Premixed insulins are a common treatment for type 2 diabetes mellitus (DM). However, their limitations and the lack of achieving glycaemic control in some patients reinforce the need to find therapeutic alternatives.
ObjectivesTo assess whether basal–prandial therapy (basal insulin, and additional pre-prandial rapid insulin boluses, when required) improves glycaemic control in patients with type 2 DM and glycosylated haemoglobin (HbA1c) >53mmol/mol (7%) treated with premixed insulin in the primary care setting.
Material and methodsA retrospective observational study in which 116 patients with type 2 DM switched from premixed insulin to basal–prandial therapy. Data on demographics, anthropometrics, laboratory results, and antidiabetic treatment were collected from the medical charts of the patients, prior to switching the treatment (baseline) and 4 months thereafter.
ResultsHbA1c significantly decreased from baseline to month 4 (65.1±5.7mmol/mol [8.1±0.5%] versus 51.9±7.2mmol/mol [6.9±0.7%]; p<.005), and 70 patients (60.9%) had an HbA1c ≤53mmol/mol (7%). Additionally, fasting blood glucose (FBG) significantly decreased (9.7±1.7mmol/l [175.4±31.2mg/dl] versus 6.9±1.4mmol/l [124.4±25.8mg/dl]; p<.005), and the number of patients with FBG<5.6mmol/l (100mg/dl) (2 patients [1.7%] versus 21 patients [18.3%]; p<.005), and with post-prandial blood glucose ≤10mmol/l (180mg/dl) (14 patients, [12.1%] versus 87 patients [76.3%]; p<.05) significantly increased. There were also significant decreases in body weight (76.3±12.9kg versus 74.8±12.5kg; p<.001) and waist circumference (96.1±16.0cm versus 94.4±14.5cm; p<.005). Only 4 patients (3.5%) had hypoglycaemia.
ConclusionsBasal–prandial therapy improved glycaemic control in patients with type 2 DM, with a low incidence of hypoglycaemia, and decreased body weight.
Las insulinas premezcladas constituyen un tratamiento habitual de la diabetes mellitus (DM) tipo2. Sin embargo, sus limitaciones y la ausencia de control glucémico en algunos pacientes refuerzan la necesidad de encontrar alternativas terapéuticas.
ObjetivosAnalizar si la terapia basal-prandial (insulina basal y bolos adicionales de insulina rápida preprandial cuando sea necesario) mejora el control glucémico de los pacientes con DM tipo2 y hemoglobina glucosilada (HbA1c)>53mmol/mol (7%) pese al tratamiento con insulinas premezcladas en atención primaria.
Material y métodosEstudio observacional retrospectivo en 116pacientes con DM tipo2 cuyo tratamiento cambió de insulina premezclada a terapia basal-prandial. Se recogieron datos demográficos, antropométricos, analíticos y tratamiento antidiabético de la historia clínica de los pacientes antes del cambio del tratamiento (basal) y 4 meses después.
ResultadosLa HbA1c descendió significativamente entre el momento basal y el mes 4 (65,1±5,7mmol/mol [8,1±0,5%] versus 51,9±7,2mmol/mol [6,9±0,7%]; p<0,005), y 70 pacientes (60,9%) mostraron HbA1c≤53mmol/mol (7%). Además, la glucemia en ayunas (FBG) disminuyó significativamente (9,7±1,7mmol/l [175,4±31,2mg/dl] versus 6,9±1,4mmol/l [124,4±25,8mg/dl]; p<0,005), y aumentó significativamente el número de pacientes con FBG<5,6mmol/l (100mg/dl) (2pacientes [1,7%] versus 21pacientes [18,3%]; p<0,005) y con glucemia postprandial≤10mmol/l (180mg/dl) (14pacientes [12,1%] versus 87pacientes [76,3%]; p<0,05). Se observaron descensos significativos del peso corporal (76,3±12,9 versus 74,8±12,5kg; p<0,001) y del perímetro de cintura (96,1±16,0 versus 94,4±14,5cm; p<0,005). Solamente 4 pacientes (3,5%) sufrieron hipoglucemia.
ConclusionesLa terapia basal-prandial logró mejorar el control metabólico de los pacientes con DM tipo2, con una baja incidencia de hipoglucemias y pérdida de peso.
Diabetes mellitus (DM) is a chronic disease that requires continuous medical care to prevent both acute and long-term complications.1 New drugs and many therapeutic combinations have been developed in an attempt to achieve glycaemic control, consisting mainly of the achievement of glycosylated haemoglobin (HbA1c) lower than 53mmol/mol (7%),1,2 with a cut-off for impaired fasting glucose of 5.6mmol/l (100mg/dl) and postprandial capillary blood glucose below 10mmol/l (180mg/dl).1 However, current management of DM has not been able to achieve and maintain the blood glucose levels required to provide an optimum state of health.2 In fact, approximately between 50% and 63% of patients do not achieve the above mentioned HbA1c levels.3,4
Lifestyle changes and administration of oral antidiabetic drugs such as metformin represent the initial approach to treatment of type 2 DM.2 In patients in whom glycaemic control is not achieved, the current clinical guidelines recommend combined administration of oral antidiabetic drugs and basal insulin.2 Indeed, several clinical trials have shown that, in patients with type 2 DM, HbA1c levels of 53mmol/mol (7%) or lower and an optimum control of baseline blood glucose may be achieved with one or two doses of insulin analogues combined with one or more oral antidiabetic drugs, mainly metformin.5–9 However, even in cases where fasting blood glucose (FBG) control is achieved, treatment optimization is often needed to control postprandial blood glucose.2 Moreover, progressive impairment of beta cell function in patients with type 2 DM may increase the difficulty for achieving glycaemic control, which would involve the need for intensifying the treatment regimen.2 The main therapeutic alternative for maintaining glycaemic control is the intensification of prandial insulin therapy by administration, in addition to basal insulin, of rapid-acting insulin analogues before each meal (basal–prandial therapy) or administration of insulin premixes.10
Although basal–prandial therapy better reproduces physiological insulin secretion and is the recommended regimen to intensify insulin treatment, administration of premixes is currently the most commonly used therapeutic regimen because of its greater simplicity.11 However, administration of insulin premixes has limitations such as the impossibility to separately adjust the doses of premix components, to use a flexible regimen of self-titration and calculation of preprandial insulin doses, and to adequately control postprandial and fasting blood glucose levels.12 In addition, this treatment regimen might be associated with higher risk of hypoglycemic episodes and weight gain.13 These limitations and the absence of achieving glycaemic control in some patients under treatment with premixed insulin led us to consider the addition of prandial insulin after optimizing the basal insulin as a practical therapeutic alternative.
The purpose of this study was to assess whether the administration of basal insulin and additional preprandial rapid insulin boluses when it was required (a step basal–prandial therapy) improves glycaemic control in patients with type 2 DM with poor metabolic control (HbA1c >53mmol/mol [7%]) treated with premixed insulin in the primary care setting.
Material and methodsThis was a multicentre, retrospective, observational study conducted in primary care centres in Ourense, Spain. The study was performed in compliance with the Declaration of Helsinki, all its amendments, and the applicable legal regulations. The study was approved by the appropriate ethics committees, and patients gave their informed consent to participate in the study.
Patients selectionThe main inclusion criteria were patients of both genders, aged 18 years or older, with a documented diagnosis of type 2 DM, who had been treated with premixed insulin (≤2 doses) for at least 6 months and who had been switched to basal–prandial therapy due to a poor metabolic control at least 4 months before study entry. Patients should have shown HbA1c levels higher than 53mmol/mol (7%) before being switched to basal–prandial therapy. Patients treated with corticosteroids or with severe concomitant diseases were excluded from the study.
Study treatmentStudy treatment consisted of administration of a step basal–prandial therapy together with oral antidiabetic drugs. Step basal–prandial therapy was defined as administration of a single dose of a long-acting insulin analogue as basal insulin and additional administration of a preprandial rapid insulin bolus at the meal where it was required. Treatment was administered according to the routine clinical practice at each centre. As this was a retrospective observational study, there was no interference with treatment decision or administration.
Patient assessmentInformation about patients’ assessments during the month prior to switching the treatment to basal–prandial therapy (baseline) and after 4 months of treatment was retrospectively collected from patients’ medical charts. The information collected included demographics, anthropometrics, laboratory results (blood glucose, HbA1c, chemistry, lipid profile), and antidiabetic treatment. Hypoglycaemia was defined as a blood glucose level lower than 3.8mmol/l (70mg/dl) or symptoms consistent with hypoglycaemia. Symptomatic hypoglycaemia was considered as an event with clinical symptoms consistent with hypoglycaemia. The measurements of glycaemia to control the hypoglycaemic episodes were performed according to routine clinical practice, measuring pre- and post-prandial capillary glycaemia at daily meals usually eaten by patients once a week, as well as when patients experienced symptoms compatible with hypoglycaemia.
Statistical considerationsQuantitative variables were described using centralization and dispersion measures (mean and standard deviation), and qualitative variables using frequencies and valid percentages. In order to assess whether statistically significant differences existed between baseline and month 4, a Student's t test for paired samples or a Wilcoxon's test was used for quantitative variables, and a MacNemar's test for qualitative variables. A value of p<0.05 was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc, Chicago, USA).
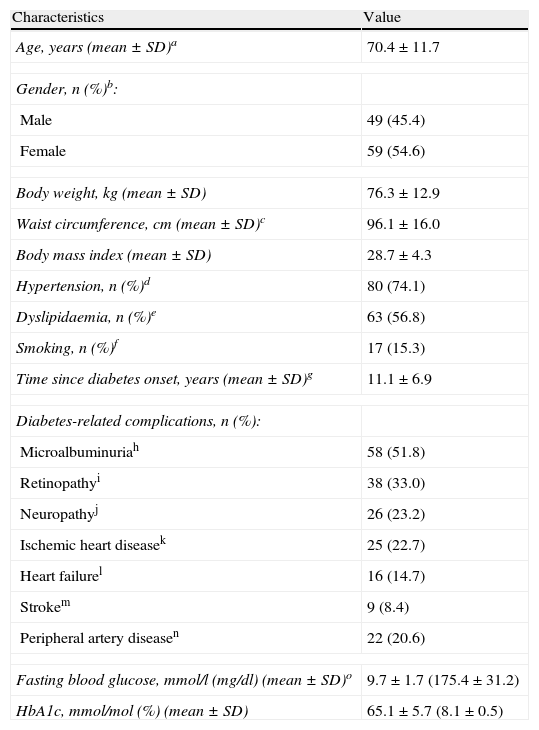
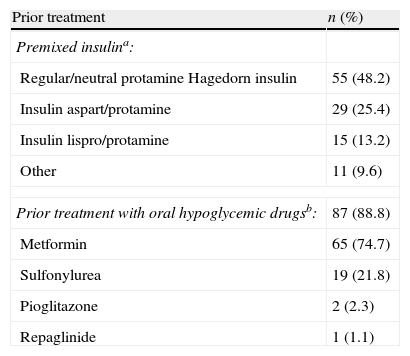
ResultsPatient populationFrom January 2009 to June 2009, a total of 116 patients were entered into the study. Patient characteristics are shown in Table 1. Mean FBG was 9.7±1.7mmol/l (175.4±31.2mg/dl), and mean HbA1c was 65.1±5.7mmol/mol (8.1±0.5%) (Table 1) despite treatment with premixed insulin and, in most cases, with oral hypoglycaemic drugs (Table 2).
Baseline characteristics of patients (n=116).
| Characteristics | Value |
| Age, years (mean±SD)a | 70.4±11.7 |
| Gender, n (%)b: | |
| Male | 49 (45.4) |
| Female | 59 (54.6) |
| Body weight, kg (mean±SD) | 76.3±12.9 |
| Waist circumference, cm (mean±SD)c | 96.1±16.0 |
| Body mass index (mean±SD) | 28.7±4.3 |
| Hypertension, n (%)d | 80 (74.1) |
| Dyslipidaemia, n (%)e | 63 (56.8) |
| Smoking, n (%)f | 17 (15.3) |
| Time since diabetes onset, years (mean±SD)g | 11.1±6.9 |
| Diabetes-related complications, n (%): | |
| Microalbuminuriah | 58 (51.8) |
| Retinopathyi | 38 (33.0) |
| Neuropathyj | 26 (23.2) |
| Ischemic heart diseasek | 25 (22.7) |
| Heart failurel | 16 (14.7) |
| Strokem | 9 (8.4) |
| Peripheral artery diseasen | 22 (20.6) |
| Fasting blood glucose, mmol/l (mg/dl) (mean±SD)o | 9.7±1.7 (175.4±31.2) |
| HbA1c, mmol/mol (%) (mean±SD) | 65.1±5.7 (8.1±0.5) |
HbA1c: glycosylated haemoglobin; SD: standard deviation; missing data: an=1; bn=8; cn=38; dn=8; en=5; fn=5; gn=49; hn=4; in=1; jn=4; kn=6; ln=7; mn=9; nn=9; on=1.
Prior antidiabetic treatment (n=116).
| Prior treatment | n (%) |
| Premixed insulina: | |
| Regular/neutral protamine Hagedorn insulin | 55 (48.2) |
| Insulin aspart/protamine | 29 (25.4) |
| Insulin lispro/protamine | 15 (13.2) |
| Other | 11 (9.6) |
| Prior treatment with oral hypoglycemic drugsb: | 87 (88.8) |
| Metformin | 65 (74.7) |
| Sulfonylurea | 19 (21.8) |
| Pioglitazone | 2 (2.3) |
| Repaglinide | 1 (1.1) |
Missing data: an=2; bn=18.
Total average dose of insulin did not significantly change from previous premixed insulin to basal–prandial therapy [31.1±11.0IU; 0.4±0.2IU/kg] versus [32.6±12.9IU; 0.4±0.2IU/kg]; (p=0.180). Even though all patients switched to basal–prandial therapy, it must be highlighted that 66 patients (61.7%) were being treated with basal insulin only and did not need any additional dose of rapid insulin four months after the switch from premixed insulin treatment, 46 of whom were also receiving oral hypoglycaemic drugs (metformin in 41 patients [89.1%], sulfonylurea in 4 patients [8.7%] and repaglinide in 2 patients [4.3%]). Forty-one patients were still receiving additional doses of rapid insulin: one dose in 9 patients (8.4%), two doses in 4 patients (3.7%), and more than two doses in 28 patients (26.2%). Data regarding the administration or not of additional doses of rapid insulin were missing in 9 patients. When only basal insulin was being administered, the mean insulin dose received by patients was 30.3±10.8IU. In patients receiving an additional dose of rapid insulin, mean basal and prandial insulin doses were 34.2±8.5IU and 9.3±3.4IU, respectively. In patients given two additional doses of rapid insulin, mean basal and prandial insulin doses were 38.0±10.6IU and 12.5±2.9IU, respectively. In patients receiving more than two additional doses of rapid insulin, mean basal and prandial insulin doses were 19.9±12.0IU and 11.5±5.9IU, respectively. The type of basal insulin used was insulin glargine in 114 patients (98.3%) and insulin detemir in 2 patients (1.7%). The most commonly used prandial insulin was insulin glulisine (34 patients [29.3%]), followed by human neutral soluble insulin (PRB) (11 patients [9.5%]) and insulin aspart (3 patients [2.6%]).
In addition, 72 patients (82.8%) reported being receiving oral hypoglycaemic drugs four months after switching the treatment (metformin in 66 patients [91.7%], sulfonylurea in 5 patients [6.9%] and repaglinide in 3 patients [4.2%]), while 15 patients (17.2%) reported not being receiving them. Data were not available in 29 patients.
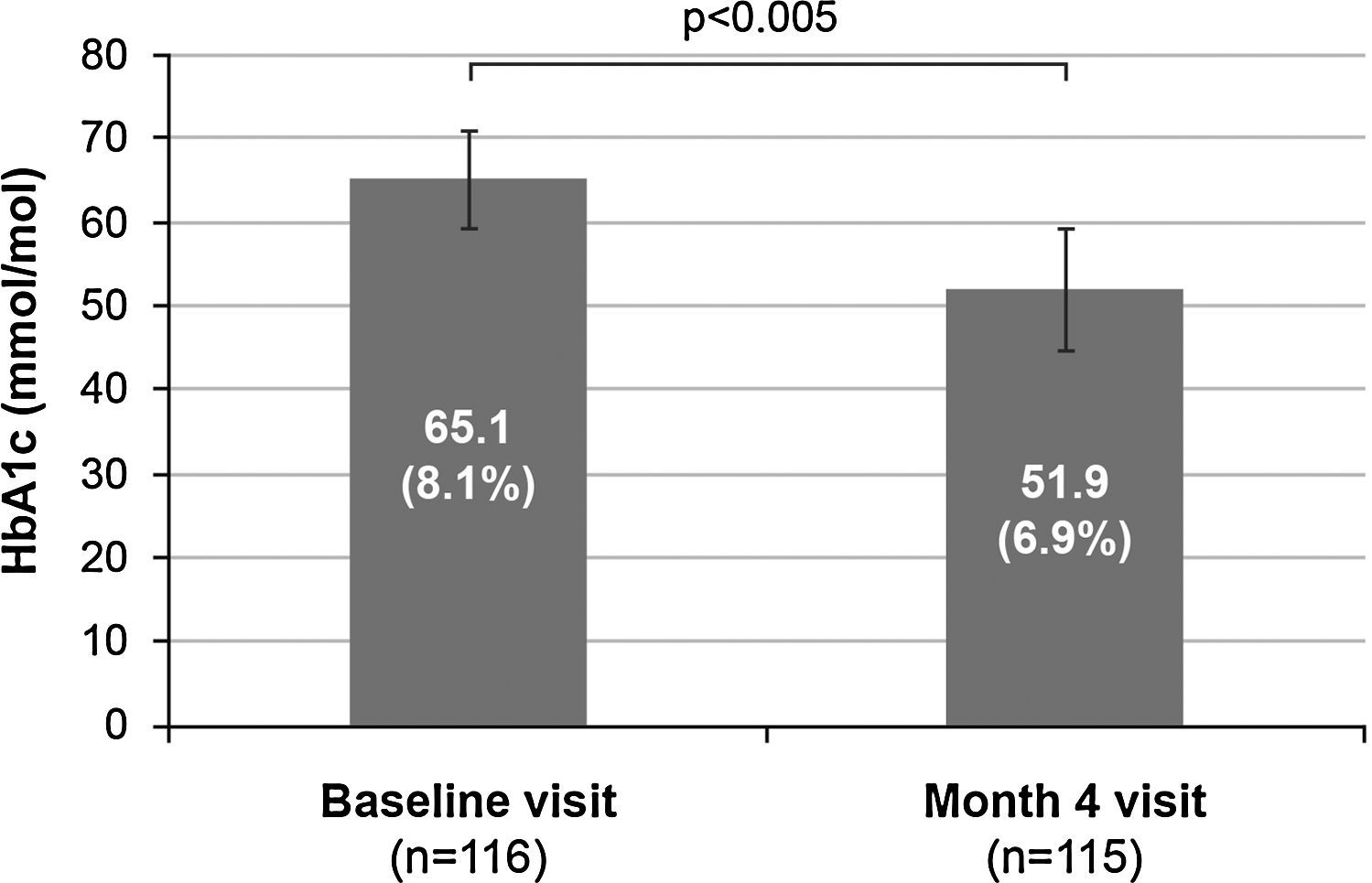
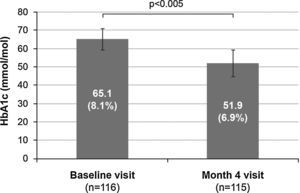
Treatment efficacyFour months after treatment was switched from premixed insulin to basal–prandial therapy, 70 patients (60.9%) achieved good metabolic control (HbA1c ≤53mmol/mol [7%]). Among these, 38 patients (54.3%) were receiving basal insulin alone at that time point, and 32 patients (45.7%) were also receiving additional doses of rapid insulin. One dose of rapid insulin was reported in 10 patients (33.3%), two doses in 3 patients (10.0%), and more than two doses in 17 patients (56.7%). The number of doses of rapid insulin received by the remaining 2 patients was missing. In addition, mean HbA1c values decreased by 13.2mmol/mol (1.2%) during the study, from 65.1±5.7mmol/mol (8.1±0.5%) at baseline to 51.9±7.2mmol/mol (6.9±0.7%) at month 4 (p<0.005) (Fig. 1).
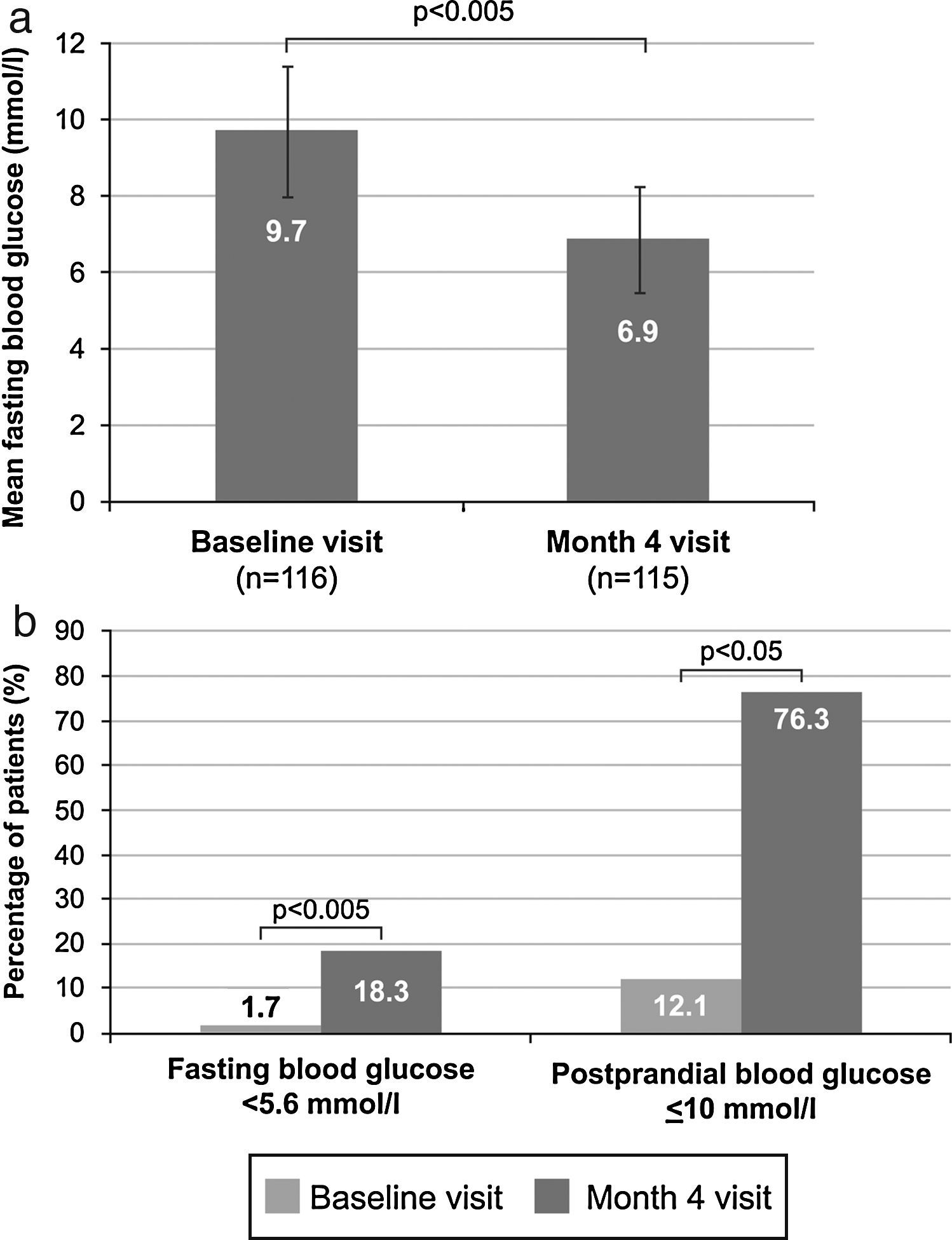
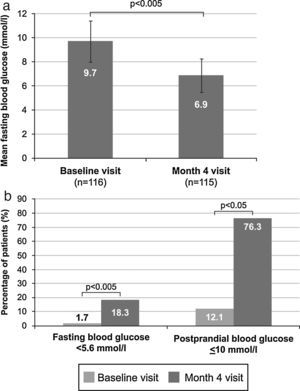
Mean venous FBG significantly decreased during the study from 9.7±1.7mmol/l (175.4±31.2mg/dl) at baseline to 6.9±1.4mmol/l (124.4±25.8mg/dl) at month 4 (p<0.005) (Fig. 2A). A significant change between these time points also occurred in the number of patients with venous FBG levels lower than 5.6mmol/l (100mg/dl), which increased from 2 patients (1.7%) to 21 patients (18.3%) (p<0.005) (Fig. 2B). Additionally, a significant increase from 14 patients (12.1%) at baseline to 87 patients (76.3%) at month 4 (p<0.05) (Fig. 2B) was observed in the proportion of patients with controlled postprandial capillary blood glucose (≤10mmol/l [180mg/dl]). Basal insulin was only being administered to 43 of these 87 patients (49.4%), and additional preprandial insulin doses were being given to 44 patients (50.6%). Only 4 patients (3.5%) experienced hypoglycaemic episodes. There were 2 asymptomatic and 2 symptomatic episodes, and one of the latter occurred at night. In addition, 81.4% of patients who achieved glycaemic control (HbA1c <53mmol/mol [7%]) after switching treatment reached it without both hypoglycaemic episodes (94.3%) and weight gain (87.1%).
A subanalysis of the 64 patients (55.2%) who did not change oral antidiabetic drug doses showed that 39 patients (61.9%) had HbA1c ≤53mmol/mol (7%), and mean HbA1c significantly decreased from 65.0±6.0mmol/mol (8.1±0.6%) at baseline to 52.0±7.8mmol/mol (6.9±0.7%) at month 4 (p<0.001). In addition, mean venous FBG significantly decreased during the study from 9.4±1.4mmol/l (169.1±25.6mg/dl) to 7.0±1.3mmol/l (126.3±23.0mg/dl) (p<0.001). Although the number of patients with venous FBG levels below 5.6mmol/l (100mg/dl) increased from 1 patient (1.6%) at baseline to 8 patients (12.7%) at month 4, statistical significance was not reached. Moreover, the proportion of patients with controlled postprandial capillary blood glucose (≤10mmol/l [180mg/dl]) increased from 10 patients (15.6%) at baseline to 50 patients (79.4%) at month 4 (p<0.05).
Anthropometric dataMean patient weight significantly decreased after switching to basal–prandial therapy, from 76.3±12.9kg at baseline to 74.8±12.5kg at month 4 (p<0.001). Waist circumference also significantly decreased from 96.1±16.0cm at baseline to 94.4±14.5cm at month 4 (p<0.005).
DiscussionThe present study was designed as observational to assess the effect of a step basal–prandial therapy according to routine clinical practice. The results of this study showed that switching the treatment from premixed insulin to a step basal–prandial therapy might improve glycaemic control in patients with type 2 DM. The advantages of basal–prandial therapy over insulin premixes were demonstrated in a recent 52-week randomized clinical trial.14 The results reported showed that administration of insulin glargine as basal insulin combined with preprandial insulin glulisine allowed for a significant reduction in HbA1c levels as compared to use of insulin premixes in patients with type 2 DM (−9mmol/mol [−1.31%] versus −15mmol/mol [−0.80%]). Such reduction in HbA1c levels was similar to that found in this study, which reinforce the beneficial effect of basal–prandial therapy under standard clinical practice conditions. In our study, the reduction was achieved in an even shorter treatment period. Another 24-week randomized clinical trial found even greater decrease in HbA1c levels.15 This clinical trial was designed to assess the hypothesis of non-inferiority of premix therapy to basal–prandial therapy with insulin glargine as basal insulin and prandial insulin lispro. Although the hypothesis could not be confirmed, the trial allowed for increasing the little information available about the comparison of both treatment schemes. In fact, both groups showed a significant decrease in glycosylated HbA1c levels from baseline to the end of treatment, from 74mmol/mol (8.9%) to 51mmol/mol (6.8%) in the group given basal–prandial therapy and from 73mmol/mol (8.8%) to 53mmol/mol (7.0%) in patients receiving insulin premixes. Moreover, the number of patients with HbA1c levels under 53mmol/mol (7%) increased during follow-up, tending to be higher after 12 weeks of treatment, and becoming statistically significant at the end of the study (69% versus 54%, p<0.05). Reduction in HbA1c levels may partially be due to the significantly lower FBG (8.2mmol/l [147mg/dL] versus 8.8mmol/l [159mg/dl], p=0.013) and postprandial glucose (8.6mmol/l [155mg/dl] versus 9.7mmol/l [174mg/dl], p=0.002) levels observed in the group receiving basal–prandial therapy. Results of our study also showed a significant decrease in FBG levels. Mean values even lower than previously reported and within the range of 3.9–7.2mmol/l (70–130mg/dl) recommended by clinical guidelines were achieved,1 as well as an increase in the proportion of patients with FBG lower than 5.6mmol/l (100mg/dl). Such decrease in FBG may have contributed to HbA1c reduction. However, such contribution is more relevant in the first years following diagnosis of type 2 DM.16 As the disease advances, contribution of postprandial blood glucose gains importance and has a progressively greater impact on HbA1c levels.16 Considering the time since onset of diabetes in our patient population, the significant decrease in postprandial blood glucose observed in the present study, together with the increased proportion of patients with controlled postprandial blood glucose at month 4 of treatment, might have significantly contributed to an improved glycaemic control of patients.
Although intensification of antidiabetic therapy may improve glycaemic control, potential occurrence of hypoglycaemia represents the greatest handicap for administration of intensive therapy.17 The reported number of hypoglycaemic episodes by patient and year varies substantially. One of the clinical trials conducted reported 48.7 episodes of hypoglycaemia/patient/year in patients treated with the basal–prandial scheme and 51.2 episodes/patient/year in patients receiving insulin premixes.15 Another clinical trial demonstrated lower mean rates of 13.9 episodes of hypoglycaemia/patient/year in patients treated with basal–prandial therapy and 18.5 episodes/patient/year in those receiving insulin premixes.14 Such hypoglycaemia rate is even higher than the one found in the present study, where only 4 episodes of hypoglycaemia were recorded during the 4 months of treatment with the basal–bolus therapy. These results are more similar to those found in another clinical trial where basal–prandial therapy was reported to be a safe regimen with only 2 episodes of hyperglycaemia in a group of 65 hospitalized patients with type 2 DM given such therapy.18
Food supplements are often recommended to prevent the occurrence of hypoglycaemia.19 Because of this, and since up to 90% of patients with type 2 DM are obese,20 impact of treatment on loss weight should be analyzed. In contrast to the results obtained in the previously conducted studies, the present study found a significant 1.5kg reduction in mean body weight and a 1.7cm reduction in mean waist circumference in patients switched from premixed insulin to basal–prandial therapy in usual clinical practice. Prior clinical trials comparing both regimens reported variable results, from higher mean weight gains in the group receiving basal–prandial therapy (3.6kg versus 2.2kg, p=0.0073)14 to similar weight gains with both treatment schemes (4.5kg versus 4.0kg, p=0.224).15 Other clinical trials have also demonstrated the potential interference of the type of basal insulin with mean weight gain.21–23 However, the results reported by another retrospective study conducted on patients switched from conventional insulin therapy to the basal–prandial regimen do not support the hypothesis that basal–prandial therapy is associated to an increase in body weight.24
In addition, health-care team may have also contributed to the benefit observed in our study. In fact, improvements in glycaemic control of patients have been achieved even though more than a half of them did not need any additional dose of rapid insulin four months after the treatment was switched. Guidelines of medical care for patients with diabetes mellitus highlight the importance of medical care received by patients from physician-coordinated multidisciplinary teams capable of providing ongoing diabetes support and self-management education1. Apart from treatment efficacy, these multidisciplinary teams have shown to confer an incremental reduction in HbA1c values.25 Besides, other strategies for diabetes care such as case management, team changes, patient reminders and patient education have also produced additional small to modest improvements in glycaemic control.25 Thus, further studies are still needed to clarify the contribution of the health-care team and basal–prandial therapy in the improvement of glycaemic control.
Some limitations should be considered when interpreting the study results. Although observational studies provide valuable information about the administration of treatments in clinical practice conditions, they are not capable of providing strong evidence or establishing cause–effect relationships. The lack of a comparator group and the retrospective collection of data from patients’ medical charts are also limitations to be taken into account, as well as the absence of patients switching from more than two doses of premixed insulin, the arbitrary use of prandial insulin that may not reflect the best clinical practice and the short study follow-up that precludes the achievement of conclusions concerning the durability of the benefit achieved by the study treatment. Additionally, biases derived from changes in oral hypoglycaemic agents cannot be excluded. Even though the results of the present study should be interpreted with caution based on the previously mentioned limitations, the author believes that they provide helpful information to clinicians.
In conclusion, results of the present study show that switching of patients with type 2 DM from treatment with premixed insulin to a basal–prandial insulin scheme might enable a significant improvement in glycaemic control to be achieved under routine clinical practice conditions. In addition, the improvement in glycaemic control observed in our study enabled almost two-thirds of patients not to need additional doses of prandial insulin four months after the treatment switch, and it was attained with a very low number of hypoglycaemic episodes and a significant decrease in body weight. However, the results of the present study should be interpreted with caution based on its previously mentioned limitations, and further studies are still needed to confirm them and to assess both the long-term maintenance of the improved glycaemic control in routine clinical practice and the effect of this treatment modification on patients’ satisfaction and quality of life.
Conflict of interestThe authors have no conflict of interest to declare.
No external funding was received for this study, which was conducted thanks to the unconditional effort of all participating investigators.
The author would like to acknowledge the following investigators from the Ourense Healthcare Area in Spain who participated in the study: Margarita Arandia-García (Centro de Salud Coles), María Jesús Arias-Gómez (Centro de Salud A Ponte), Inmaculada Casado-Gorriz (Centro de Salud Allariz), Gabriel Díaz-Grávalos (Centro de Salud Cea), Aurora Eiris-Cambre (Centro de Salud Valle Inclán), María del Carmen Fernández-López. (Centro de Salud Xinzo), Francisco Fernández-Marcos (Centro de Salud Viana do Bolo), María Jesús Fernández-Silva (Centro de Salud El Puente), Mercedes González-Ferreira (Centro de Salud Xinzo), Isabel Gómez-Fernández (Centro de Salud Xinzo de Limia), Antonio González-Álvarez (Centro de Salud Novoa Santos), José Luis López-Álvarez (Centro de Salud Mariñamansa), María del Carmen López-Gómez (Centro de Salud Novoa Santos), Belén Novoa-Rodríguez (Centro de Salud A Ponte), Antonio Pedreira-Penedo (Centro de Salud Irixo), Luis Prieto-Robisco (Centro de Salud A Carballeira), Antonio Quintela-Fernández (Centro de Salud Castrelo de Miño), Ángeles Reinoso-Rey (Centro de Salud Valle Inclán), Ana Isabel Sala-López (Centro de Salud Ponte Noalla), Jesús Valverde-Leis (Centro de Salud Verín), Aquilino Vázquez-Fernández (Centro de Salud Cartelle), Francisco Vázquez-Rodríguez (Centro de Salud Irixo), Carmen Vidal-García (Centro de Salud Xinzo), Rubén Vilariño-Méndez (Centro de Salud A Ponte), and Ana Veiga-Vázquez (Centro de Salud Verín).
Medical writing support was provided by Esther Álvarez-García at Dynamic S.L. during the preparation of this paper. Responsibility for opinions, conclusions and interpretation of data lies with the author.
Data from the manuscript were presented at the following meeting: 71st Scientific Sessions of the American Diabetes Association, June 24–28th, 2011, San Diego, USA.