This study aimed to scale the process of encapsulation in clay of the heavy metals Ni, Cr, and Cu, traditionally carried out at the laboratory level, up to industrial conditions. For this purpose, sludge from the galvanic industry, considered highly polluting, was mixed with the clay used to make traditional ceramics. The sludge concentrations were 2%, 5%, and 8% by weight. Two shaping processes traditionally used in the ceramic industry were used: extrusion and dry pressing, and the sintering was carried out in industrial gas kilns at 2 firing cycles: fast, lasting 34min in a roller gas kiln, and slow, lasting 42h in a tunnel gas kiln. The specimens thus obtained were characterized in terms of mechanical and esthetic properties. In the first case, the following characteristics were evaluated: flexural strength at three points, shrinkage, calcination losses, and water absorption. In the second, the color change obtained in the finished product generated by the presence of sludge was evaluated. Additionally, by means of the toxicity characteristic leaching procedure (TCLP) method, the impact that the finished product would have on the environment by leaching was determined. The results showed that the two processes of forming and sintering are viable for the treatment of galvanic sludge contaminant.
En este estudio se busca escalar en condiciones industriales el proceso de encapsulación en arcilla, de metales pesados Ni, Cr y Cu, realizado tradicionalmente a nivel de laboratorio. Para ello, se mezcla con arcilla empleada para la elaboración de cerámica tradicional, lodo proveniente de la industria galvánica, considerado altamente contaminante. Las concentraciones de lodo fueron 2%, 5% y 8% en peso. Se emplearon dos procesos de conformado tradicionalmente usados en la industria cerámica: extrusión y prensado en seco y la sinterización se realizó en hornos industriales a 2 ciclos: rápido de 34 minutos en horno de rodillos y lento de 42 horas en horno túnel. Las probetas así obtenida se caracterizaron en cuanto a propiedades mecánicas y estéticas. En el primer caso se evalúo: resistencia a la flexión en tres puntos; contracción, perdidas por calcinación, absorción de agua. En el segundo se evalúo el cambio de color obtenido en el producto terminado generado por la presencia de lodo. Adicionalmente, mediante el método Toxicity characteristic leaching procedure (TCLP), se determinó el impacto que desencadenaría en el ambiente el producto terminado por lixiviación. Los resultados muestran que los dos procesos de conformado y sinterización son viables para el tratamiento de lodos galvánicos contaminantes.
The development of any activity carried out by human beings involves the generation of waste. Much of it, accumulated and treated in an inappropriate way, constitutes a risk to the health of living beings and the health of the environment. For the treatment and/or correct disposal of these hazardous wastes, it is a priority to mitigate the damage generated in the environment or to contain it permanently, the latter process being the least convenient for stopping the serious environmental impact generated by these types of materials [1]. Permanent storage is the most widely used method for treating sludge from galvanic processes (GS) in industrial wastewater treatment plants [2,3]. The accumulation of sludge in safety cells from the galvanic industry is becoming an active problem with the exponential growth of the industry and the resulting impact on the environment, which will undoubtedly have strong repercussions for present and future generations [4]. It is therefore important to carry out research studies aimed at solving the problem of the accumulation of GS and the treatment of what will be generated in the future.
In the research papers published on the subject, the various methods of metal extraction (pyrometallurgy, hydrometallurgy, and electrodeposition) that provide added value due to the recovery of metals [5–8], including those consisting of the synthesis of inorganic pigments, where GS is used as a raw material [9–13], those using encapsulation or adsorption in a ceramic matrix, such as cement [14,15], asphalt [16], sintered clay [17–21], and aluminosilicate inorganic polymers [3], and more recently phytomining, which takes advantage of the affinity of some plants for the absorption of heavy metals, from where they are later recovered from their ashes [22], stand out. However, the selection of the method and its efficiency depends on the origin of the contaminated material, since the chemical composition with respect to heavy metals changes from one industry to another, and sometimes a fractional separation is necessary prior to the final recovery.
Among adsorption methods, their efficiency is determined by factors such as the temperature, the initial concentration of metals in the solution, the porosity of the adsorbent, and the contact time. The adsorption concentrations obtained are relatively low: 26–182mg/g. Recently, Bednarik et al. have achieved an adsorption capacity of 280mg Cd2+/g and rapid adsorption kinetics (6h) using a simple synthesis method with an aluminosilicate inorganic polymer [3]. Metal recovery processes, through leaching followed by electrodeposition, incur the problem of low recovery efficiency, with the associated high consumption of electrical energy. Although they are a viable alternative in metal recovery, they should be improved in terms of efficiency and electrical energy consumption. Dornelles, Amaral et al. recovered 63% of copper from galvanic sludge using sulfur at 550°C treated by means of pyrometallurgical processes [5]. These methods can be a viable alternative for the clean recovery of metals such as copper, which is becoming alarmingly scarce worldwide due to the high demand for electronic devices, which has worsened as a result of the pandemic generated by the Covid-19 pandemic. This will result in copper shortages in the coming decades [23,24], so without a doubt, the recovery of valuable metals for industry will become crucial.
Another alternative is phytomining. This was initially developed for Ni, and it is a way to take advantage of heavy metals that are absorbed by some plant species. Phytomining is the process whereby plants capable of accumulating metals in their shoots are cultivated on heavy metals-rich substrates, with the objective of recovering the metal from their biomass (by calcination) for commercial gain. Tognacchini et al. have shown that the Ni, Cu, and Co hyperaccumulator species Odontarrhena chalcidica is capable of growing on toxic galvanic sludge in order to produce a biomass comparable to that on soils that are naturally rich in metals. This is an alternative for the recovery of heavy metals from galvanic sludge with contents lower than 13%, since the pyrometallurgy and hydrometallurgy processes require higher heavy metal content [7]. However, during the treatment of the sludge product of the galvanic industry, many tons of CaCO3 are required, which during the calcination process of the plant material decompose into CO2, which on a planet already affected by climate change generated by the product of the accumulation of CO2 due to the greenhouse effect could substantially alter the ecosystem [5,25].
The chemical composition of the sludge is directly associated with the type of industry of origin, and is usually very rich in heavy metals such as nickel, zinc (Zn), copper (Cu), chromium (Cr) and/or cadmium (Cd). The methods in which the metals contained in the sludge are encapsulated in a cement matrix solidification/stabilization (S/S) can improve the physical characteristics of wastes, reduce their leaching, and limit the solubility of their heavy metals [14,15]. For GS encapsulated in a cement matrix, amounts of galvanic sludge close to 40% have been used without affecting the mechanical properties of the cement [14,15]. Monoliths have been manufactured using asphalt that incorporates GS. In both cases, cement and asphalt with added GS meet the leaching standards stipulated by the standard [16]. For sintered clay in processes carried out at the laboratory level in muffle furnaces, amounts of sludge of up to 20% have been incorporated into the ceramic matrix, with sintering temperatures of 900–1200°C, residence times at a maximum temperature between 1 and 72h, and warming ramps from 1°C to 10°C [17–21]. In this case, the conditions in which the tests are carried out are far from the process conditions at an industrial level, and the esthetic variables of the finished product are not analyzed. It is essential to evaluate the mechanical, technical, esthetic, and environmental properties of the finished product at the industrial level. Variables such as the chemical and mineralogical composition of the raw material, the forming method of the specimens, the sintering cycles, and the ramps and temperatures determine the characteristics of the final product [21,26–28].
The present research investigation shows a method the industrial level for the use of GS in the production of tablets stoneware. Different concentrations of GS mixed with the clay used for the manufacture of tablets and stoneware were used, from the dry pressing and extrusion processes. The first constitutes the method used by the ceramic industry for the shaping of pieces with low thickness<6mm and low humidity<8%, where the drying of the pieces is facilitated by eliminating the drying contraction. This makes it possible to obtain pieces with great dimensional accuracy sintered in fast firing cycles [28]. The second (extrusion) is the method used by the ceramic industry for the shaping of pieces with high thickness, >10mm, and high humidity, >20%, which allows obtaining pieces with a constant cross section and using sintering processes in slow firing cycles [27]. The previously formed samples were sintered in industrial gas kilns at two different firing cycles: a fast cycle in a roller kiln and a slow cycle in a tunnel kiln. The different samples obtained were evaluated for their mechanical, technical, esthetic, and environmental properties. Satisfactory results were obtained for the retention of heavy metals in the ceramic matrix, but the mechanical, technical, and esthetic characteristics of the product were compromised with the addition of the sludge.
Materials and methodsGalvanic sludge from the treatment of industrial wastewater from a chrome plating process for sanitary fittings and clay from a local industry dedicated to the manufacture and marketing of traditional ceramic products for floor and wall cladding were used as raw material. The selected sludge concentrations were taken from a previous study at the laboratory level [29]. The chemical composition of the sludge was determined by means of X-ray fluorescence (XRF) using portable X-MET 7500 equipment from Oxford Instruments. The content of the metals Cu, Ni, and Cr, was confirmed using flame atomic absorption spectrometry (FAAS) on Thermosolar S series equipment. The quantification of Cu, Ni, and Cr extracted in the TCLP test was carried out on the same equipment. The elemental analysis of C and N was carried out using LECO equipment.
The technical properties, calcination losses and water absorption were determined by means of gravimetric methods. The flexural strength at three points was evaluated on a Shimadzu brand Universal Testing Machine, the linear shrinkage was measured with a caliper, and the colorimetric coordinates were determined with a Konica Minolta C400 colorimeter. The determination of the crystalline structure of the phases present in the clay and in the finished product after the sintering process was carried out by means of X-ray diffraction (XRD) on an X’Pert Pro MPD diffractometer equipped with a copper anode, with an intensity of 30mA, voltage of 40kV, 2θ from 10° to 90° at a speed of 0.2°/min and step of 0.02. For the identification of phases, PANalytical High Score Plus software and the PDF2 database of the XPowder program were used. Anorthite (Calcium Aluminum Silicate) CaAl2(SiO4)2 00-002-0523, Cristobalite (Silicon Oxide) 00-001-0424, Quartz (Silicon Oxide) SiO2 01-089-8936, Hematite (Iron Oxide) Fe2O3 00-001-1053, Mullite Al4.8O9.6Si1.2 01-079-1276, Mullite Cr-doped (Chromium Aluminum Silicate Oxide) Cr0.5 (Al3.93Si1.57)O9.79 01-070-3372, Copper Silicate Cu(SiO3) 01-070-3326, Nickel Iron Oxide (NiOFe2O3) 00-044-1485 and Copper Iron Oxide (Cu2OFe2O3) 00-004-0758.
For the cross-sectional analysis, the Phenon ProX integrated scanning electron microscope (SEM) with an EDS detector (silicon drift detector, SDD) was used. The density of the powder for the different concentrations of sludge was determined via the Archimedean method in an analytical balance and with a volumetric balloon. The bulk density of the specimens was measured by means of the mercury immersion method and the plasticity of mixtures via the Pfefferkorn method.
Preparation of the sampleIn order to reduce any interference present due to differences in the granulometric distribution of the clay, a single 50kg sample was taken at the exit of the milling process. The sludge was dried at 105°C for 24h and then ground in a ball mill and passed through a #120 Tyler sieve. With the clay thus obtained, the different concentrations of sludge/clay were prepared in triplicate: 2%, 5%, and 8% of sludge by weight, clay balance. Equivalent samples were used for the shaping and firing process. They were mixed in a ball jar and shaken for 30min. Concentrations were determined based on a previous paper [25]. For the process of dry pressing and sintering in a roller gas kiln, the mixture was moistened to 6.5% and compacted by dry pressing in a NANNETTI model MIGNON SSN/EA hydraulic press at a pressure of 28MPa. On that basis, for each concentration of sludge used, 6 cylindrical specimens were obtained with dimensions of 100mm in diameter by 6.5mm in thickness. For this process the samples were engobed and glazed in the production line.
For the extrusion process and tunnel kiln firing, the mixture was humidified to 20% and extruded under a vacuum in a Verdés clay extruder Mod. 050-D. For each concentration of sludge used, 6 rectangular specimens were obtained with dimensions of 120mm in length, 60mm in width, and 10mm in thickness; the reduction ratio was 13.09. The samples were dried in a convection oven at 105°C to constant weight. For the sintering of the samples obtained by dry pressing, an industrial continuous single-layer roller kiln, SACMI model FMA 250, was used at a firing cycle of 34min and a maximum temperature of 1150°C. For the sintering of the samples obtained by extrusion, a Metalcértima industrial tunnel gas kiln was used in a 42-h firing cycle at a temperature of 1150°C. After the sintering process, the losses due to calcination, linear shrinkage (ASTM C326-14), water absorption (ASTM C20-15), and flexural strength at three points (ASTM C674-13) were determined. Additionally, a leaching test was carried out in order to determine the toxicity characteristics (TCLP) according to US EPA 1311 [30]. The influence of the addition of sludge on the structure of the ceramic material was determined in selected specimens after the flexural strength test.
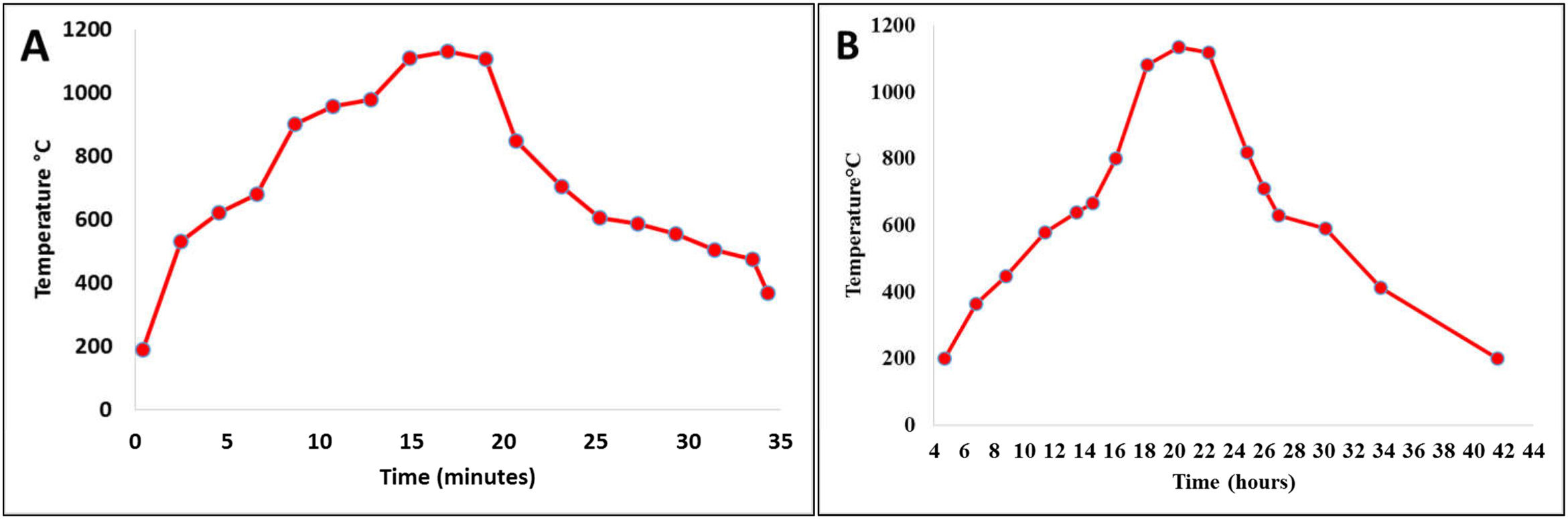
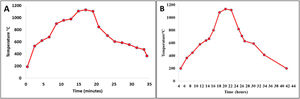
Firing cyclesDepending on the type of material and the shape and dimensions of the final product, the sintering process is perhaps one of the most important ones in the consolidation of ceramic products. The sintering curves and cycles will determine the properties of the material put into service. Fig. 1 shows the sintering curves at the industrial level to which the ceramic pieces of the present study were subjected: Fig. 1a roller kiln and Fig. 1b tunnel kiln. It is observed that, unlike muffle furnaces, where tests are carried out at laboratory level, in industrial furnaces the curves do not show a linear behavior; there are zones characterized by different heating and/or cooling ramps, each of which behaves according to the physicochemical characteristics of the mixture and the dimensions and shapes of the parts to be sintered.
The curve recorded in the roller kiln (Fig. 1a) with a fast-firing cycle of 34min at 1150°C and a residence time of 4min showed its highest heating ramp at 164°C/min and lowest at 10°C/min. After firing, there is a cooling zone, where the highest cooling ramp is −157°C/min and the lowest is at −10°C/min. In this process, the specimens formed by dry pressing, whose thickness was 6.5mm, were sintered, see Image 1. Fig. 1b corresponds to the tunnel kiln with a slow firing cycle of 42h at a temperature of 1150°C, where the residence time at this temperature was 2h. The highest warming ramp was 2.25°C/min and the lowest 0.4°C/min. On the other hand, the highest cooling ramp was −1.92°C/min and the lowest was −0.2°C/min. In this process, the specimens formed by extrusion, whose thickness was 10mm, were sintered, see Image 1. It should be noted that this is one of the differences that this study shows with respect to others carried out at the laboratory level, where furnaces were used under conditions that differ greatly from the conditions of an industrial process. In other studies, the fastest warming ramp found was 10°C/min and the slowest was 1°C/min [31,32]. The residence time at maximum temperature is another parameter where the process at the laboratory level differs greatly from the industrial process; in the laboratory, it can vary from 10min to 30h [32,33].
Results and discussionThe chemical composition of the sludge determined by XRF and corroborated by FAAS, which is a quantitative technique, is shown in Table 1, where a concentration of heavy metals from 4.24% for Ni to 13.65% for Cr can be seen, with chromium being the one with the highest concentration. (This study considers the data obtained by FAAS). The Ca and C contents come from the treatment of wastewater and sludge, to which CaCO3 was added; its concentration in the sludge was 52%. In the clay, there is no presence of CaCO3. On the other hand, the organic matter content in the sludge and clay was determined gravimetrically from the calcination loss test at 550°C, and the value obtained was 23.12% for the sludge and 2.38% for the clay. It is important to mention that one of the difficulties in treating sludge is that its chemical composition depends on the type of process and the treatment from which it comes [34].
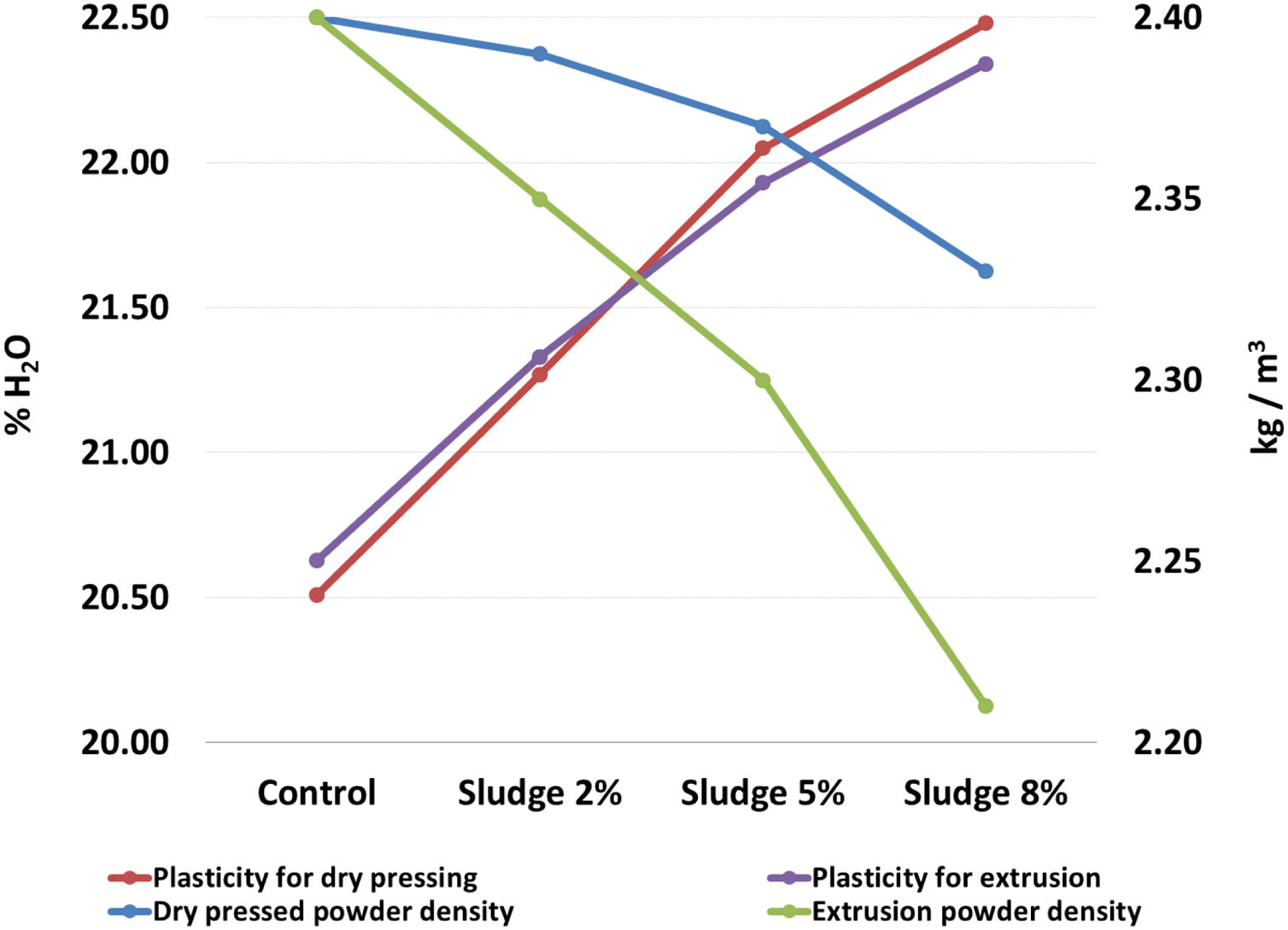
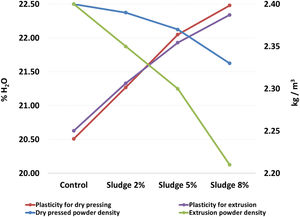
Table 2 and Fig. 2 show the results corresponding to the average and standard deviation of the three repetitions carried out by sludge concentration for the density of the powders and plasticity. It can be seen that the density of the powder decreases and the plasticity increases as the concentration of sludge increases. In both cases, for the two processing methods evaluated here, the slope of the curve is approximately of the same order of magnitude, which indicates that the decrease in density and the increase in plasticity are a direct function of the sludge concentration added to the mix. These results are directly related to the content of organic matter present in the sludge, with a carbon concentration of 6.1%, as seen in Table 1. The above results agree with those reported by [35], who found that the organic matter content in the sludge affects the plasticity and density of the clay mixture. It is known that density and plasticity are very important for the shaping process. The variation in the density of the powder directly affects the filling of molds in the pressing process [28], while plasticity affects the degree of compaction of the pieces, which can cause problems both in the wet transport and in the drying process [36].
Density of powders and plasticity of the mixtures.
| Sludge concentration | Dry pressed powder density (kg/m3) | Plasticity for dry pressing (% H2O) | Extrusion powder density (kg/m3) | Plasticity for extrusion (% H2O) |
|---|---|---|---|---|
| Control | 2.40±0.09 | 20.51±0.11 | 2.40±0.05 | 20.63±0.15 |
| Sludge 2% | 2.39±0.12 | 21.27±0.15 | 2.35±0.09 | 21.33±0.10 |
| Sludge 5% | 2.37±0.17 | 22.05±0.13 | 2.30±0.10 | 21.93±0.06 |
| Sludge 8% | 2.33±0.08 | 22.48±0.07 | 2.21±0.09 | 22.34±0.08 |
Table 3 presents a summary of the data and their standard deviation obtained for the measurement of the technical characteristics, carried out for 6 replicates of each sludge concentration.
Summary of technical characteristics.
| Process | Sample | Shrinkage (%) | Water absorption (%) | Calcination losses (%) | Flexural strength (n/mm2) | Colorimetric coordinates | |||
|---|---|---|---|---|---|---|---|---|---|
| ΔL* | Δa* | Δb* | ΔE* | ||||||
| Dry pressing | Control | 3.24±0.1 | 7.06±0.1 | 4.14±0.1 | 23.64±2.6 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sludge 2% | 3.34±0.1 | 7.54±0.1 | 4.69±0.1 | 22.04±1.6 | −0.88 | −1.52 | 1.35 | 2.22 | |
| Sludge 5% | 3.30±0.1 | 8.79±0.2 | 5.58±0.1 | 18.43±1.3 | −2.16 | −3.48 | 3.09 | 5.13 | |
| Sludge 8% | 2.92±0.1 | 10.50±0.1 | 6.41±0.2 | 18.14±2.6 | −4.09 | −4.86 | 3.95 | 7.48 | |
| Extrusion | Control | 9.77±0.2 | 5.43±0.6 | 4.58±0.0 | 30.38±2.0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sludge 2% | 10.17±0.1 | 5.68±0.3 | 4.97±0.0 | 29.66±2.2 | −5.47 | −0.49 | −3.31 | 6.41 | |
| Sludge 5% | 9.42±0.4 | 8.71±0.5 | 5.93±0.0 | 26.43±1.6 | −11.70 | −0.84 | −6.07 | 13.20 | |
| Sludge 8% | 8.82±0.1 | 8.94±0.4 | 6.92±0.0 | 23.55±3.4 | −13.43 | −2.14 | −9.44 | 16.56 | |
Linear shrinkage is a parameter associated with the dimensional reproducibility of the product. Its variation with respect to the control can cause the finished product to be larger or smaller, making the installation process difficult. In Table 3, it can be seen that for the pieces obtained by dry pressing and sintering in a roller kiln, those dosed with 2% and 5% of sludge exhibited an increase in shrinkage of 3.08% and 1.85%, respectively, with relation to the control, so that the pieces obtained are smaller. On the contrary, those with 8% added sludge exhibited an expansion of 9.88% with respect to the control, which leads to larger pieces. Similar results were obtained by [18], who obtained samples by dry pressing and with galvanic sludge concentrations up to 5%. In the case of the pieces obtained by extrusion with a content of 2% sludge, a contraction of 4.09% with respect to the control is shown, which decreases their thickness, while those with 5% and 8% of added sludge showed an expansion of 3.58% and 9.72%, respectively, increasing their final size with respect to the control. These results differ from those presented by [16], who obtained samples by extrusion mixing sludge at 1%, 5%, and 10% by weight with respect to clay and sintered at temperatures between 800°C and 1200°C. In all cases, the higher the sludge concentration, the greater the shrinkage undergone by the samples.
The absorption of water allows establishing the open porosity present in a ceramic material and, together with the forming process, allows classifying ceramic products according to the ISO 13006 standard. In Table 3, it can be seen that regardless of the forming process, with an increase in the percentage of sludge, there is an increase in water adsorption and therefore an increase in the open porosity for the specimens dosed with sludge. For those formed by dry pressing, the increase in absorption with respect to the standard varies by 6.80%, 24.50% and 48.73% for the concentrations of 2%, 5%, and 8% of sludge, respectively. For those formed by extrusion, the variations with respect to the control are 4.60%, 60.41%, and 64.64%, and there is a much higher water absorption capacity compared to the sludge percentages of 5% and 8%. Here, not only the percentage of sludge added to the clay but also the shaping process used in its processing has an influence. The above results agree with those reported by [16,18,20], who found that the greater the amount of sludge added to the clay, the greater the water absorption undergone by the samples.
On the other hand, the losses due to calcination constitute a parameter that allows determining what quantity of material decomposes due to the action of the increase in temperature. The greater its magnitude, the more rigorous the control that should be used in the heating ramps during sintering. This makes it possible to avoid defects in the finished product due to a loss of gases caused by decomposition. In general, from the results shown in Table 3, it can be seen that with an increase in the percentage of sludge, the losses due to calcination increase and that according to the forming process, the losses due to calcination are higher if the process is carried out by pressing compared to extrusion. For the pieces formed by dry pressing, the variations with respect to the control are 13.29%, 34.78%, and 54.83%, for 2%, 5%, and 8% of sludge, respectively, and for those formed by extrusion, the variations with respect to the standard are 8.52%, 29.48%, and 51.09% for the same sludge concentrations. The results obtained are consistent with those presented by [37,38], which show that the increase in calcination losses is directly proportional to the increase in sludge dosage. Regardless of the forming and sintering process, the result shows the same trend.
On the other hand, flexural strength is an important parameter in ceramic pieces, according to how the material is classified according to ISO 13006, since a minimum resistance value must be met. Table 3 shows that regardless of the forming process, with an increase in the percentage of sludge, the flexural strength decreases. For the pieces formed by dry pressing, the variations with respect to the control are: 6.77%, 22.04%, and 23.27% for 2%, 5%, and 8% of sludge, respectively. In the case of those formed by extrusion, the variations with respect to the control are 2.37%, 13.00%, and 22.48%. These results agree with those reported in different studies [17,37,38], which show that without taking into account the process variables, the addition of galvanic sludge to the clay decreases the flexural strength of the samples.
For the ceramic material put into service, color is a determining characteristic in the esthetics of the finished product. For this reason, controlling the variations that may occur both in the same piece and between different pieces is essential. Table 3 shows the color variations experienced by the specimens with sludge versus the control. The average values of the colorimetric coordinates seen in the specimens are presented It can be seen that in the samples formed by dry pressing in the ΔL* and Δa* coordinates, the values of the 3 doses are negative, and become more negative with increasing sludge percentage, indicating that the addition of sludge modifies the color toward dark and green tones. In the colorimetric coordinate Δb*, the values of the 3 doses are positive, and become more positive with increasing sludge percentage, indicating that the addition of sludge modifies the color toward yellow. In the samples formed by extrusion, the ΔL*, Δa*, and Δb* coordinates all show negative values, and become more negative with increasing sludge percentage. This indicates that the addition of the sludge to the clay modifies the color toward dark tones and green and blue colors. The total color change ΔE* shows that regardless of the forming and firing process, the addition of sludge modifies the color, with the change becoming greater as the percentage of added sludge increases. In this section, it should be mentioned that the samples obtained by dry pressing and that were engobed and glazed with a glossy glaze allow us to observe the presence of pinholes in the glaze. This is an indication that there was outgassing while the glaze was molten.
As mentioned in the paragraph concerning water adsorption, ISO 13006 Ceramic tiles – Definitions, classification, characteristics and marking [39]. It classifies the tiles taking into account the forming process and water absorption (see Table 1 of standard 13006). According to this table, dry pressed samples, regardless of the added sludge concentration, can be classified in group BIIb, and because their water absorption is between 6% and 10%, the flexural strength should be at least 18N/mm2. Regarding the samples obtained by extrusion, those with 2% added sludge can be classified in group AIIa, because their water absorption is between 3% and 6%, and the flexural strength should be at least 20N/mm2. The samples with 5% and 8% added sludge can be classified in group AIIb1, because their water absorption is between 6% and 10%, and the flexural strength is at least 17.5N/mm2.
The final properties of a ceramic material made of clay will depend on the raw materials and their combination, the forming processes, and mainly the firing cycles. The latter must be suitable for the shapes and thicknesses of the samples to be sintered [40]. As to the results of the technical characteristics obtained in this research, the evidence suggests that they are strongly associated with the presence of CaCO3 in the sludge. During the firing process, the carbonate decomposes into its oxides CaO and CO2, where the release of carbon dioxide leads to the formation of porosity and the dimensional expansion of the support [41]. This process directly affects the losses due to calcination, since by increasing the percentage of sludge, the amount of carbonate in the raw material increases. The generation of pores, in turn, strongly influences the water absorption of the material, so that the higher the percentage of sludge, the more empty spaces are generated, which can eventually be occupied by water during the process of absorption. In addition, this directly affects the behavior of the flexural strength; as the concentration of sludge increases, the flexural strength decreases, due to the generation of pores as a result of the release of CO2. Changes in the color of the material can be associated with the presence in the sludge of the chromophore metals Cr, Ni, and Cu, which, due to the effect of temperature and the reactions that occur in the process, can generate colored compounds such as oxides of these same metals [42]. Color generation is economically advantageous from an industrial point of view, as it could reduce the consumption of pigments such as MnO2, used to give brown tones to stoneware tiles. In the case of glazed tiles, dark colors could decrease the pigment load in the glaze, as the bisque has a darker tone.
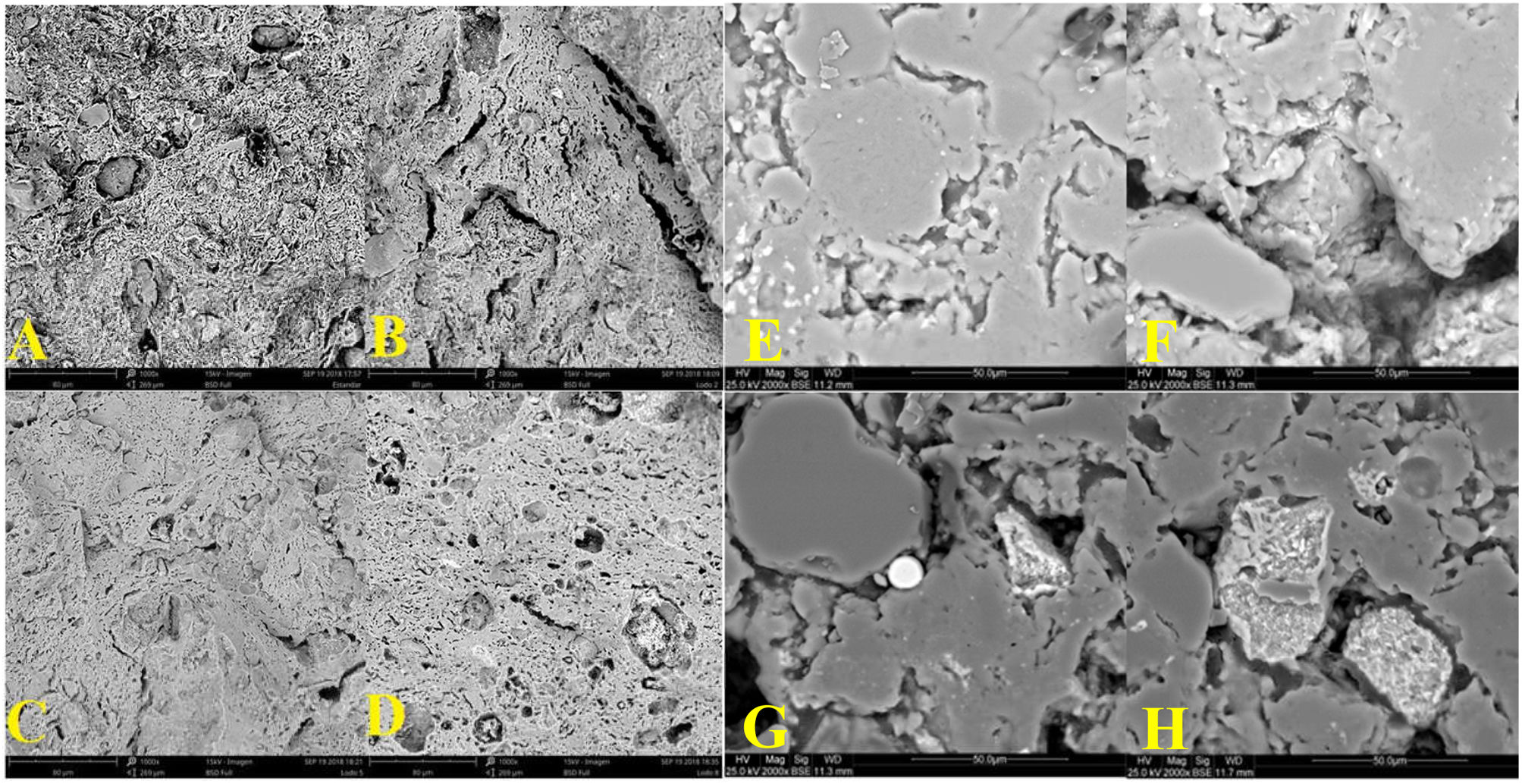
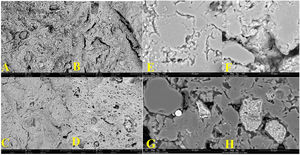
MicrostructureFig. 3 compares the internal structure of the ceramic body, taken by SEM at 2000X. The micrographs (A) control, (B) 2% sludge, (C) 5% sludge, and (D) 8% sludge correspond to the samples processed by extrusion. The micrographs (E) control, (F) 2% sludge, (G) 5% sludge, and (H) 8% sludge correspond to the samples processed by dry pressing. It can be seen that regardless of the shaping process and/or the firing cycle in the pieces with added sludge, the internal structure shows evidence of the formation of pores, channels, and holes, which are larger and longer compared to the structure of the control. It can also be seen that some grains in the specimens with sludge have channels around them, evidencing a lack of cohesion between grains. Likewise, it can be seen that the structure in the extruded samples is more compact than that of the dry-pressed ones. These observations agree with the results of the mechanical and technical characterization of the specimens, where the samples with added sludge exhibit higher water absorption than do the controls, and the mechanical resistance in the specimens with sludge is lower than that of the corresponding control. Additionally, the flexural strength is also higher in the samples obtained by the extrusion process compared to the specimens synthesized by dry pressing.
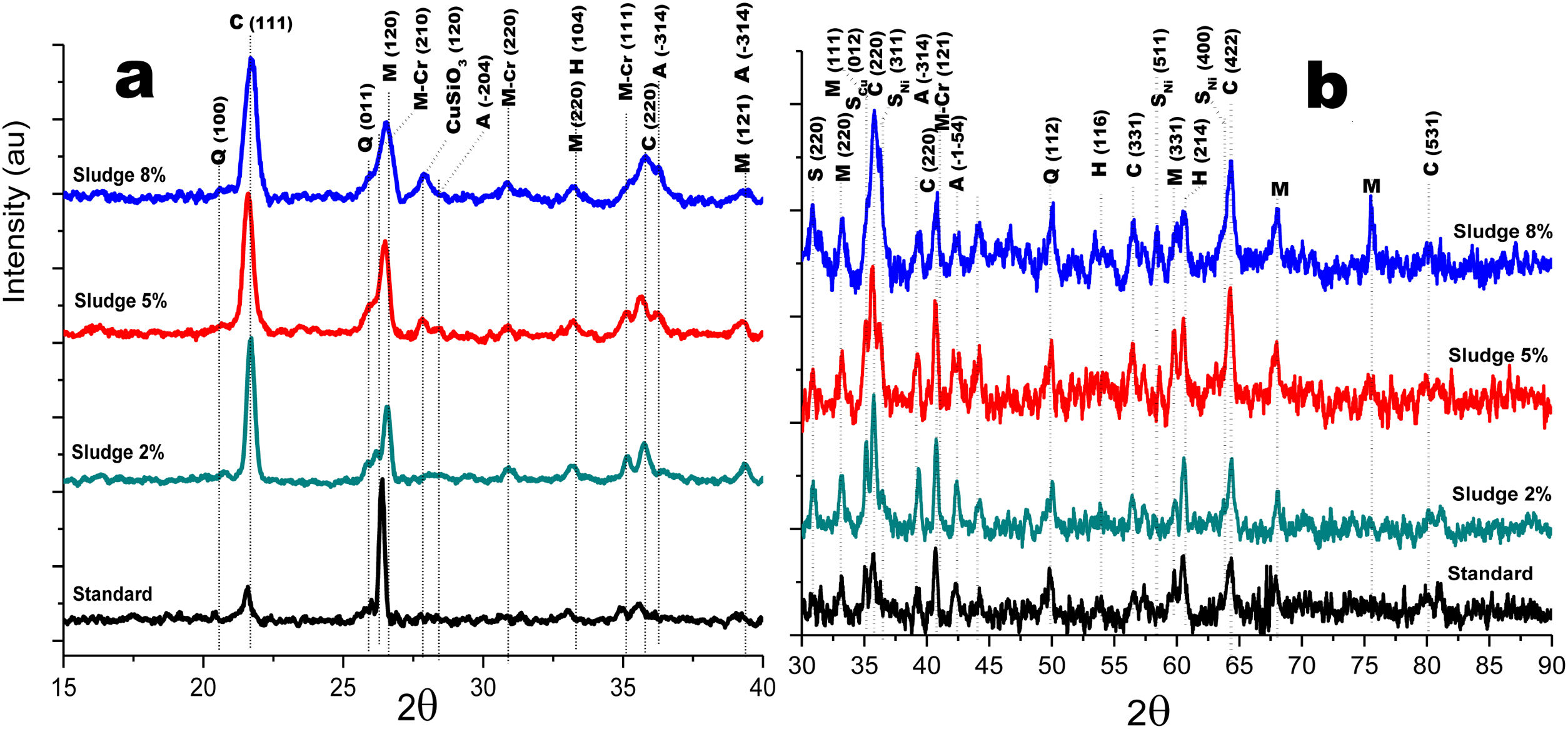
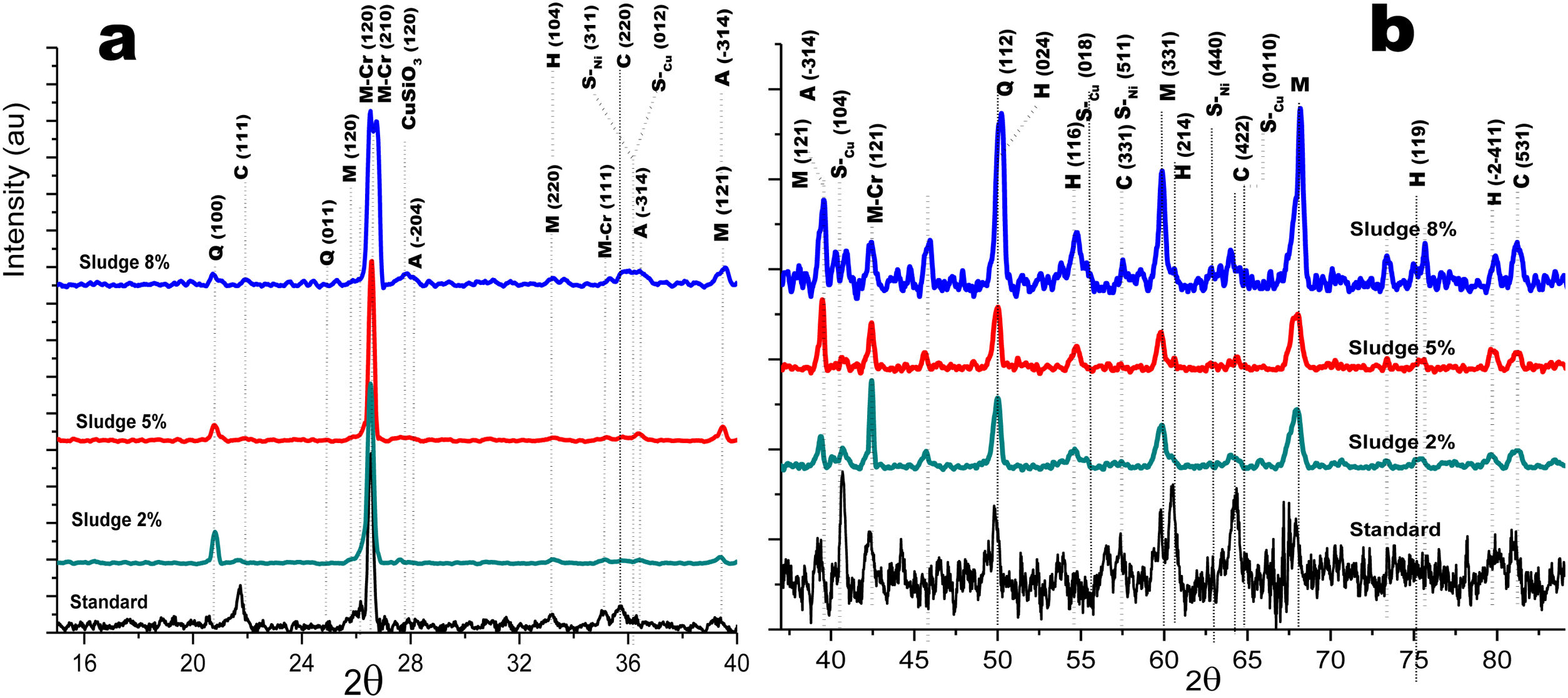
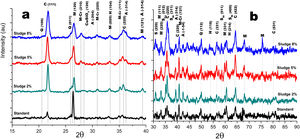
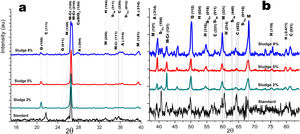
Crystalline structureFigs. 4 and 5 show the normalized diffractograms for the clay without added sludge (standard) and for the samples obtained at an industrial level by dry pressing and sintering in a fast cycle. For each of them, the diffractogram was separated into two ranges in order to facilitate their interpretation. In the standard (clay without added sludge), four phases were identified: quartz, cristobalite, hematite, and mullite. In the samples with added sludge, nine different crystallographic phases were identified, which were indexed according to the JCPDS charts related to the methodology, namely anorthite (A) (CaO·Al2O3·2SiO2), cristobalite (C) (SiO2)-c), quartz (Q) (SiO2-H), hematite (H) (Fe2O3)-R, mullite (M) (Al4.8O9.6Si1.2-O), Cr-doped mullite (M-Cr) (chromium aluminum silicate (Al3.93Cr0.5O9.79Si1.57-O), copper ferrite spinel (SCu) (Cu2O·Fe2O3-R), nickel ferrite spinel (SNi) (NiO·Fe2O3-C), and copper silicate Cu(SiO3-O). Similar phases have been reported by other authors in the sintering of tailing bricks in a temperature range of 700–1100°C [43,44].
Anorthite is absent in the standard and in the 2% sludge spiked sample. Anorthite is formed during the sintering of clay with added sludge; since it is processed with CaCO3 and above 700°C, it forms CaO. In the presence of mullite and above 900°C, it would give rise to the formation of (A). The growth of (A) is more evident at 2θ=27.9 for the plane with Miller indices (−204), clearly differentiated for the 5% sludge. In this same area at 2θ=26.2, the plane with Miller indices (210) denotes the presence of the mullite phase doped with chromium with an orthorhombic crystalline structure; this phase is present for the three concentrations of sludge studied here and increases its intensity with its concentration. A similar behavior is observed for cristobalite and mullite, the first at 2θ=21.4, 35.4 and 63.7 and the second at 2θ=26.2, 35.1 and 75.2. The presence of copper is evidenced by the copper silicate Cu (SiO3) phase, with an orthorhombic crystal structure at 2θ=28.0 for the plane with Miller indices (120). Additionally, evidence of copper and nickel spinels was found at 2θ=35.7 for the two spinels (I=100) and 40.4 and 55.0 for the copper spinel; these planes are of greater intensity for the samples with 8% added sludge.
Fig. 5a and b shows the diffractograms corresponding to the standard and the different concentrations obtained by dry pressing. The same phases were identified and shown in Fig. 4. However, the width toward the middle of the peak and its intensity ratio for the normalized samples in the zone 2θ=25–27 for chromium mullite, around 2θ=50 for quartz and hematite and 2θ=67 for mullite, shows that the samples obtained by dry pressing are more crystalline compared to those obtained by the extrusion method. This behavior can be explained by solid state diffusion processes in the extruded samples for the residence time in the furnace of 4h at 1150°C, which favors the development of larger diameter crystals, while those of dry pressing only remain at this temperature for 4min [43–45].
TCLP testThe toxicity characteristic leaching procedure (TCLP) technique determines the mobility of the contaminants present in a sample and the degree of toxicity, which allows the material to be classified as “dangerous”. The leachate is determined based on the acid neutralization capacity of the sample. Table 4 shows the results of the average and the standard deviation of the 3 repetitions carried out for each sludge concentration in the TCLP tests. It can be seen that regardless of the shaping process or the firing cycle, the higher the percentage of added sludge the higher the concentration of Cr, Ni, and Cu extracted by the fluid, and that the metal with the greatest leaching is chromium, followed by copper and nickel. The order of the extraction level coincides with the concentration of metals in the sludge, in which Cr has the highest concentration followed by copper and finally nickel (see Table 1). These results are comparable to those reported at the laboratory level by different authors [16,18,19]. On the other hand, it can be seen that the samples formed by extrusion and fired in a slow cycle exhibit a lower extraction of metals in the fluid compared to the results obtained for the specimens synthesized by dry pressing and fired in a fast cycle. It should be pointed out that in all cases the extraction values are within the maximum allowed Cr 5mg/L [46]. These results could be related to the presence of the glassy phase formed in the specimens subjected to the long sintering cycle [19], which could facilitate the macro- and micro-encapsulation of the sludge in the ceramic matrix during solidification. Additionally, the chemical reactions inherent to the firing process facilitate the loss of water by the metals contained in the sludge in the form of hydroxides, becoming less soluble oxides, as evidenced by the XRD results [47]. These processes guarantee the immobilization of heavy metals, preventing them from reaching the environment. In this regard, Singh and collaborators [48] found that the firing temperature is a relevant factor in the immobilization of the metals contained in the solid waste, and that at temperatures above 950°C their leaching becomes negligible.
Metal extraction result in the TCLP test.
| Process | Sample | Cr mg/L | Ni mg/L | Cu mg/L |
|---|---|---|---|---|
| Dry pressing – roller kiln | Control | 0.000 | 0.000 | 0.000 |
| Sludge 2% | 1.184±0.010 | 0.093±0.003 | 0.376±0.008 | |
| Sludge 5% | 2.379±0.010 | 0.165±0.003 | 0.630±0.010 | |
| Sludge 8% | 3.270±0.121 | 0.224±0.020 | 1.039±0.029 | |
| Extrusion – tunnel kiln | Control | 0.000 | 0.000 | 0.000 |
| Sludge 2% | 0.776±0.027 | 0.060±0.004 | 0.255±0.011 | |
| Sludge 5% | 1.523±0.043 | 0.077±0.002 | 0.627±0.012 | |
| Sludge 8% | 1.626±0.020 | 0.092±0.001 | 0.734±0.008 | |
The present study demonstrates that the process of encapsulation in a ceramic matrix of the heavy metals Cr, Ni and Cu, which are in gel form, contained in a galvanic process sludge scaled to industrial level constitutes an excellent alternative for the industrial use of galvanic sludge as a raw material in the ceramics industry. The use of these sludges at an industrial level will mitigate the environmental impact generated by the accumulation of sludge contaminated with the heavy metals studied here. The results show that the two shaping methods traditionally used in the industry, dry pressing and extrusion at fast or slow firing cycles, allow obtaining a good retention of heavy metals in the ceramic matrix. In the two forming and firing processes studied in this investigation, the amount of metals extracted in the TCLP test was below the allowed limits, obtaining better retention results in the specimens shaped by extrusion and sintered in a slow cycle.
The use of galvanic sludge as a raw material in the production of traditional ceramics at an industrial level is limited by a maximum concentration of 2% of sludge. Higher concentrations would have negative consequences on the mechanical, technical, and esthetic properties of the finished product. On the other hand, the change in coloration obtained by the addition of sludge, with a tendency toward dark tones, constitutes an advantage for the ceramic tile and stoneware industry, where pigments such as iron and manganese oxides are used to give different tones to the pieces. On the other hand, for glazed tiles with dark colors, if the bisque is darker, fewer pigments may have to be consumed in the glaze, lowering production costs.
The shaping process used at an industrial level induces changes in the technical properties of the final product and therefore its use when put into service. The results obtained in the present study show that under controlled conditions it is possible to incorporate sludge from the galvanic industry in the production of traditional ceramics at an industrial level and offers an alternative for the use of a new, low-cost raw material, mitigating the damage generated in the environment by the constant and growing accumulation of hazardous waste, which can come to have economic value.