Sodium liquid glass silicate compositions modified with boron cluster anions [B10Cl10]2− or [B10H10]2− or SiO2 nanoparticles have been studied. It is shown that in the silicate matrix, the presence of the polyhedral anions results in nanoscale supramolecular structures formed due to their interaction with silanol groups of silicates. In the range of 20–600°C, the TMA method is used to study the formation of supramolecular associates, as well as the deformation stability of the modified silicate matrix and the softening temperature of the modified polysilicates formed upon thermal exposure.
Se han estudiado composiciones de silicato de vidrio líquido de sodio modificadas con aniones poliédricos [B10Cl10]2− o [B10H10]2− o nanopartículas de SiO2. Se muestra que en la matriz de silicato, la presencia de los aniones poliédricos da como resultado estructuras supramoleculares a nanoescala formadas debido a su interacción con grupos de silicatos de silanol. En el rango de 20–600°C, TMA se ha utilizado para estudiar la formación de asociados supramoleculares, así como la estabilidad a la deformación de la matriz de silicato modificado y la temperatura de reblandecimiento de los polisilicatos modificados formados tras la exposición térmica.
The boron cluster anions [BnHn]2− as well as their analogs (carboranes, metallocarboranes) are unique objects in boron chemistry [1–5] because of their structures and properties. From fundamental point of view, they are interesting because they possess the 3D aromatic structure, tendency to act as ligands in coordination chemistry [6–8] and form a great variety of substituted derivatives of terminal hydrogen atoms with various functional groups [9–11].
Traditionally, the most studied areas of the application of compounds and materials based on boron cluster anions and their derivatives have been considered fields of science associated with the high power intensity of boron hydride compounds. Later, interest in boron clusters increased due to the high neutron-absorbing ability of the boron atom; boron cluster anions were proposed to be used for manufacturing heat-resistant polymers, neutron-protective coatings with good adhesion to various materials, neutron-protective tissues and materials, and contrast agents for MRI diagnostics [12–14]. In addition, complex metal compounds with boron cluster anions can be used as extractants of heavy metals, precursors of complex metal boride compounds, coordination polymers, compounds with high energy intensity, etc. [15–18].
A relative new field of application of boron clusters anions is their use to create compositions with liquid sodium glass to manufacture materials promising to be used as neutron-protective materials. We succeeded in preparing first compositions based on sodium liquid glass (LG) and triethylammonium salt of the [B10H10]2− anion (Et3NH)2[B10H10] with its high content 30–60wt% [19–21] which can be used to develop neutron-absorbing materials resistant to deformation up to 600°C [22]. The compositions were studied by IR spectroscopy and the data revealed non-bonding interactions between BH-groups of the boron cluster and OH-groups of silicates. Analogous compositions containing 15–60% of the [B12H12]2− anion in LG revealed that the substituted derivative of the closo-dodecaborate anion [B12H11NH3]− is formed in the system resulting in plasticizing effect of materials prepared [23]. At the concentration of boron clusters higher that 45%, crystals of Na2[B12H12] can be formed on the surface of the silicate matrix [24].
In the present work, we have prepared borosilicate composites based on sodium liquid glass (LG) and the decahydro-closo-decaborate anion [B10H10]2− or its perchlorated analog [B10Cl10]2−; the latter is first used to prepare LG/[An] compositions. Both clusters were added at low concentrations (2–30wt%). Supramolecular structure of composites has been discussed based on IR spectroscopy data. Thermomechanical properties of compositions have been studied in comparison with properties of compositions LG/nanoSiO2 containing model SiO2 nanoparticles.
ExperimentalSynthesisSilicate matrix used in this work is a 30% aqueous solution of sodium silicate (silicate module 2.9, pH=10) [25]. [Et3NH]2[B10H10] was synthesized from decaborane-14 according to the known procedure [26]. The reaction between [Et3NH]2[B10H10] and KOH afforded K2[B10H10] [27]. Chlorination of K2[B10H10] by chlorine in water was carried out in accordance with the known procedure [28] to form K2[B10Cl10]. Triethylammonium decahloro-closo-decaborate [Et3NH]2[B10Cl10] precipitated from reaction solutions containing K2[B10Cl10] and [Et3NH]Cl [29].
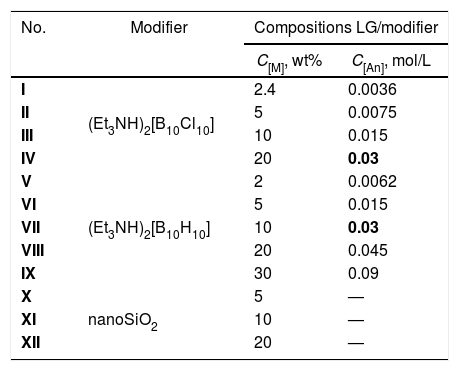
LG/[An] compositions I–IX were prepared by dissolving [Et3NH]2[An] (where An=[B10H10]2− or [B10Cl10]2−) in an aqueous solution of liquid glass (LG) in air at room temperature using the procedure similar to that described for [B10H10]2− used at high content (30–60wt%) [19]. The reaction solutions of starting compositions were allowed to stand at 95–100°C for 3–4h to remove triethylamine (detected by organoleptic method) and water. The process was regulated by IR spectroscopy. The resulting solids were heated at 200°C for 2h to release water adsorbed. The composition of samples I–IX are shown in Table 1.
Compositions of liquid glass with modifiers (Et3NH)2[B10Cl10] (I–IV), (Et3NH)2[B10H10] (V–IX), and nanoSiO2 (X–XII). C[M] is weight (wt%) or molar (mol/L) content of the modifier in the composition.
| No. | Modifier | Compositions LG/modifier | |
|---|---|---|---|
| C[M], wt% | C[An], mol/L | ||
| I | (Et3NH)2[B10Cl10] | 2.4 | 0.0036 |
| II | 5 | 0.0075 | |
| III | 10 | 0.015 | |
| IV | 20 | 0.03 | |
| V | (Et3NH)2[B10H10] | 2 | 0.0062 |
| VI | 5 | 0.015 | |
| VII | 10 | 0.03 | |
| VIII | 20 | 0.045 | |
| IX | 30 | 0.09 | |
| X | nanoSiO2 | 5 | — |
| XI | 10 | — | |
| XII | 20 | — | |
Nanosized particles of silicon oxide SiO2 were obtained in situ by acid hydrolysis of tetraethoxysilane in isopropyl alcohol. The concentration of SiO2 particles in isopropanol was 6.6wt%. The solution was optically transparent. Dynamic light scattering data confirm that the particle size does not exceed 15nm. The content of the ethoxy groups in the process of hydrolysis was monitored by IR spectroscopy; the reaction was carried out until the absorption band of stretching vibrations ν(CH) with a maximum at 2976cm−1 disappeared. After hydrolysis, the particles formed were stabilized with a 0.15M HCl solution.
LG/nanoSiO2 compositions X–XII were prepared through a co-solution. NanoSiO2 in isopropanol (6.6wt%) was added in 10wt% LG, and the solvent was removed at 100°C. The composition of samples X–XII are shown in Table 1.
MethodsIR spectra of solid samples were recorded in thin layer on a Bruker Vertex 70 IR-Fourier spectrophotometer equipped with a GladiATR diamond crystal plate from Pike Technologies. Spectra were recorded in the range of 4000–600cm−1 at a resolution of 4cm−1. IR spectra of starting compounds and compositions I–IX are shown in Figs. S1–S8 (see Supporting Information).
Thermal and thermooxidative properties were studied on a Netzsch 449F3 derivatograph at a heating rate of 10K/min in air. Thermomechanical properties were studied on a Netzsch TMA-420 testing machine (Germany) equipped with a spherical indenter of a diameter of 3mm when loading 50g at a heating rate of 10K/min. Measurements were carried out in air. Compressed samples with a height of 1mm were used in the experiments. TGA and TGA/DSC data for starting compounds and samples I–IX are shown in Figs. S9–S26 (see Supporting Information). Softening temperature Tsoft was determined as the intersection of the “linear” portion of the started section of the thermomechanical curve and the “linear” portion of the curve at the maximum slope point.
Scanning electron microscopy (SEM) for II, IV, and IX was carried out on a CAMSCAN-S2 instrument, accelerating voltage 20kV, focal length 10mm. Images were obtained in the secondary electron mode. Transmitting electron microscopy (TEM) for samples II, IV, and IX was performed on a Jem-1011 instrument at an accelerating voltage of 80kV. The samples were applied on carbon films by ultrasonic dispersion. The TEM and SEM data are shown in Figs. S27–S32 (see Supporting Information).
Light scattering on colloidal SiO2 particles was studied using a PhotocorComplex spectrometer [30]. The principle of the operation of the spectrometer is based on dynamic light scattering (photon correlation spectroscopy). The essence of the method is as follows: when a laser beam passes through a test liquid containing dispersed particles, part of the light is scattered by fluctuations of particles. Scattered light is recorded by a photodetector, and the signal is processed by a digital correlator. From the obtained autocorrelation function, the average particle size was calculated by the DynaLS program.
X-ray powder diffraction was recorded on a Bruker D8 Advance Vario diffractometer indicated that the compositions LG/[An] are amorphous. As an example, Fig. S33 shows the X-ray powder diffraction pattern of sample IV.
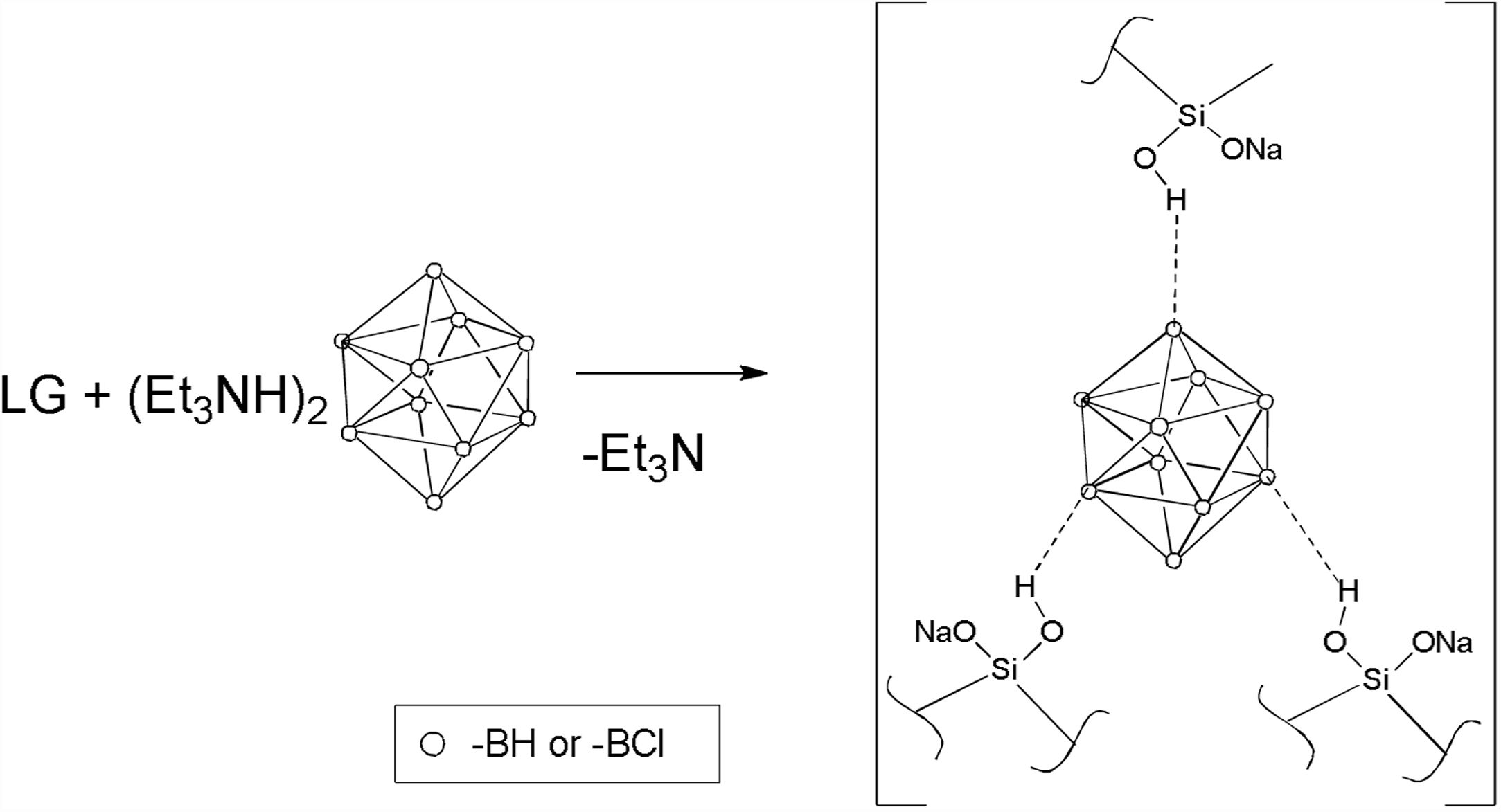
Results and discussionPreparation of compositions LG/[An] and LG/nanoSiO2In this work, we used polysilicates of sodium liquid glass. Liquid glass is a solution of sodium silicates with different degree of condensation; under temperature, polymeric derivatives are formed due to their condensation of silicates accompanied with the releasing of water and formation of siloxane bonds [25]. To modify polysilicates, we used water-soluble triethylammonium salts of boron cluster anions [Et3NH]2[An] (where An=[B10H10]2− or [B10Cl10]2−), the content of which in the initial LG compositions was 2–30wt%. When (Et3NH)2[An] was allowed to react with sodium silicates (which contain NaOH), the [Et3NH]+ cations transformed to triethylamine Et3N which released from the reaction mixture (Scheme 1). The interaction between (Et3NH)2[B10H10] and LG was discussed early [19]. Therefore, the formed compositions I–IX contained the silicate matrix with the boron cluster anions distributed in it.
For comparison of thermomechanical properties, compositions LG/nanoSiO2 were prepared with different amount of nanoparticles (samples X–XII). The content of initial components used to prepare target compositions are listed in Table 1.
IR spectra of compositions LG/[An] and their structureIn the IR spectra of compositions I–IX, bands characteristic of sodium liquid glass ν(SiO) and boron clusters ν(BH) (for the [B10H10]2− anion) or ν(BCl) (for the [B10Cl10]2− anion) are observed; however the bands ν(BH) or ν(BCl) are very weak because of small amounts of the boron modifiers. IR spectra of starting compounds and compositions prepared are shown in Figs. S1–S8 (see Supporting Information).
Previously, for composition LG/(Et3NH)2[B10H10] (40/60wt%) with high content of the boron cluster [19], it was shown that in the IR spectra, the stretching vibration band of the Si–O bonds is shifted to the high-frequency region as compared to initial LG. Moreover, a strong splitting of the ν(BH) band is also observed, which indicate the formation of dihydrogen interactions between BH groups of the borohydride cluster and OH groups of the silicate matrix.
Fig. S1 (see Supporting Information) shows the IR spectra of compositions LG/(Et3NH)2[B10Cl10] (IV) and LG/(Et3NH)2[B10H10] (IX) formed in the presence of 20 and 30wt% of boron clusters, respectively, and the data were compared with started reagents (salts (Et3NH)2[B10Cl10], (Et3NH)2[B10H10], and LG) in the region 1300–700cm−1. The shift of the ν(Si–O) band to the high-frequency region for both samples in comparison with initial LG indicates a deep structuring of the silicates due to the formation of associates with boron clusters. The splitting of the ν(BH) band (in the 2400–2500cm−1) cannot be discussed because of its low intensity, therefore this region is not shown in Fig. S1.
We assume that the [B10H10]2− form spatially-branched supramolecular three-dimensional structures due to specific multicenter contacts with silicates (B–Hδ−…Hδ+–O–Si) and water molecules (B–Hδ−…Hδ+–O), which schematically are shown in Scheme 1. The property of the borohydride [B10H10]2− anion to form dihydrogen contacts B–H…H–X (X=C, O, N) was found previously in its salts with organic cations (tetraphenylphosphonium Ph4P+, alkylammonium R4–xNHx+, where R=Me, Et, Pr, Bu; x=1–4) and/or N-and O-containing solvent molecules [31]. We believe that the interaction between LG and [Et3NH]2[B10Cl10] proceeds similarly with the distribution of the [B10Cl10]2− anion in the silicate matrix, because this anion is also known to form non-bonding interactions in solids. Non-covalent interactions with the Cl atoms of the [B10Cl10]2− anion cannot be identified by IR spectroscopy because no shifts or splitting of the ν(BCl) bands are observed in the spectra. Nevertheless, Cl…H–X and Cl…X (X=C, N, O) in salts and complexes containing the [B10Cl10]2− anion were first found and identified by 35Cl nuclear quadrupole resonance (NQR) which correlate with the X-ray diffraction data [27,29,32–34]. Here, we cannot measure and discuss 35Cl NQR spectra of compositions because of low concentration of the perhalogenated cluster in samples. Nevertheless, we concluded that supramolecular associates can be formed in compositions LG/[An] with both boron clusters because of these specific non-bonding interactions.
Thermomechanical properties of compositions LG/[An]The effect of the boron cluster content on thermomechanical properties of the resulting LG/[An] compositions was studied. Thermomechanical analysis (TMA) was used as the main method to study the mobility of the LG/[An] systems and initial reagents. TGA and TGA/DSC data for starting compounds and samples I–IX are shown in Figs. S9–S26 (see Supporting Information).
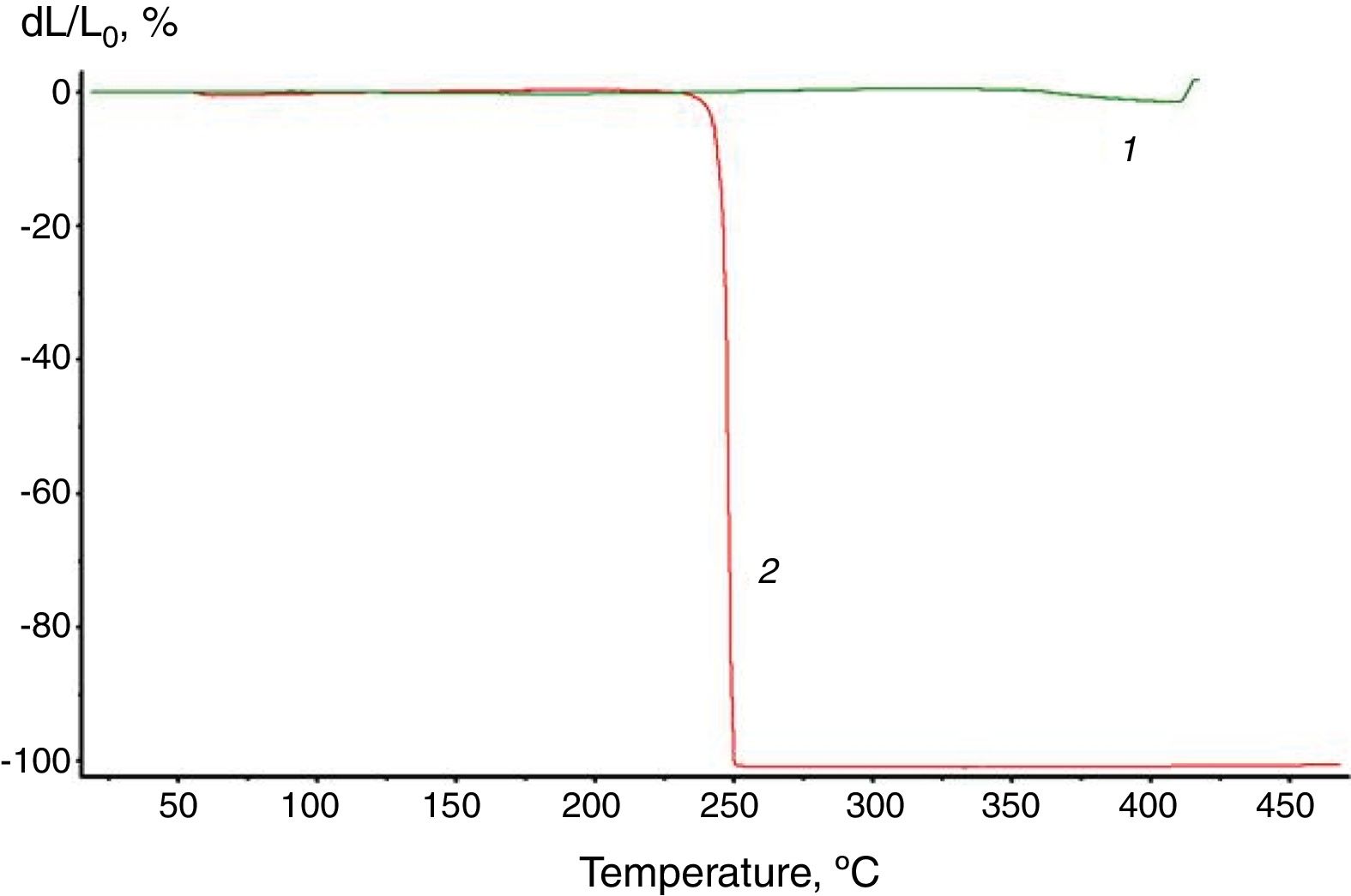
Analyzing the TMA curves of the starting modifiers (Fig. 1), it was found that [Et3NH]2[B10Cl10] does not soften up to the destruction temperature of 420°C, whereas for [Et3NH]2[B10H10] the softening point is Tsoft=245°C. The results obtained are in good agreement with the properties of triethylammonium salts (Et3NH)2[An] to participate in specific interactions with the CH and NH groups of organic cations.
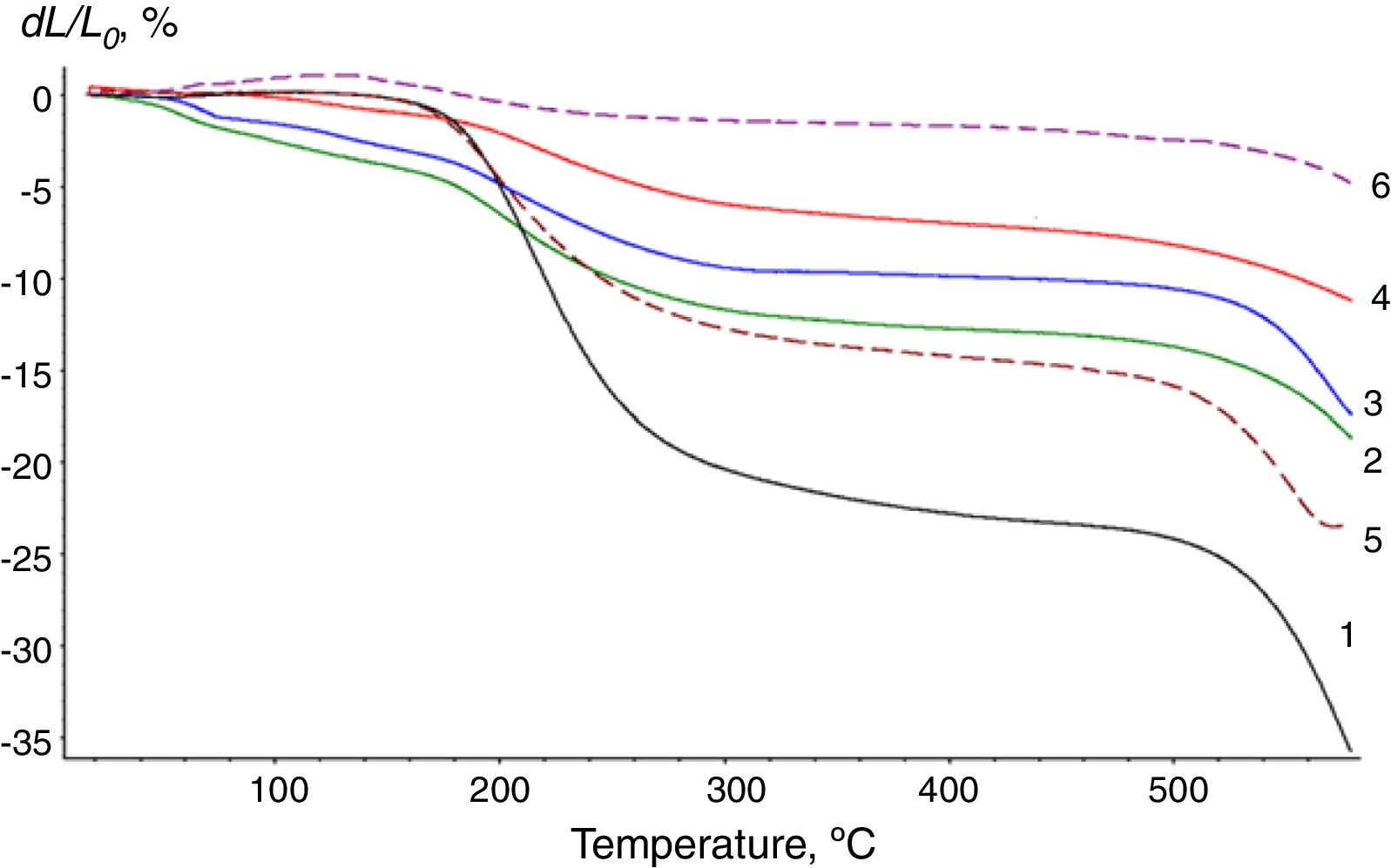
Fig. 2 shows the TMA curves for the LG/[An] compositions. It is clear that the boron modifier affects the molecular mobility of the compositions. Two distinct transitions with Tsoft=196 and 531°C are observed on the TMA curve of initial LG (Fig. 2, curve 1) characterizing the softening process. It is known that in the range of 180–220°C, polycondensation of silicates with the participation of silanol groups intensively proceeds, which is accompanied by the release of water, in which silicates are dissolved partially to form a viscous mass. Thus, in this temperature range, water plasticizes LG as evidenced by an increase in the dL/L0 values. The polycondensation of silicates proceeds up to 500°C, and Tsoft=531°C on curve 1 characterizes the softening of polysilicates with the maximum polycondensation degree [25].
As can be seen from Fig. 2, the shape of the TMA curves for the prepared compositions modified with low amounts of additives (curves 2–6) differs significantly from curve 1. In the presence of the modifier, the first transition is observed in the same temperature range as for initial LG. The shape of the curves changes with a further increase in temperature. As the content of the modifier increases, values dL/L0 decrease. Even for sample II with only 5% of the perchlorated modifier, the value of dL/L0 characterizing the softening degree is almost two times lower as compared with initial LG, which may result from a decreased amount of water released during the condensation of silanol groups. Reduced molecular mobility can be associated not only with a decreased amount of the plasticizer (water), but also with the structuring of the system due to the presence of the modifier capable to form long-range contacts and act as a cross-linking agent for polysiloxane chains. Therefore, TMA studies demonstrated that LG silicates predominantly participate in the formation of supramolecular structures LG/[An]. At the same time, the [B10Cl10]2− anion forms a larger number of energetically more stable contacts with LG silicates as compared with the [B10H10]2− anion.
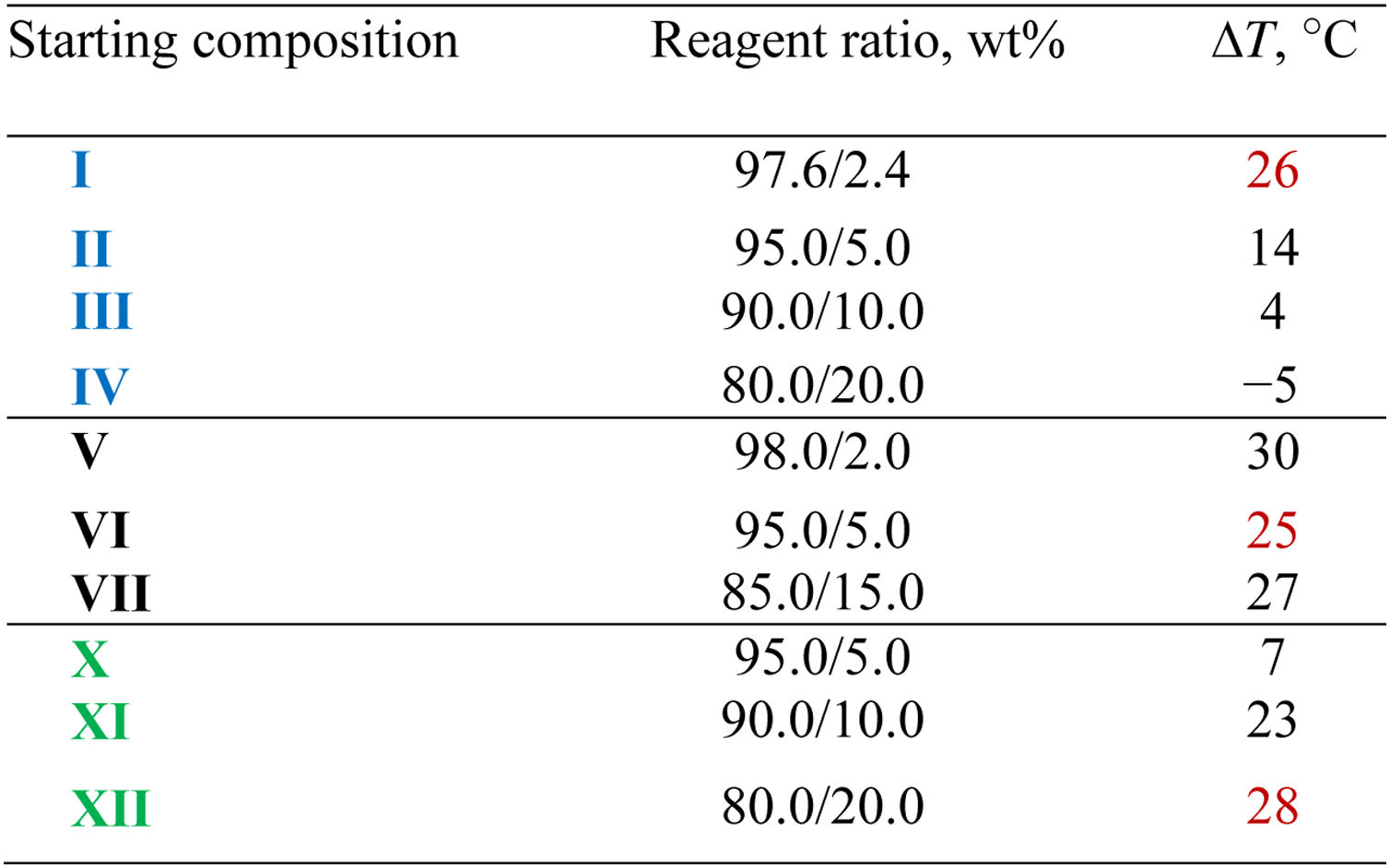
For polysilicates with the maximum polycondensation degree, special attention should be paid to transitions observed in the range 500–600°C. Two opposite factors affect Tsoft of polysilicates, which is related to structural features of the systems. The first is the loosening of the package of polysiloxane chains with the modifier, which may result in a decrease in Tsoft. The second is the cross-linking of polysilicate chains due to contacts with the modifier, which leads to an increase in Tsoft. The prevailing influence of one of these factors can be given by the value ΔT=Tsoft(LG)−Tsoft(LG/[An]).
Analyzing the results obtained, it is obvious that for the equimolar content of the modifier (being 0.03mol/L, sample IV for [B10Cl10]2− and sample VII for [B10H10]2−, shown in bold in Table 1), the deformation stability of the LG/[B10Cl10]2− system is significantly higher as compared to the LG/[B10H10]2− system. This indicates a deeper structuring of the system when forming supramolecular ensembles in the presence of the perchlorated anion. It should be noted that with the increased concentration of the perchlorated modifier in the initial composition from 5 to 20wt%, the deformation resistance of the LG/[B10Cl10]2− system increases as indicated by decreased dL/L0 values (Fig. 2).
From the result obtained, it was suggested that a higher degree of structuring of the composition obtained in the presence of the [B10Cl10]2− anions is associated not only with larger dimensions of the perchlorated boron cluster as compared to the [B10H10]2− anion but also with the presence of more electronegative atoms Clδ−. As a result, larger and more stable associates are formed in the LG/[B10Cl10]2− system. The resulting compositions can be considered as inorganic polymers modified with boron clusters giving boron-containing supramolecular aggregates. Different nature of the boron clusters defines higher molecular mobility of the system LG/[B10H10]2− as compared with LG/[B10Cl10]2−.
The structuring degree in the LG/[An] system increases with the increasing content of the modifier. For samples containing the [B10H10]2− anion, when increasing the modifier content to 30% (sample IX), spatially branched network structure can be formed with supramolecular structures built around the boron clusters. Such composites have increased deformation resistance; the values of dL/L0 remain unchanged up to 600°C (Fig. 2, curve 6).
Thermal stability of the supramolecular structures formed in the LG/[An] systems up to 600°C is provided by the presence of the boron clusters encapsulated in the silicate matrix. Therefore, a protective layer is formed, which prevents the diffusion of oxygen inside the composition preventing their oxidation. Note that triethylammonium salts of the boron clusters are thermally stable at temperatures significantly lower than 600°C; the TGA/DSC data for initial salts (Et3NH)2[B10Cl10] and (Et3NH)2[B10H10] are shown in Figs. S20 and S21, respectively. Thus, the supramolecular structures formed by boron cluster anions in the silicate matrix are responsible for not only the deformation resistance of the LG/[An] system but also thermo-oxidative stability of the system.
Comparison of properties of compositions LG/[An] and LG/nanoSiO2The largest linear dimensions of the boron cluster anions are 6 and 7nm for [B10H10]2− and [B10Cl10]2− respectively. For comparison, similar compositions based on LG containing model SiO2 particles 15nm in size were prepared and their thermomechanical properties were studied. Both types of modifiers, the boron cluster anions and silicon nanoparticles, act as loosening agents for siloxane chain packing.
In the process of thermomechanical analysis, at a temperature increase from 200 to 600°C, silicates with reactive silanol groups condense to form polysilicates, which soften near 500°C. Consider the effect of small concentrations of bulk modifiers (supramolecular structures based on LG/[An] as well as LG/nanoSiO2) on Tsoft.
Table 2 shows the values of ΔT (“plasticizing effect”) for modifiers of different nature in a wide range of concentrations. As follows from Table 2, for LG/[B10Cl10]2− systems (samples I–IV), in the temperature range 500–600°C, the highest plasticizing effect is achieved when the content of [B10Cl10]2− in the starting composition is 2.4wt% (sample I). With an increase in the content of the perchlorated modifier in LG, the degree of structuring increases and the system mobility decreases leading to decreased ΔT values. For sample IV, ΔT is negative, since in the presence of a large amount of the active [B10Cl10]2− modifier, the compaction of the packing proceeds due to cross-linking rather than its loosening, resulting in increased Tsoft.
For LG/[B10H10]2− compositions (samples V–VII), when the concentration of the [B10H10]2− anion ranges from 2 to 15%, ΔT values vary insignificantly, which agrees with the above-considered features of the reactivity of polyhedral anions. The [B10Cl10]2− anion forms a greater number of energetically more stable contacts with LG silicates as compared with the [B10H10]2− anion.
According to the TMA data, the plasticizing effect in the LG/SiO2 systems (samples X–XII) is comparable to this effect in the LG/[An] system (Table 2). Polycondensation reactions involving silanol groups occur in the temperature range 200–500°C, and bulk particles can be inserted into the structure of polysilicates, loosening the polysiloxane chain packing. The supramolecular systems LG/[An], in contrast to LG/nanoSiO2, are formed below the temperature of intense polycondensation of silicates, at T<200°C [19]. It should be noted that near 200°C under conditions of increased molecular mobility of the silicate matrix, the formation of additional interactions between modifiers and silanol groups of silicates should be taken into consideration in addition to the polycondensation reaction of silicates. Therefore, it is clear from Table 2 that ΔT for systems LG/[An] and LG/nanoSiO2 are comparable (the optimal values at different concentration of the modifiers are indicated by red color in Table 2). In this case, we can assume similar distribution of all three modifiers over the LG matrix resulting in similar properties of final samples.
Taking into account the fact that in the LG/[B10H10] systems, polyhedral anions are non-oxidized up to 600°C [20,21], it can be concluded that supramolecular structures are stable up to 500–600°C and can have a loosening effect on the packing of polysiloxane chains, which leads to a decrease in Tsoft in the presence of small additives.
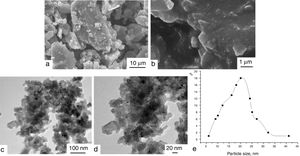
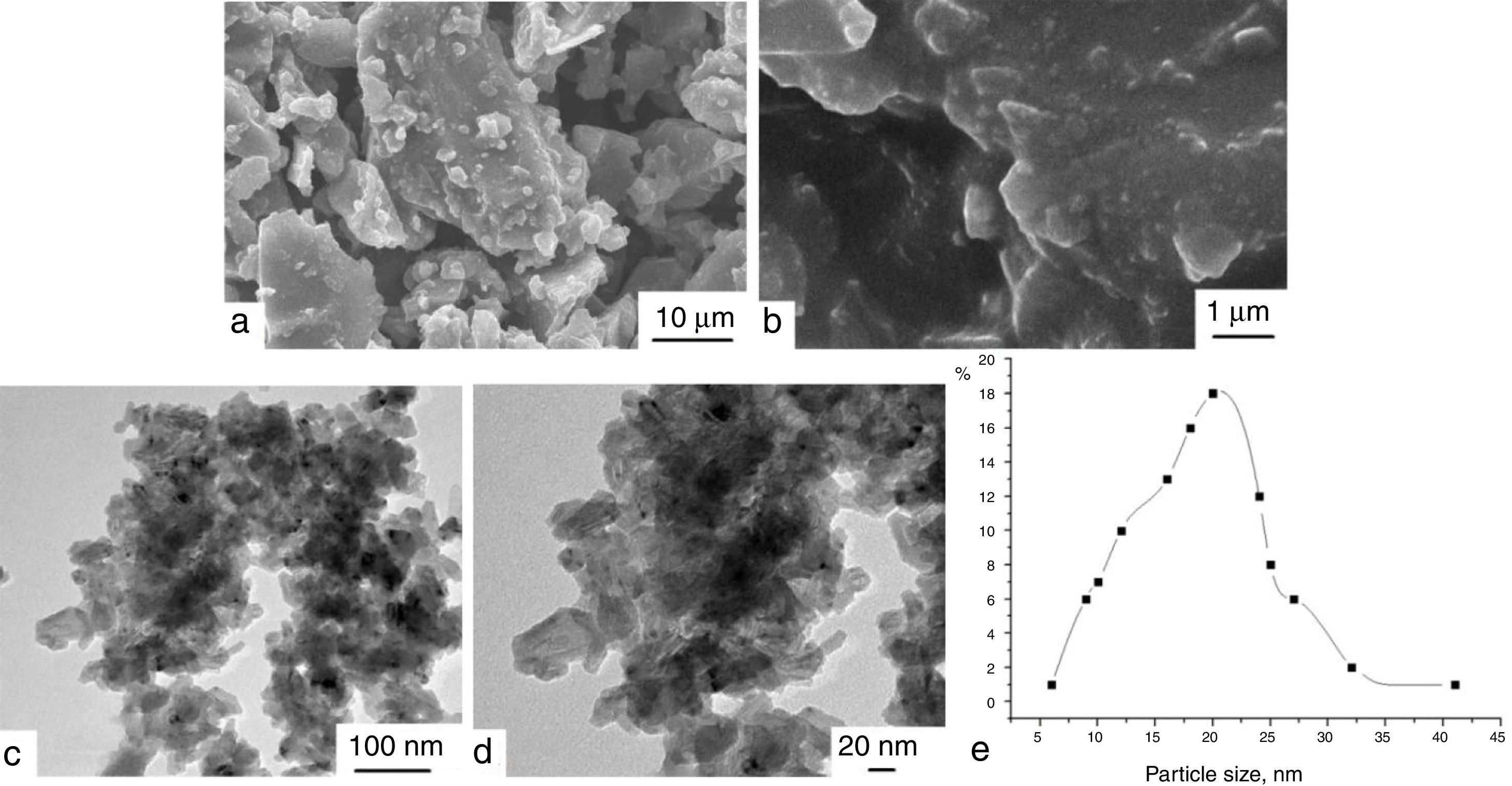
Morphology of compositions LG/[An]The morphology of samples II, IV, and IX was studied by TEM and SEM (see Supporting Information, Figs. S27–S32). According to TEM, the compositions are large blocks with irregular shape and sizes ranging from 5 to 40μm. The SEM and TEM data as well as size distribution curve for sample IV are shown in Fig. 3. According to SEM, it is clear that large blocks are formed by particles with irregular shape and sizes falling in the range from 10 to 30nm. The data obtained correlate with the X-ray powder diffraction data, which indicate that samples studied are amorphous and contain no crystalline phases.
ConclusionsIn summary, compositions based on sodium liquid glass LG and triethylammonium salts of boron cluster anions [B10H10]2− and [B10Cl10]2− added as Et3NH+ salts in amounts 2–30wt% have been synthesized and studied by TMA and IR spectroscopy. It was shown that for the LG/[An] systems, TMA is highly sensitive to structural transformations involving silanol groups: the association of the components of the initial system to form supramolecular systems and the condensation of silicates to form polysilicates. These processes occur in different temperature ranges: the first 20–200°C; the second 180–500°C. Using TMA, the degree of participation of silanol groups of LG in the formation of supramolecular structures as well as the softening temperature of polysilicates formed by increasing the temperature during TMA to 600°C have been estimated. It has been shown that the perchlorinated [B10Cl10]2− anion actively interacts with silicates, which seems to result in the formation of larger supramolecular associates. Based on the electron microscopy data, it has been suggested that supramolecular structures are nanoscale associates. This assumption is confirmed by the close results of TMA for LG/[An] and LG/nanoSiO2 compositions, prepared for comparison. It has been shown that, at the optimal content, the bulk supramolecular structures formed in the LG/[An] and LG/nanoSiO2 systems reduce the softening point of polysilicates.
This work was supported by the State Assignments of the Kurnakov Institute and the Semenov Institute in the field of fundamental research. The synthetic part of the work is supported by the Russian Federation President Council on Grants for Governmental Support for Leading RF Scientific Schools and Young Scientists (grants NSh-2845.2018.3 and MD-265.2019.3). Thermomechanical properties were performed within the Program of Fundamental Research of the Presidium of the Russian Academy of Sciences (program no. 35) “Scientific foundations for the creation of new functional materials”.




![Scheme of preparation of compositions LG/[An]. Scheme of preparation of compositions LG/[An].](https://static.elsevier.es/multimedia/03663175/0000005900000005/v1_202010080725/S0366317519300846/v1_202010080725/en/main.assets/thumbnail/sc1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![TMA curves for starting (Et3NH)2[B10Cl10] (1) and (Et3NH)2[B10H10] (2). TMA curves for starting (Et3NH)2[B10Cl10] (1) and (Et3NH)2[B10H10] (2).](https://static.elsevier.es/multimedia/03663175/0000005900000005/v1_202010080725/S0366317519300846/v1_202010080725/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![TMA curves of LG (1, black) and samples II (2), III (3), IV (4), VII (5), and IX (6). Compositions LG/[B10Cl10]2− are shown with color solid lines (2–4); compositions LG/[B10H10]2− are shown with dashed lines (5, 6). TMA curves of LG (1, black) and samples II (2), III (3), IV (4), VII (5), and IX (6). Compositions LG/[B10Cl10]2− are shown with color solid lines (2–4); compositions LG/[B10H10]2− are shown with dashed lines (5, 6).](https://static.elsevier.es/multimedia/03663175/0000005900000005/v1_202010080725/S0366317519300846/v1_202010080725/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)