Drug therapy to improve prognosis and quality of life of patients living with MASLD remains a major unmet need. Dietary intervention based on Mediterranean diet and an increasement in structured physical activity have demonstrated an improvement in liver features of MASLD from steatosis to steatohepatitis and a regression of at least one stage of fibrosis. Indeed, losing 10% of body weight has been associated with an impressive response of 90% of patients with resolved steatohepatitis, 100% with improved steatosis and 80% whose fibrosis has regressed at least in one stage [1]. Unfortunately, only 10% of the cohort achieved a 10% loss of body weight, which in turn supports the idea that lifestyle intervention is highly useful but very difficult to achieve. Nevertheless, lifestyle intervention could stratify patients in responders and non-responders to the intervention, which may aid in selecting the subgroup requiring drug therapy [2].
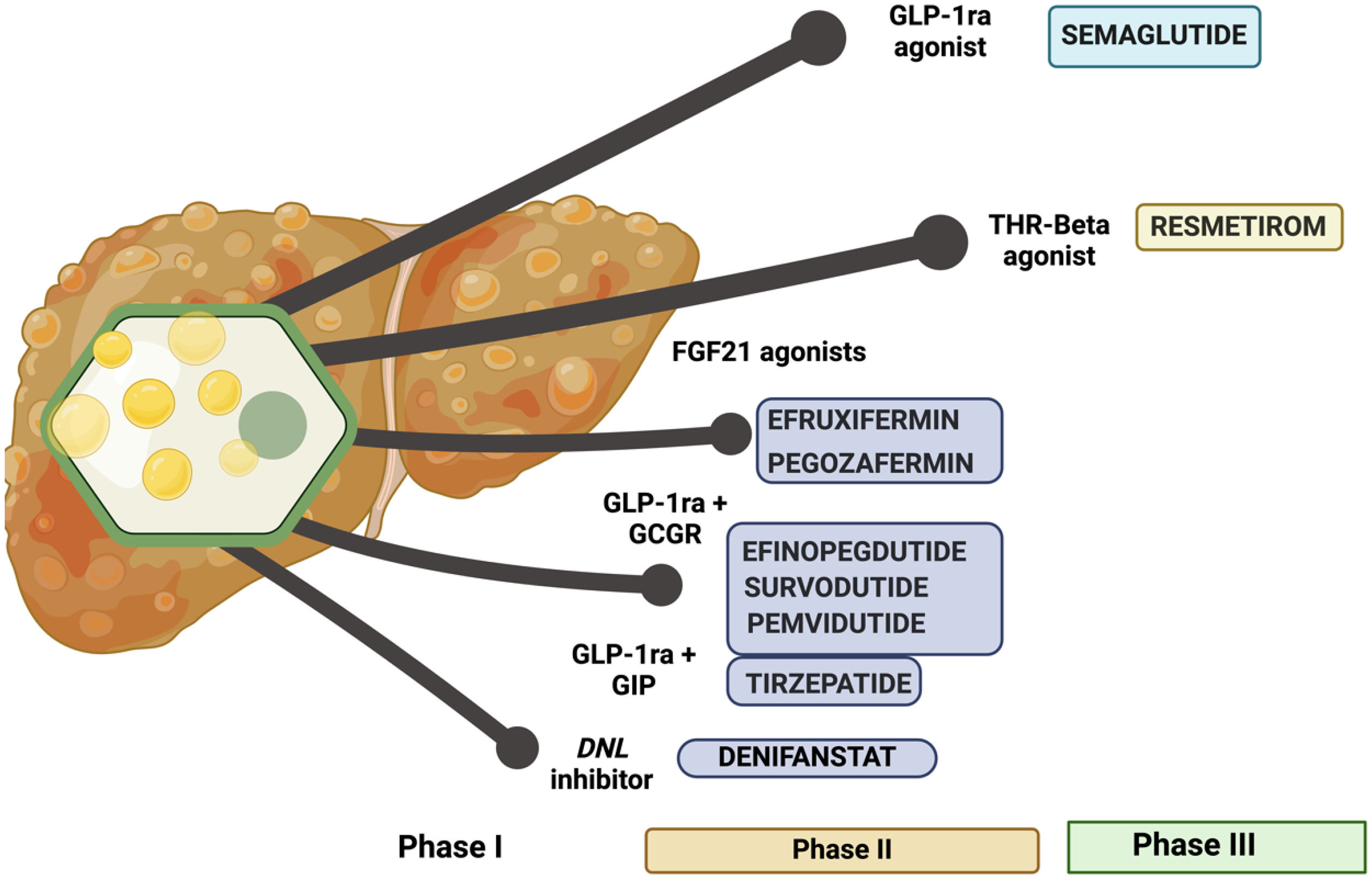
During the design of drug interventions in MASLD, pathophysiological events were taken one by one and have been targeted through different approaches with varying success rates. Insulin resistance, lipid metabolism imbalance to bile acids metabolism, gut-liver axis, apoptosis, and mitochondrial dysfunction were targeted separately, and as a result some drugs reached phase III clinical trials but failed at demonstrating superiority against placebo. Such was the case with selonsertib, an ASK-1 inhibitor; cenicriviroc, a CCR2/CCR5 antagonist; or elafibranor, a dual alpha-delta PPAR agonist [2,3]. Resmetirom achieved this goal and received a fast-track approval by FDA. Semaglutide demonstrated superiority to placebo in phase III Essence trial; however, it has neither been approved nor published yet. Several other drugs, including FGF-21 agonists [4] (efruxifermin, Pegozafermin), de novo lipogenesis inhibitors like denifanstat [5] or dual incretins analogs like GLP-1/Glucagon receptor [6,7] (efinopegdutide, pemvidutide and survodutide), or GLP-1/GIP (tirzepatide) agonists have also been tested in phase II RCT. These drugs demonstrated a positive effect with patients achieving MASH resolution from a half to two thirds of the cohort receiving active arm and promoting at least one stage fibrosis regression around a quarter to a third of treated patients and they are currently moving on to phase III randomized clinical trials [8]. Moreover, testing the usefulness of these drugs on liver cirrhosis, a stage without any approved drug therapy; as such, it remains an unmet need.
Resmetirom is an agonist of beta fraction of thyroid hormone receptor (THR-β) that is highly expressed in the liver but not in other organs, precluding adverse events related to hyperthyroidism in which the alpha fraction is involved. Intrahepatocyte hypothyroidism has been reported in patients with MASLD; in such cases, resmetirom could restore thyroid tone, promoting fat removal and decreasing oxidative stress and inflammation by modulating de novo lipogenesis, fatty acid beta oxidation, mitophagy and mitochondrial biogenesis, cholesterol metabolism, and carbohydrates metabolism [9]. In a phase II RCT including 125 patients with biopsy-proven MASLD and fibrosis F1-F3 and fat infiltration in the liver with at least 10% by MRI-PDFF received placebo or resmetirom 80 mg or 100 mg per day. In comparison with placebo, MRI-PDFF improved (differences in least square means) in patients receiving resmetirom at week 12 (-22,5; CI95%: -32,9 to -12,2) and week 36 (-28,8; CI95%:-42,0 to -15,7). Moreover, some biomarkers of liver damage and fibrosis also improved. Safety was similar in resmetirom and placebo arm [10]. Furthermore, four RCT have been designed (two have already been reported and two remain ongoing) to demonstrate safety and efficacy beyond fibrosis regression on hepatic decompensation rate [11]. In trial MAESTRO-NAFLD-1, 1143 patients with at least 3 metabolic risk factors and MRI-PDFF higher than 8% were randomized to three double-blind arms (100 mg resmetirom (n = 325), 80 mg resmetirom (n = 327) or placebo (n = 320)) or open-label 100 mg resmetirom (n = 171). The adverse event rate was similar across four arms and the drug was well tolerated. Gastrointestinal adverse events were more commonly observed in patients receiving resmetirom, but diarrhea was self-limited and did not promote drug withdrawal. Resmetirom promoted a decline of 10% to 20% in LDL-cholesterol, ApoB, triglycerides, and 30%-40% hepatic fat infiltration and modest changes in liver stiffness [12]. In MAESTRO-NASH, a phase III RCT recruiting 966 biopsy-proven MASLD patients with MASH and F2-F3 fibrosis, were randomized to three arms: placebo (n=321), resmetirom 80 mg (n=322) and resmetirom 100 mg (n=323), one pill per day. The main aim was to achieve a histological response at week 52 defined as ≥2 points of improvement in NAS score without impairment of fibrosis and a fibrosis regression of at least one stage without impairment of steatohepatitis. The trial remains ongoing, and a third liver biopsy was planned for month 54. MASH resolution was observed to be significantly higher in patients receiving resmetirom (25,9%, 29,9% and 9,7% in resmetirom 80 mg, 100 mg, and placebo arms respectively). An improvement of at least one stage of fibrosis was also significantly observed in patients receiving resmetirom (24,2%, 25,9% and 14,2% in resmetirom 80 mg, 100 mg, and placebo arms respectively). However, both goals of MASH resolution and fibrosis regression were only achieved in 14,2%, 16% and 4,9% in resmetirom 80 mg, 100 mg, and placebo arms respectively. Adverse events showed a similar distribution (10%-12%) among arms, thus confirming an optimal safety profile for the drug. These preliminary results were promising and allowed a fast-track approval by FDA; however, as it was restricted to 52 weeks of therapy, more information is required regarding long-term safety and efficacy, as well as the performance of a final analysis calculating number needed to treat (NNT) and number needed to harm (NNH). Nevertheless, fibrosis improvement has been associated with lower rates of clinical outcomes [13]. Two more points need to be addressed: a) the stratification of patients according to their sensitivity to resmetirom, identifying features associated with resmetirom response; and b) definition of futility rules that could allow us to withdraw the drug when a response is not achievable. Response to resmetirom therapy should also be addressed in a specific subgroup of patients after stratifying them according to their lack of response to lifestyle intervention with Mediterranean diet and structured aerobic physical activity. Indeed, monitoring sex-hormone binding globulin levels resmetirom compliance could also be addressed. Lastly, resmetirom could represent a potential useful drug in lean-MASH patients, in whom weight-lowering drugs could not be preferred, but it remains to be demonstrated.
Semaglutide has also demonstrated superiority compared to placebo in fibrosis-MASLD. GLP-1 agonist has been breaking many barriers to reach a full position on the management of metabolic-associated diseases. Due to its pleiotropic effect on the brain, pancreas, gastrointestinal tract and muscle, promotes an overall metabolic improvement rising lipolysis, fatty acid oxidation, ketogenesis and blocking hepatic de novo lipogenesis, overexpression of LDL receptor promoting a decline in LDL-cholesterol levels, and increasing energy expenditure to promote diabetes control and weight loss. In a phase II study (n=320), semaglutide 0,4 mg daily (2.8 mg/week) demonstrated a significantly high ability to promote MASH resolution in comparison with placebo [14]; however, it did not promote fibrosis regression. However, in a phase III study with a larger cohort (N=800) enriched on F3 patients, an intermediate analysis demonstrated that both steatohepatitis resolution (62,9% vs. 34.1%;p<0.001) and the fibrosis regression rate (37% vs. 22.5%;p<0.001) were superior to placebo without safety concerns [15]. These results support a fast-track approval of semaglutide as drug therapy for MASLD.
Questions regarding the potential combination of these two drugs have also arised. More than 14% of patients treated with resmetirom were being provided with stable doses of semaglutide, which had no specific impact on preliminary analysis and no issues were raised about safety or interactions. Regardless, this data suggests we could combine both in clinical practice when indicated and available, as further studies are guaranteed to demonstrate the efficacy of combination therapy in this setting.
Management of MASLD-fibrosis requires taking short- and long-term. Its beneficial effect, which has apparently been modest in the first year, could be subjected histological improvement, thus becoming the starting point in a change in the trajectory of patients; however, we cannot anticipate how different it would be in comparison with no treatment or placebo at the moment. Further analysis of the ongoing trials should eventually demonstrate the impact of these drugs on the natural history of the disease and their potential for improving quality of life, promoting fibrosis regression, and reducing extrahepatic and hepatic cancer along with a decline in hepatic decompensation rates.
In summary, resmetirom is an approved and already promising drug. However, it is also worth noting that semaglutide will become available soon for MASLD and fibrosis treatments, showing a safe profile and promising preliminary clinical results. This could give way to an increasement in quality of life and survival rates in patients living with MASLD. (Figure 1)