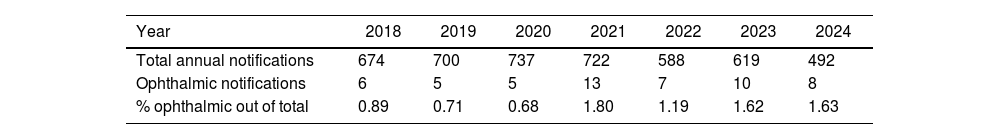
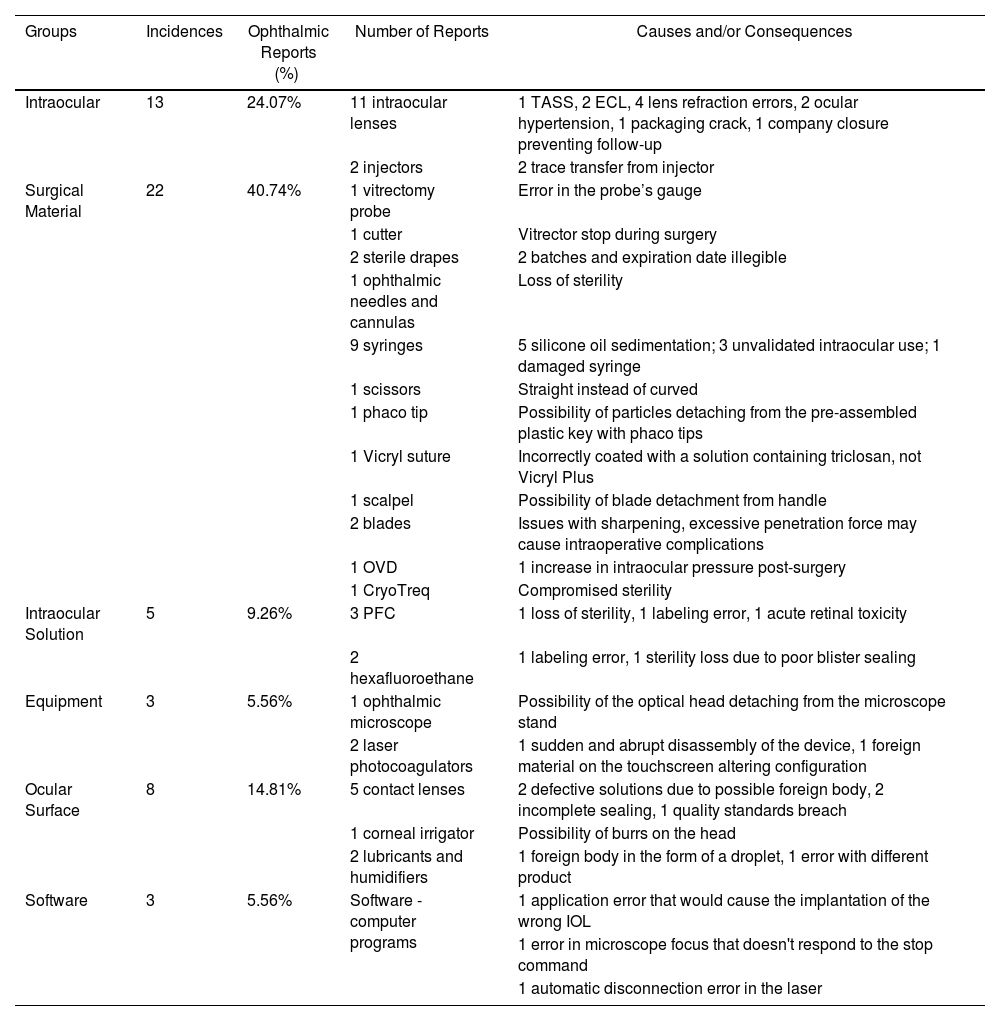
The implementation of the Medical Device Regulation and Royal Decree 192/2023 not only requires manufacturers to enhance safety measures in the production of medical devices but is also complemented by additional regulations impacting hospitals, clinics, and healthcare personnel. Among these is the requirement to identify and report serious incidents to the Spanish Agency of Medicines and Medical Devices (AEMPS). To establish a baseline and evaluate the dissemination efforts of these concepts at the level of scientific societies and other organizations, serious incidents involving ophthalmic medical devices reported to AEMPS since 2018 and subject to health alerts have been identified. Although the percentage remains minimal, it is notable that issues with software or equipment such as surgical microscopes and Optical Coherence Tomography (OCT) are now being reported.
La entrada en vigor del Medical Device Regulation y del Real Decreto 192/2023 obliga no solo a los fabricantes a extremar las medidas de seguridad en la fabricación de productos sanitarios, sino que se complementa con otras que afectan a hospitales, clínicas y personal sanitario. Entre ellas destaca la obligación de identificar y notificar a la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) los denominados incidentes graves. Para tener una idea de partida y evaluar el esfuerzo de divulgación de estos conceptos tanto a nivel de sociedades científicas como de otros organismos, se han identificado los efectos graves en productos sanitarios de uso ocular, comunicados a la AEMPS desde 2018 y objeto de alertas sanitarias. Aunque el porcentaje es todavía testimonial, llama la atención que ya empiezan a notificarse problemas con software o con equipamientos, como microscopios quirúrgicos y tomografía de coherencia óptica (TCO).