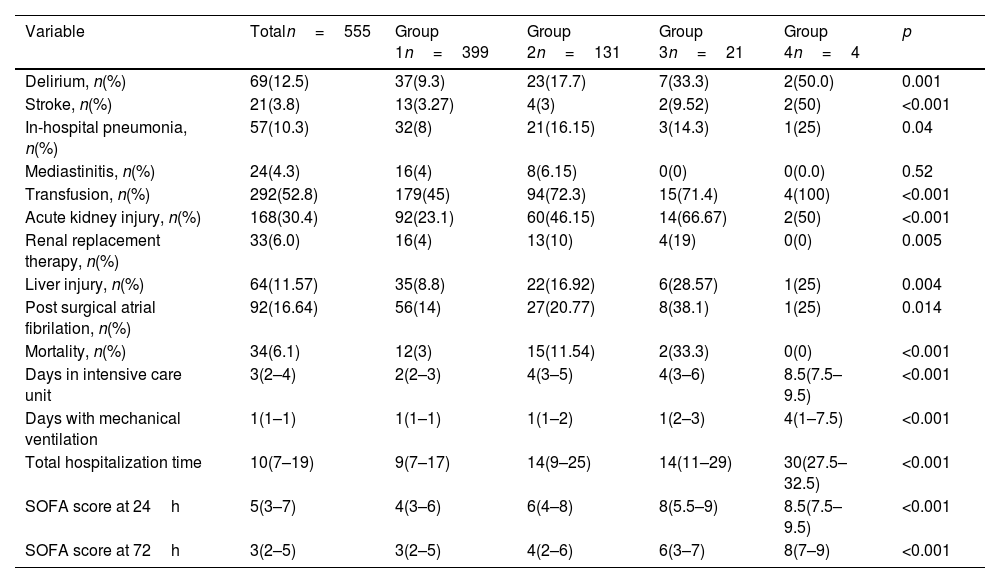
Groups 3 and 4 exhibited significantly higher rates of postoperative complications compared to the other groups. Specifically, this group experienced the highest incidence of delirium (50%), cerebrovascular events (50%), pneumonia (25%), transfusion requirements (100%), acute kidney injury (66.67%), renal replacement therapy (19%), liver injury (28.57%), postoperative atrial fibrillation (38.1%), and mortality (33.3%). Additionally, Group 4 required longer stays in the intensive care unit (ICU), prolonged mechanical ventilation, and extended total hospitalization, as well as the highest SOFA scores at 24 and 72h (Table 5).
Outcomes.
| Variable | Totaln=555 | Group 1n=399 | Group 2n=131 | Group 3n=21 | Group 4n=4 | p |
|---|---|---|---|---|---|---|
| Delirium, n(%) | 69(12.5) | 37(9.3) | 23(17.7) | 7(33.3) | 2(50.0) | 0.001 |
| Stroke, n(%) | 21(3.8) | 13(3.27) | 4(3) | 2(9.52) | 2(50) | <0.001 |
| In-hospital pneumonia, n(%) | 57(10.3) | 32(8) | 21(16.15) | 3(14.3) | 1(25) | 0.04 |
| Mediastinitis, n(%) | 24(4.3) | 16(4) | 8(6.15) | 0(0) | 0(0.0) | 0.52 |
| Transfusion, n(%) | 292(52.8) | 179(45) | 94(72.3) | 15(71.4) | 4(100) | <0.001 |
| Acute kidney injury, n(%) | 168(30.4) | 92(23.1) | 60(46.15) | 14(66.67) | 2(50) | <0.001 |
| Renal replacement therapy, n(%) | 33(6.0) | 16(4) | 13(10) | 4(19) | 0(0) | 0.005 |
| Liver injury, n(%) | 64(11.57) | 35(8.8) | 22(16.92) | 6(28.57) | 1(25) | 0.004 |
| Post surgical atrial fibrilation, n(%) | 92(16.64) | 56(14) | 27(20.77) | 8(38.1) | 1(25) | 0.014 |
| Mortality, n(%) | 34(6.1) | 12(3) | 15(11.54) | 2(33.3) | 0(0) | <0.001 |
| Days in intensive care unit | 3(2–4) | 2(2–3) | 4(3–5) | 4(3–6) | 8.5(7.5–9.5) | <0.001 |
| Days with mechanical ventilation | 1(1–1) | 1(1–1) | 1(1–2) | 1(2–3) | 4(1–7.5) | <0.001 |
| Total hospitalization time | 10(7–19) | 9(7–17) | 14(9–25) | 14(11–29) | 30(27.5–32.5) | <0.001 |
| SOFA score at 24h | 5(3–7) | 4(3–6) | 6(4–8) | 8(5.5–9) | 8.5(7.5–9.5) | <0.001 |
| SOFA score at 72h | 3(2–5) | 3(2–5) | 4(2–6) | 6(3–7) | 8(7–9) | <0.001 |
Median (interquartil range); mean±standard deviation.
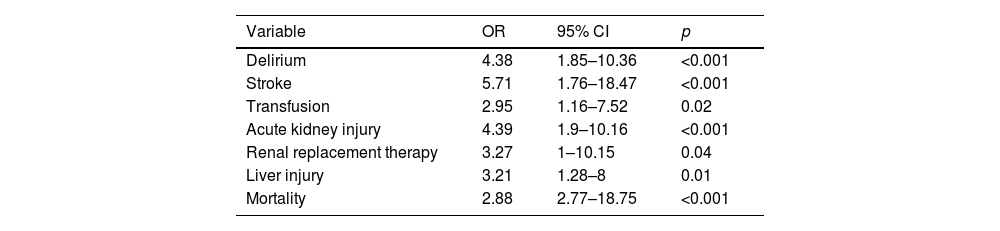
The logistic regression model identified the presence of two or more postoperative syndromes (Groups 3 and 4) as a significant predictor for several adverse outcomes. Specifically, delirium was associated with an odds ratio (OR) of 4.38 (95% CI 1.85–10.36), cerebrovascular events with an OR of 5.71 (95% CI 1.76–18.47), transfusion requirements with an OR of 2.95 (95% CI 1.16–7.52), acute kidney injury with an OR of 4.39 (95% CI 1.9–10.16), renal replacement therapy with an OR of 3.27 (95% CI 1–10.15), and in-hospital mortality with an OR of 2.88 (95% CI 2.77–18.75). Additionally, liver injury was significantly predicted by the presence of two or more postoperative syndromes, with an OR of 3.21 (95% CI 1.28–8) (Table 6).
Logistic regression for the prediction of outcomes according to the presence of two or more postsurgical syndromes.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Delirium | 4.38 | 1.85–10.36 | <0.001 |
| Stroke | 5.71 | 1.76–18.47 | <0.001 |
| Transfusion | 2.95 | 1.16–7.52 | 0.02 |
| Acute kidney injury | 4.39 | 1.9–10.16 | <0.001 |
| Renal replacement therapy | 3.27 | 1–10.15 | 0.04 |
| Liver injury | 3.21 | 1.28–8 | 0.01 |
| Mortality | 2.88 | 2.77–18.75 | <0.001 |
This study demonstrates clear associations between postoperative syndromes and adverse outcomes in patients undergoing cardiac surgery with CPB. A significant portion of the population either did not experience postoperative syndromes (71.9%) or experienced one (23.6%), while only a small proportion developed two (3.8%) or three or more syndromes (0.72%). The average patient age was 57 years, and comorbidities such as hypertension, heart failure, type 2 diabetes, and atrial fibrillation were common, suggesting that patients with multiple postoperative syndromes often have more complex medical histories that may contribute to the severity of their postoperative conditions.11,12
Patients with more than one postoperative syndrome (Groups 3 and 4) had longer ECC and aortic clamping times, higher EuroSCOREs, and a greater incidence of complex surgeries. These factors indicate that the patients in these groups had more challenging surgical procedures, which likely predisposed them to worse outcomes.10,11 The elevated lactate levels and greater need for vasoactive drugs in these groups suggest more pronounced hemodynamic instability.2,9
The clinical outcomes were markedly worse for patients in Group 4, who experienced the highest rates of complications, including delirium, cerebrovascular events, pneumonia, acute renal injury, and in-hospital mortality. These findings align with the idea that the number of postoperative syndromes is directly proportional to the severity and frequency of complications.5,6 The logistic regression analysis further substantiated this, identifying the presence of two or more postoperative syndromes as a significant predictor of severe complications.7,9
Study limitationsThis study was conducted at a single medical center and should be replicated at other centers to assess the reproducibility of our results. In addition, study outcomes should be interpreted with caution due to the small sample size and because the inclusive study design leads to significant variability in the evaluated variables, including types of procedures performed, CPB and cross-clamp times, and procedure urgency, among others.
ConclusionsThe study's findings highlight the significant association between multiple postoperative syndromes and increased morbidity and mortality in patients undergoing cardiac surgery with ECC. Patients with a higher burden of postoperative syndromes are at greater risk for severe complications, including delirium, renal injury, and intrahospital mortality. These results underscore the importance of tailored, intensive postoperative management for patients with multiple syndromes to optimize recovery and reduce the incidence of adverse outcomes.
CRediT authorship contribution statementJAZP: original draft writing, review, and editing; RGN: methodology, analysis; GRV: review; DMS: original idea, methodology, analysis, original draft writing, review, and editing.
Ethical approvalThe local institutional research and ethics committees (Instituto Nacional de Cardiología Ignacio Chávez) waived approval for this study.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that there are no conflicts of interest to disclose.
To all the staff of the Cardiovascular Critical Care Unit of the Instituto Nacional de Cardiología Ignacio Chávez.