One of the limitations of clinical trials lies in the difficulty of extrapolating their results to populations other than the one investigated initially. There are two main limitations: the first arises when the inclusion and exclusion criteria are so strict that a non-negligible number of patients in daily clinical practice would not be eligible, for one reason or another, to take part in the trial. The second limitation lies in the extrapolation of the results to a population within a community whose epidemiological characteristics differ from those of the community chosen for the initial trial.
One example, now widely debated in the medical literature on the extrapolation of the results to other communities, is the case of clinical trials of the primary prevention of cardiovascular disease with lipid-lowering drugs.
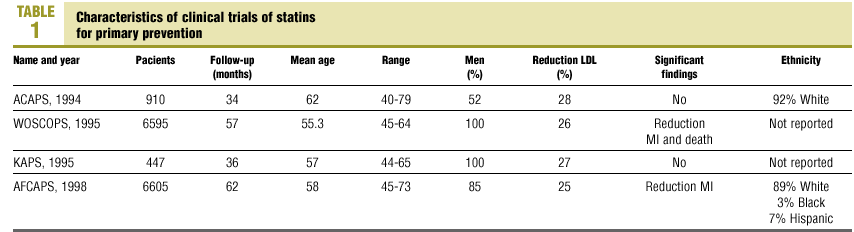
The first problem that raises concerns about the external validity of these clinical trials is age-bias and sex-bias. In an analysis of clinical trials of primary prevention published in the last 10 years, we found 4 studies (Table 1) that involved a total of 14 557 randomly assigned patients, with a mean age of 56.9 years. The WOSCOPS1 and AFCAPS2 trials included the largest numbers of participants. The WOSCOPS and KAPS3 trials recruited men only; in the AFCAPS trail 85% of the participants were men, and women (48%) were best represented in the ACAPS4 trial.
Overall, of the 14 557 participants, 13 129 (90%) were men. The WOSCOPS and AFCPAS trials were the only ones to yield significant findings; the latter included patients up to 73 years of age. Information on ethnicity was included only in the reports of the ACAPS and AFCAPS trials. Both were carried out in the USA, and white persons made up 92% and 89% of the population, respectively.
Primary prevention trials have therefore been based on populations consisting predominantly of white males -- a feature that makes it difficult to extrapolate the findings to populations of older persons, women, and non-white persons. A meta-analysis of primary prevention studies with statins confirmed the lack of benefit in terms of overall mortality, probably because the reduction in coronary heart disease was too small to influence overall mortality.
The WOSCOPS trial was the first to find net benefits of statins for primary prevention. The AFCAPS trial confirmed the benefits and extended them to persons at lower risk (mean cholesterol 5.7 mmol/L). The number needed to treat (NNT) to prevent 1 myocardial infarction was 42 in the WOSCOPS study, and 50 in the AFCAPS trial. A study that simulated the application of the results of the WOSCOPS trial in a Spanish population estimated that if the trial had been carried out in Spain, and assuming the same reduction in relative risk (RRR 31%), the NNT would be 161, or four-fold as high as in the original study, because the baseline risk for the Spanish population is much lower than in Scotland, where the WOSCOPS trial was done.5 Moreover, cohort studies such as the Seven Countries Study showed that for a given cholesterol level, the risk of death from coronary heart disease varied in different countries6. Specifically, for a cholesterol level of 5.2 mmol/L the risk of death from coronary causes was five-fold as high in Northern Europe as in Mediterranean countries (15% vs 3%). This was the origin of the so-called «French paradox» (probably also applicable to Spain), which showed that with an equally unfavorable risk profile (for example, a mean level of total cholesterol of 6.1 mmol/L in men and 6.5 mmol/L in women in France from 1985 to 1990, and practically identical values in the UK), the rate of ischemic heart disease in France was around 25% the rate found in the UK.
More recent studies with lipid-lowering drugs7,8 have been carried out in high-risk populations that included patients with a history of cardiovascular disease or other risk factors without a history of disease. The MRC/BHF Heart Protection Study and Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm represent a new approach to the design of trials with lipid-lowering drugs, as the aim of treatment was not to reduce cholesterol levels, but to reduce risk. The HPS7 trial enrolled more than 20 000 persons in the UK, and subgroup analyses were possible for age, sex, total cholesterol level and previous history of coronary heart disease. Although the benefits were spectacular in practically all subgroups, analysis of the application of the results in clinical practice is more complex than for the «pure» clinical trials of primary prevention measures noted above. For example, if we wished to compare patients without previous coronary disease assigned to receive simvastatin or placebo, we have no information on the number of patients with non-coronary vascular diseases who were included in each subgroup. Likewise, if we wished to compare the results in the subgroup of women, or the subgroup of participants more than 70 years old, no information is available on the proportion of these participants who had a previous history of coronary heart disease. These subgroups probably contained patients with prior vascular disease, given that 41% of the entire study population had a previous history of myocardial infarction, and 24% had a history of other coronary heart disease. Of the remaining 35% -- participants with no history of coronary heart disease -- 25% had a history of cerebrovascular disease, and 38% had a history of peripheral vascular disease. Consequently, about 87% of the population in the study had one or more cardiovascular diseases, meaning that the HPS trial should perhaps be considered a secondary prevention study. The results of the HPS trial showed that all patients who had a vascular event probably benefited from treatment with a statin regardless of their cholesterol level.
The ASCOT8 study enrolled patients in the UK and Scandinavian countries with hypertension who were at high risk for cardiovascular disease but who did not have a prior history of coronary heart disease. This study can thus be considered a test of the primary prevention of coronary heart disease (but not of cardiovascular disease, as nearly 20% of the patients in both the group assigned to receive atorvastatin and the placebo group had antecedents of some other cardiovascular disease).
In the ASCOT study a cholesterol level below 6.5 mmol/L (approximately 260 mg/dL) was the criterion, with mean values of 5.5 mmol/L (approximately 220 mg/dL) in both groups. These values were slightly lower than those obtained in the AFCAPS trial described above. The ASCOT study reported a significant 36% reduction in the combined endpoint of death and myocardial infarction after 3.3 years´ follow-up in a population aged 40 to 79 years. However, it is notable that no significant differences were found in certain subgroups such as women, patients with diabetes, or patients with a prior history of vascular disease.
Thus it is that some issues raised by now classical studies of primary prevention, such as the benefits for women or for persons older than 75 years, remain unresolved. The questions surrounding the benefits that would be expected if these trials had been done in populations with a lower baseline risk, such as the inhabitants of Mediterranean areas, also remain unanswered.
Key points
* Primary prevention trials have been based mainly on populations of white men up to 79 years of age.
* For a given level of total cholesterol, the risk of dying from coronary heart disease can be up to five-fold as high in Northern Europe as in Mediterranean countries.
* New studies of lipid-lowering drugs are focussed differently: treatment aims to reduce risk levels rather than cholesterol levels.
* New evidence regarding secondary prevention suggests that all patients might benefit from statins, whereas evidence from primary prevention studies has not shown clearly that all patients at high risk -- regardless of their cholesterol level -- benefit from lipid-lowering treatment.