Liver transplantation (LT) is a well-established therapy for patients with decompensated cirrhosis and early-stage hepatocellular carcinoma. Liver transplantation activity varies sharply across Latin American (LATAM) countries due to differences in resources, expertise, and funding and local attitudes toward organ donation and transplantation. This current guidance of postoperative care after LT is the first position paper of the Latin American Association for the Study of the Liver (ALEH) Special Interest Group (SIG), drawing evidence-based recommendations regarding immediate and long-term postoperative care of LT recipients, taking into consideration their applicability in Latin America.
The burden of liver diseases has been increasing over the past decades worldwide, leading to 2 million deaths per year (4% of all deaths; 1 out of every 25 deaths worldwide), mainly due to complications of cirrhosis and hepatocellular carcinoma (HCC).1 The highest burden of chronic liver disease (CLD) is found in the Eastern Mediterranean and European regions, followed by South-East Asia, the Western Pacific, Africa, and the Americas.2 Latin America (LATAM) is a region composed of 20 countries, representing around 8% of the global population (approximately 651 million population) with culturally and ethnically diverse populations and huge economic and developmental diversity leading to large differences in health care provision and investment.3 Regional disparities are also reflected in inhabitant lifestyles, dietary patterns, physical activity, and alcohol consumption, leading to high variability in the burden of CLD, with alcohol-related liver disease (ARLD), metabolic dysfunction-associated steatotic liver disease (MASLD) and viral hepatitis B and C, being the main causes for CLD and the second cause of years of working life lost in the region.4
The burden of liver diseases has led to an increase in the need for organs for liver transplantation (LT) since it remains the only definitive treatment for those with end-stage liver disease.5 Over the years, great progress has been made in the field of LT, with improvements in surgical technique and medical management, becoming a routine procedure with excellent results and a five-year survival of more than 75–80%.6
Although the total number of LT worldwide and their rates have steadily increased, access to transplantation remains highly variable across different regions. Furthermore, mortality rates on the waiting list remain high due to a shortage of available organs.7 The use of living donors (LDLT), donation after circulatory death donors (DCD), and marginal and extended criteria donors with the use of machine perfusion technology represent significant advances for expanding the donor pool and improving patient and allograft survival; they have not become widely available in developing countries.8
Globally, there were 28.343 deceased donor LT (rate 3,48 pmp), and 9.060 living donor LT (rate 1,1 pmp) performed in 20,22.9 Developed countries with higher donation rates have a considerably higher LT rate: United States (9528 total LTs in 2022, rate 28,4 pmp) and Spain (1159 total LTs in 2022, rate 24,8 pmp). In Latin American (LATAM) Countries, there were 3011 deceased donor LT (rate 4.62pmp) and 246 living donor LT (rate 0.37 pmp), performed in 167 active LT centers in 12 out of the 20 LATAM countries in 20,22.9 The number of LT per country is depicted in Fig. 1 and Table 1. It is important to highlight that LT activity, nowadays below 10 LT per million people in most LATAM countries, is still far below the region's current needs.9
Liver transplantation activities in Latin American countries in 2022.
LT, liver transplantation; DD, deceased donor; LDLT, living donor liver transplantation.
Major barriers to the sustained development of LT across different LATAM countries include a poor organ donation rate, lack of funding, education, legislation and organization issues.10-14 Access to LT in the region is very heterogeneous, and even countries with long-standing transplantation activity, such as Argentina and Brazil are still facing challenges, particularly in organ donation and financial coverage for LT.15-16 Today 8 out of the 20 countries of LATAM, representing 93,2 million (14,3% of LATAM population), do not have real access to LT.
2The current practice of LT in LATAMTo assess current practices regarding the management of LT recipients across different transplant centers in LATAM, the ALEH LT SIG invited all their members from active LT centers to answer a web-based survey with questions regarding current practices concerning the management of LT recipients. Twenty-two centers performing 35 (5–160) LT per year answered the survey (Table 2).17
Results of survey regarding current practice concerning immunosuppression after LT (n = 22).
AIH, autoimmune hepatitis; ATG, anti‐thymocyte globulin; HCC, hepatocellular carcinoma; LT, liver transplantation; m-TOR, mammalian target of rapamycin; OKT3, muromonab-CD3; PBC, primary biliary cholangitis.
Currently, used immunosuppressive (IS) drugs are reported to be available in almost all centers. Tacrolimus, mycophenolate, and prednisone were the main agents employed. Basiliximab was also used for selected patients. Weaning of corticosteroids at three, six and 12 months were reported, respectively, by 41%, 36%, and 23% of the centers, but the policy for lifelong corticosteroid use in AIH-transplanted subjects was commonly observed. Tailoring of IS was accepted, particularly in autoimmune hepatitis (AIH) (59%), hepatocellular carcinoma (HCC) (54%), acute kidney injury (AKI) or chronic kidney disease (CKD) (77%) and primary biliary cholangitis (33%). All centers reported using renal-sparing regimens for AKI or CKD, particularly mTOR-inhibitors (50%). Just four centers are currently performing protocol liver biopsies either in subjects with AIH or for weaning of immunosuppression, while 18 of them are considering liver biopsy prior to steroid pulse therapy (Table 3).
Results of survey regarding current practice concerning management of LT recipients (n = 22).
HBIG and nucleos(t)ide analogs (NUCs) are used in most instances for HBV recurrence prevention, whereas CMV prophylaxis was shown to vary (Table 3). In the occurrence of de novo malignancies (DNMs), most centers adopted the strategy of tacrolimus reduction in association with mTOR-inhibitors (50%) or immunosuppression minimization (27%). Most centers referred to major changes in LT practice over the years due to economic restraints. These data disclosed that most LT centers are employing standard care practices in the management of LT recipients in accordance with international guidelines.18-24 Heterogeneities regarding HBV recurrence and CMV prophylaxis may reflect financial restraints and point to the importance of developing these ALEH guidelines to support LT activity in LATAM.
Most physicians involved in LT in LATAM have engaged in the Latin American Association for the Study of the Liver Special Interest Group (ALEH LT SIG) to discuss major issues related to transplantation activity in LATAM. This current ALEH guidance is the first position paper of this group of experts drawing evidence-based recommendations regarding immediate and long-term postoperative care of LT recipients taking into consideration their applicability in LATAM. This guidance was written by members of ALEH LT SIG to LATAM healthcare providers involved in LT practice as well as civil society organizations and policy makers. For each of these topics, a thorough review of the medical literature was performed, and a series of recommendations were issued taking into consideration their applicability in LATAM. A summary of the most important recommendations is reported here.
3Perioperative issues of liver transplantationManagement of LT recipients in the intensive care unit (ICU) is based on assessment of graft function, prevention, and treatment of dysfunction of other organs as well as screening and treatment of surgical complications, infections, and allograft rejection.17-21,25 Some considerations regarding ICU management, allograft dysfunction, and technical surgical complications are highlighted below.
Early allograft dysfunction (EAD) is a syndrome characterized by marked abnormalities in AST and/or ALT and INR within the first postoperative week usually resolving thereafter with graft recovery, whereas primary graft non-function (PNF) is defined as a rapid increase in AST and/or ALT, coagulopathy and multiorgan failure requiring emergency re-transplantation due to massive liver necrosis. In general, they are both forms of ischemia-reperfusion injury, due to a variety of recipient, donor, and perioperative factors. In this respect, EAD was reported to occur in 30–35% of LT.9 It is associated with lower graft/patient survival, longer stay at the intensive care unit (ICU), and increased morbidity/mortality. The incidence of PNF was shown to vary between 4% to 8%. Besides vascular complications, it is a common cause of early re-transplantation.17-21,26 Several perioperative factors have been related to EAD or PNF including the use of grafts from donors with severe hypernatremia and/or more than 30% of steatosis, sodium > 150–155 mmol/L, long-standing perioperative hypotension or requirement for vasoactive drugs, prolonged cold and warm ischemia times, and recipient ICU length of stay more than 4 to 5 days.26-29
PNF is a life-threatening condition and relisting for LT should be considered in the presence of a) AST levels greater than 3000, b) INR levels greater than 2.5, c) arterial pH lower than 7.3, or lactate levels greater than 4 after exclusion of vascular complications.
Recommendations
- A.
Benzodiazepines should be avoided in immediate postoperative care as they could impact neurologic recovery in LT recipients.
- B.
Proper assessment of subjacent etiology of shock after LT is mandatory. Its management should be focused in correcting oxygen supply, increasing vascular tone, and optimizing blood volume and/or cardiac output.
- C.
Lactic acid levels should be closely monitored in patients experiencing graft dysfunction.
- D.
Weaning from mechanical ventilation should be performed as soon as possible.
- E.
Nutrition should be started in a timely manner, even before weaning low-dose vasopressors. Serum glucose levels should be screened since hyperglycemia is common.
- F.
Hypervolemia or hypovolemia should be avoided with careful evaluation of fluid balance.
- G.
Strategies for the minimization or delayed use of nephrotoxic drugs should be attempted whenever possible, particularly in the context of AKI.
- H.
The use of blood products should be guided by viscoelastic methods whenever available. In the absence of viscoelastic tests, we suggest a hemoglobin threshold of 7g/dl to recommend transfusion of red blood cells. In the presence of bleeding, fibrinogen and platelet count thresholds from 100 to 150mg/dl and 50×109/L, respectively, could be used to guide proper replacement with fibrinogen concentrate, cryoprecipitate or platelet transfusions.
- I.
Removal of central vein and arterial lines, urinary catheters and drains are advisable as soon as possible to avoid healthcare-associated infections.
- J.
Daily monitoring of AST and ALT levels and INR, as well as other parameters associated with organ dysfunction, is essential for the early recognition of EAD or PNF. All affected patients must be managed in an ICU by a multidisciplinary team since an urgent re-transplantation may be necessary.
The incidence of vascular complications varies from 7% in deceased donor liver transplantation (DDLT) to 13% in living donor liver transplantation (LDLT).30,31 Hepatic artery thrombosis (HAT) has been reported to occur in 0%-12% of LT procedures.32 Early HAT (in less than 30 days after LT) may lead to biliary tract injury, sepsis, multiorgan failure, and death, while late HAT usually has a more insidious onset, frequently resulting in biliary complications.32-34 Risk factors related to HAT include donor age of more than 60 years, extended cold ischemia time, ABO incompatibility, smoking, hypercoagulability state, CMV status, allograft rejection, and retransplantation.33
Doppler ultrasound (DUS) is recommended for screening of vascular complications, but the occurrence of arterial spasm, vasopressor use, or hypotension may limit arterial flow assessment by DUS in the postoperative period.35 Whenever HAT is suspected on DUS, contrast-enhanced computer tomography, magnetic resonance imaging, or conventional angiography is recommended for diagnostic confirmation.
Hepatic artery stenosis (HAS), on the other hand, is reported in 3%-15% of LT procedures. It is associated with a wide spectrum of clinical presentations, ranging from asymptomatic to liver failure.18,30 DUS is also recognized as the best screening modality.33
The most frequent venous complications after LT are portal vein thrombosis (PVT) or stenosis (PVS) inferior vena cava (IVC) and hepatic vein thrombosis or stenosis. Portal vein thrombosis or stenosis affects 2%-7% and is more common in LDLT.18,31,36,37 It can be entirely asymptomatic, but early PVT may evolve into liver failure and graft loss.30 Clinical features of late PVT are dependent on its extent and the presence of residual portocaval collateral circulation. Risk factors for PVT include technical issues during the LT, preoperative PVT, hypercoagulable state, prior splenectomy, and large portosystemic collaterals.30,31,38 Therapeutic approaches usually vary according to clinical presentation, time since transplantation, and local expertise, ranging from anticoagulation to endovascular or surgical interventions.
Most cases of PVS are asymptomatic and incidentally diagnosed. Some patients may have complications of portal hypertension.30,39
Venous outflow complications are rare, with a higher incidence of LDLT.38 Their occurrence is usually related to technical issues leading to kinking or thrombosis of veno-venous anastomosis in the early post-operative course.40
Biliary complications may affect up to a third of LT recipients18,38 early in the first month or later than 30 days after LT. Biliary leaks are the result of injury to the bile ducts during the surgery, ischemia, infection, or rejection. Early biliary leaks may manifest as bile peritonitis, intra-abdominal abscess, biloma, or external biliary fistula. The frequency ranges from 3% to 15% in DDLT and 10% to 40% in LDLT.38,41,42 In contrast, biliary strictures often present later, with features of biliary obstruction: jaundice, pruritus, and cholangitis.43
Recommendations
- A.
Screening for vascular complications with Doppler ultrasound should be routinely performed within the first 24 h and seven days after LT. It should also be repeated whenever vascular complications are suspected.
- B.
Vascular complications identified by a Doppler ultrasound should be further evaluated by contrast-enhanced imaging.
- C.
Evidence for routine aspirin use for HAT prevention is limited. However, it should be considered in cases of high risk of this complication, such as donor age greater than 60 years, bench reconstruction of anatomical variants of the hepatic artery, employment of a donor iliac artery interposition graft to the aorta, and graft from a donor who died of a cerebrovascular accident.
- D.
Patients with early HAT should be considered for retransplantation due to its association with rates of graft loss.
- E.
Late HAT may lead to biliary complications. Revascularization is usually not recommended. Retransplantation may be required.
- F.
Although HAS may present with a more insidious clinical course, treatment is usually required. Surgical or endovascular approaches can be recommended according to local availability/expertise. Use of antiplatelet and/or anticoagulant agents to prevent HAT may be attempted in asymptomatic patients with an incidental diagnosis of HAS without signs of significant liver injury.
- G.
Early PVT or PVS with clinical deterioration should be aggressively managed with surgical or intervention radiology approaches. Emergent retransplantation may be an option for those patients with progressive deterioration despite therapy. In asymptomatic patients, anticoagulation, preferably with endovascular intervention, should be employed according to local resources and expertise.
- H.
Asymptomatic patients with late PVT or PVS and stable liver function can be followed and treated accordingly in the development of portal hypertensive complications.
- I.
Management of IVC or hepatic vein obstruction may be conservative, with anticoagulation and regular follow-up with DUS in asymptomatic patients with partial thrombosis. In the remaining cases, endovascular approaches are recommended according to local availability or expertise.
- J.
To avoid biliary complications, cold ischemia time should be shortened, and duct-to-duct anastomoses should be performed whenever possible.
- K.
Magnetic resonance cholangiopancreatography (MRCP) is useful to identify biliary complications, but endoscopic retrograde cholangiopancreatography (ERCP) is the first-line approach for their treatment. Percutaneous transhepatic drainage is an option when ERCP is not feasible. Cholangioscopy can be a useful resource when available.
- L.
Complex biliary strictures or strictures refractory to endoscopic or percutaneous treatment may require surgical intervention.
- M.
Retransplantation is the treatment of choice for biliary complications leading to graft failure.
Immunosuppression (IS) has markedly improved in the last decades, leading to enhanced graft and patient survival.18,44 In LATAM, many centers adopt immunosuppressive regimens with a combination of two or three agents for dosage minimization to avoid undesirable toxicity and side effects.18,24 The most used regimens for induction and maintenance of IS in LATAM countries are shown in Tables 2 and 4. Several parameters are used to tailor immunosuppression, including 1) etiology of liver disease, 2) time-lapse after LT, 3) history of CKD, HCC, and cardiovascular comorbidities, 4) previous rejection episodes and 5)occurrence of de novo malignancies (DNM) and pregnancy.45-51
Characteristics of the main immunosuppressive drugs used in liver transplantation.
CNI, Calcineurin inhibitor; TAC, tacrolimus; CsA, cyclosporine; mTOR, mammalian Target of Rapamycin; PO, oral route; EV, intravenous route.
Despite recent advances in IS, acute rejection (AR) and chronic rejection (CR) remain frequent complications after LT. Acute rejection is observed in 20% to 25% of the cases, mainly during the first weeks after LT. It can be categorized into T-cell-mediated ACR and antibody-mediated rejection (AMR), which generally occurs in setting of ABO-incompatible LT.18,52 The diagnosis of acute AMR is established by the presence of high-titer donor-specific antibodies, characteristic histological findings, and microvascular C4d deposition in the absence of other causes of injury.
Chronic rejection occurs in less than 5% of the patients and it is characterized by progressive ductopenia and obliterative arteriopathy, generally leading to progressive cholestatic graft dysfunction usually months to years after LT. However, it is wise to mention that CR may occur even a few months after LT and may lead to graft failure in the first year after surgery.51-53 Chronic rejection is more common in patients with previous episodes of AR or steroid-resistant AR, subjects submitted to retransplantation due to CR, or in DDLT when compared to LDLT.53
Late-onset AR (LAR) generally occurs 3–6 months after LT, usually due to IS weaning or lack of proper adherence to immunosuppressive therapy. Its incidence varies between 7% and 40%. Unless properly treated, LAR may impair graft and patient survival.52,54
Many risk factors for AR are often associated with an increased frequency of CR, including underlying autoimmune liver disease, CMV infection or reactivation, low levels or noncompliance to IS, extremes of recipient age, male-donor-into-female recipient, and prolonged cold ischemia time.51,52
Most mild episodes of AR can be managed with baseline adjustment IS.18 In the presence of severe AR, steroid boluses (intravenous methylprednisolone 500–1000 mg per day for one to three days) are usually needed.55Steroid resistant AR has been reported in less than 10% of the cases of AR. Patients not responding to the initial three boluses of steroids may receive a second course of methylprednisolone or be switched to other therapy, usually anti-thymocyte globulin(ATG).56 Chronic ductopenic rejection may be treated with escalation of baseline IS, and conversion of cyclosporin- based to tacrolimus-based IS, besides the addition of mycophenolate or mTOR-inhibitors.50
Recommendations
- A.
Calcineurin inhibitors (CNI), including tacrolimus or cyclosporin, are the cornerstone of the drug regimens in both the induction and maintenance phases of IS. They are usually used with corticosteroids with or without antimetabolites or mTOR-inhibitors for IS induction and as monotherapy for IS maintenance in the long-term. Antimetabolites or mTOR-inhibitors may also be added to reduce CNI dosages for the minimization of IS.
- B.
Given their adverse side effects, corticosteroids should be tapered for weaning 3 to 6 months after LT. They should be maintained at low doses in those patients transplanted with AIH to avoid disease recurrence or in those with severe or recurrent rejection.
- C.
Induction IS with anti-interleukin-2 agents (basiliximab) in combination with corticosteroids and mycophenolate to delay CNI use is a proper renal-sparing strategy for those patients with AKI or known CKD before LT.
- D.
The use of mTOR-inhibitors may also be required in those patients with AKI after LT to minimize nephrotoxicity due to CNI exposure.
- E.
Acute or chronic rejection should be suspected in the presence of clinical and or biochemical signs of graft dysfunction. It is important to rule out vascular and or biliary complications of LT in the appropriate setting. A liver biopsy may be required for a definite diagnosis, particularly before therapy with a bolus of corticosteroids.
- F.
Episodes of moderate to severe AR should be treated with steroid boluses or with escalation of baseline IS.
- G.
Escalation of immunosuppression should be employed for CR management, but retransplantation may be required in absence of response.
Pre-transplant assessment is mandatory in LT candidates to identify their individual risks for post-LT infections to better adopt suitable preventive strategies, including immunization and disease prophylaxis.57
The recommended immunizations for candidates/recipients vary according to each country/region and can include the administration of attenuated vaccines against measles, mumps, and rubella; chickenpox, yellow fever, and dengue fever vaccines and inactivated, recombinant, viral vector or RNA vaccines against Covid-19, influenza, hepatitis A and B, human papillomavirus, pneumococcus, meningococcus, recombinant zoster, tetanus, diphtheria, pertussis, and polio. There must be a 30-day interval between the application of attenuated vaccines and LT. They are also contraindicated after LT. On the other hand, inactivated, recombinant, viral vector or RNA vaccines can be given before (preferably 14 days before) or after LT (ideally after 3–6 months). Depending on the epidemiological situation, influenza, and COVID-19 vaccines may be indicated 30 days after LT.57-59
The incidence of tuberculosis in solid organ transplantation (SOT) recipients is 20 to 74 times higher when compared to the general population. Most cases are due to reactivation.57,60 Treatment of latent tuberculosis infection (LTBI) prevents reactivation of tuberculosis after LT. There are two tests available to screen for LTBI: tuberculin skin test (TT) and interferon-gamma release assay (IGRA). Both tests are less sensitive in immunocompromised individuals. They can be performed simultaneously to increase the accuracy of the detection of LTBI. Indications for tuberculosis prophylaxis include: 1) untreated LTBI or lack of documentation of adequate therapy, 2) history of contact with tuberculosis prior to LT, and 3) recipients of donor organs with a history of untreated tuberculosis.
Treatment regimens for LTBI and their duration vary and can include isoniazid, isoniazid-rifampicin, and rifampicin for periods after LT ranging from 3 to 9 months. Patients who have completed a cycle of adequate therapy for LTBI do not need to repeat prophylaxis unless they subsequently have one of the last two indications above.61
Bacterial and fungal infections are frequently reported in hospitalized patients with decompensated cirrhosis awaiting LT. They should be actively treated or controlled whenever possible before LT. Better outcomes are reported when patients with bacterial or fungal infections are afebrile, with improved inflammatory tests under appropriate antimicrobial for at least 48–72 h before LT.57,62,63 Liver transplantation candidates are frequently colonized by antimicrobial-resistant microorganisms and may require specific prophylaxis in the perioperative period. Colonization with resistant pathogens is frequent in patients with previous hospitalizations and exposure to broad-spectrum antimicrobial agents in the past 3 to 6 months.
Prophylaxis should also be adopted against Pneumocystis jirovecii-associated pneumonitis (PCP) for six months after LT, usually with trimethoprim-sulfamethoxazole. It can substantially reduce the risk of other infections such as Toxoplasma gondii, Listeria monocytogenes, and infections caused by Nocardia species.57
IgG anti-cytomegalovirus (CMV) serological status should be evaluated in donors and LT recipients for management decisions regarding CMV prophylaxis after LT. Subjects of high and intermediate risk for CMV infections after LT were reported, respectively, when the donor is IgG-CMV positive, the recipient is negative (D+/R-) and when the recipient is IgG-CMV positive (R+). Other risk factors for CMV infection include the use of a bolus of corticosteroids and ATG.57,64
Strategies to prevent invasive CMV infection after LT include universal prophylaxis of at-risk patients instituted usually after the first week of LT until 3–6 months thereafter or preemptive therapy only in those subjects with increasing viral CMV loads above predefined thresholds or in the presence of symptoms.65 The latter strategy requires routine monitoring of CMV viremia after LT. Drugs used for prophylaxis of CMV after LT are depicted in Table 5.
Currently available antivirals for management of CMV infection in liver transplantation.
IV: intravenous.
The timeline of infections after LT can be divided into three different periods: 1) perioperative period (within 30 days after LT), 2) early postoperative period (between one and 6 to 12 months after LT), and 3) late postoperative period (more than 6 to 12 months after LT).
During the first month, bacterial and fungal infections are more common, usually due to surgical complications, donor-derived infections, pre-existing infections of the recipient, and nosocomial infections. Early nosocomial infections are often associated with vascular or biliary complications or with the presence of indwelling medical devices. Antimicrobial treatment should preferably be directed at the isolated agent or, if empirical, according to the results of donor/recipient previous isolates or local microbiologic profiles.
Multidrug-resistant-microorganisms (MDRO) are common in LT recipients.57 Carbapenem-resistant organisms (CRO) pose a threat to patient survival.63,66 Identifying such potential pathogens before LT may allow appropriate perioperative prophylaxis. There is no consensus on the ideal approach for screening MDROs before transplantation. Antimicrobial therapy should be based on in vitro susceptibility data coupled with adequate infection source control.
The most common infections in the first year after LT are 1) persistent infections beyond the perioperative period including Clostridioides difficile colitis, residual pneumonia or prolonged or recurrent infections due to technical complications such as biliary fistulas or leaks, 2) viral infections due to herpes virus such as CMV, Epstein-Barr virus and varicella zoster virus, and community-acquired respiratory viruses such as adenovirus, influenza and parainfluenza and 3) opportunistic infections due to PCP, Listeria monocytogenes, Toxoplasma gondii, Nocardia and Aspergillus species.
CMV infection is one of the most common infections occurring after 1 month of LT. Its prevalence varies between 20% and 30%.65,67 In a no-prophylaxis scenario, the majority of CMV infections occur by the end of the fourth month after surgery. Some definitions may be pointed out regarding CMV infection after LT.64 Latent CMV is related to seropositivity without signs of viral replication, while active infection refers to the presence of viral replication (detection of the virus in blood and/or organs by molecular methods or antigen detection). Symptomatic infection or disease occurs in the evidence of infection with attributed symptoms, while clinically significant infection is defined by active infection requiring therapeutic intervention, according to previously defined levels of replication. After transplantation, the occurrence of infection may result in reduced graft and/or host survival due to its direct and indirect effects. The two current main diagnostic methods for CMV infection that are widely available are antigenemia (pp65) and quantitative nucleic acid amplification tests (NAT). Both can be applied in the context of disease investigation or surveillance. The value of viral replication associated with significant infection in general is at least 1000 IU/ml.65 For the diagnosis of CMV disease, it is necessary to detect viral replication associated with specific symptoms or viruses in tissues or other biological samples. There may be disease even without the identification of viral replication in blood at that time.68Table 5 shows the currently available antivirals used for the treatment of CMV infection after LT.64,65,69 Resistance is rare. However, the risk increases in recipients of prophylaxis.69 Refractoriness to treatment, even without resistance, is detected more commonly and is generally associated with excessive immunosuppression.70
CMV infection could be treated with intravenous ganciclovir or oral valganciclovir. In refractory or resistant CMV infection, a higher dose of ganciclovir may be used, as well as adjustments in baseline IS. Oral maribavir is superior in efficacy and safety for the treatment of refractory or resistant CMV infection when compared to foscarnet or cidofovir.70
After 6–12 months of LT, community-acquired infections turn out to become more prevalent in LT patients with low-level baseline IS, but severe bacterial, viral, or endemic infections, such as tuberculosis, may occur in LT patients.
LT recipients were initially associated with an increased risk of severe COVID-19. However, the findings across studies have been inconsistent.71,72 Elevated transaminases were observed in COVID-19 infections.73,74 A Spanish registry of LT recipients reported abnormal transaminases in 14.7% of cases, with only 2.7% experiencing graft rejection.75Immunosuppression in LT recipients with COVID-19 presents a challenge, as severe cases of COVID-19 are often associated with an imbalance in the host response.75 Recipients receiving mycophenolate faced an increased risk of developing severe COVID-19. Notably, this association was not observed with the use of CNI or mTOR-inhibitors.75 Low seroconversion rates have been reported in LT recipients after SARS-CoV-2 vaccines.76,77 Over half of those patients who did not exhibit humoral response after two vaccine doses developed a response following a third dose.77 Mycophenolate appears to be a factor contributing to vaccination failure.78,79
Recommendations
- A.
Liver transplantation candidates should update their immunization status according to local guidelines. Latent tuberculosis infection should be additionally screened in all of them.
- B.
After LT, the risk of infection is directly related to graft function, surgical complications, use of indwelling medical devices, and baseline IS. Treatment must be directed at the specific etiological agents and following institutional protocols.
- C.
Universal prophylaxis of CMV should be instituted at risk LT patients using either oral or preferably intravenous ganciclovir or oral valganciclovir according to availability. Preemptive therapy strategies may be preferable but require frequent CMV viral load monitoring to be initiated before the onset of symptomatic CMV disease.
- D.
CMV infection should be treated with oral valganciclovir or preferably with intravenous ganciclovir in life-threatening infections. Refractory and resistant CMV are better managed with high-dose intravenous ganciclovir or oral maribavir. Lower levels of baseline IS are also a cornerstone for the treatment of CMV infection.
- E.
Immunosuppression in recipients with SARS-CoV-2 infection should be tailored individually. It may be reasonable to discontinue mycophenolate until the infection resolves. Complete withholding of immunosuppression is not recommended, and close monitoring of tacrolimus and/or mTOR-inhibitor trough levels is advised.
- F.
LT recipients should be vaccinated according to local infectious guidelines, including influenza, pneumonia, and SARS-CoV-2, among others.
Improvement in patient/graft survival over the years reflects the advances in therapeutics to control HBV infections after LT. The combination of hepatitis B immune globulin (HBIG) and NUCs has been shown to prevent recurrent infections and to be superior to HBIG alone. Different HBIG doses, administration routes, and treatment duration early after LT have been described, with initial high doses of 10.000 IU, intravenously, in the hepatic phase, followed by daily administration for 7 days, then monthly to keep anti-HBs >500 IU/L in the first 3 months, >250 IU/L until one year and >100 IU/L afterwards.80
With the advent of 3rd generation of NUCs, entecavir and tenofovir, some studies used significantly lower doses of HBIG and demonstrated HBV recurrence of 1%.81 More recently, it was demonstrated that monotherapy with NUCs is safe and effective in some individuals who are at low risk of HBV reactivation.82
To prevent HBV-HDV recurrence, a combination of long-term HBIG with a NUC is currently recommended instead of NUC monotherapy.18
Recurrent hepatitis C (rHCV) infection after LT is universal in patients who are viremic at the time of transplant.83 All recipients with rHCV should receive treatment with oral direct antiviral agents (DAAs), with an overall sustained virological response rate (SVR)>95% and outcomes significantly improved.
Patients infected with HEV live in endemic regions, where the main route of transmission is fecal-oral. However, an increasing number of infections has been reported in developed countries, with the main route of dissemination through the consumption of undercooked pork.84,85 Although the diagnosis of hepatitis E should be considered in the population of LT who develop hepatitis of unknown etiology, some authors showed that the prevalence of HEV circulation, at least in Brazil, is low and no signs of chronic liver disease due to HEV infection were observed.86
Recommendations
- A.
HBV prophylaxis can be undertaken based on the risk of HBV recurrence. The discontinuation of HBIG after one year is reserved for patients at low risk for recurrence, who should continue monotherapy with an NUC. High-risk patients should receive combination therapy for at least one year or lifelong.
- B.
Recurrent hepatitis C should be treated with DAAs once the patient is stable, usually within 3 months after LT.
- C.
In LT patients, cases of acute hepatitis of unknown etiology should be investigated using molecular tests for HEV-RNA since anti-HEV IgG seroconversion can be delayed or never occur. In case of progression to chronic infection, the use of ribavirin for an initial course of 3 months can be considered.
Risk factors for relapses of alcohol consumption after LT due to alcoholic liver disease (ALD) include previous or current psychiatric illness, short pre-LT duration of abstinence, family history of alcohol dependence, and lack of social support.87-92
The risk of alcohol relapse after LT ranges from 10% to 30% and is usually associated with the risk of AR, CR, and graft loss.87,88 Patients who resumed heavy drinking after LT were shown to have 5- and 10-year survival rates of 69.5% and 20.1%, respectively, compared to 90.3% and 81.5% seen in abstinent patients.89,90
Liver transplantation for patients with cirrhosis due to autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) are usually associated with higher survival rates when compared to their counterparts with ALD or viral hepatitis, however, disease recurrence remains an important issue in the long-term.18 Recurrence of AIH (rAIH) has been reported in 18% to 20% of those patients within 5 years and in 31% of them 10 years after LT.93-96 The most important risk factors for disease recurrence were pre-LT disease activity or decompensated cirrhosis, retransplantation for rAIH and baseline immunosuppression after LT. The type of CNI employed has no impact on rAIH rates and the influence of corticosteroid withdrawal is still a point of debate in the literature.95
The prevalence of PBC recurrence (rPBC), on the other hand, has a wide worldwide variability, ranging from 0% to 35%.97-99 One study evidenced recurrence rates of 22% after 5 years and 36% after 10 years. The risk factors for rPBC were age at diagnosis (<50 years), age at LT (<60 years), and use of tacrolimus. Use of cyclosporine, instead of tacrolimus, was shown to reduce the risk of rPBC, but those results are still controversial. Some studies corroborate the preemptive use of UDCA, not only to reduce the risk of recurrence but also to improve graft/patient survival.97,100,101
Recurrence of PSC (rPSC) is detected in 20% to 60% of those patients undergoing LT. The recurrence rates approach 20% at 5 years.102 The risk factors for rPSC include severity of IBD, repetitive/resistant episodes of AR, and CMV reactivation/infection. There appears to be no difference in recurrence rates according to immunosuppression, type of biliary anastomosis (duct-to-duct anastomosis, Roux-em-Y choledochojejunostomy), or preemptive use of UDCA.103
Recommendations
- A.
Strengthening social support may decrease alcohol relapse in patients transplanted for ALD. Patients should enroll in a substance abuse treatment program such as Alcoholics Anonymous and/or psychiatric follow-up to ensure long-term abstinence.
- B.
Disease recurrence should be suspected in the presence of clinical and or biochemical signs of graft dysfunction in patients submitted to LT due to AIH, but AR should always be ruled out.
- C.
The diagnosis of rPBC should be histologically confirmed, as liver tests are nonspecific, and AMA reactivity may persist after LT. It is important to exclude other causes of bile duct damage. Preventive UDCA after LT for PBC is recommended as it is associated with a reduced risk of disease recurrence, graft loss, and death.
- D.
Diagnosis of rPSC requires a confirmed diagnosis of PSC before LT, a cholangiogram showing nonanastomotic biliary strictures, and or histologic findings of obliterative fibrous cholangitis after the exclusion of other etiologies of biliary injury.
- E.
Although UDCA is frequently used in rPSC, there is no evidence of improvement in outcomes. Dominant stenosis and recurrent cholangitis should be treated in the same way as in pre-LT.
Several factors are associated with bone disease in post-LT, including age, menopause, cholestatic disease, alcohol, smoking, steroid use, and previous fractures.104 In the first 3–6 months, there is an increase in bone resorption and osteopenia/osteoporosis are still clinically relevant.
Metabolic syndrome (MetS) is observed in over half of the LT recipients within 3 years of LT, with cardiovascular disease being one of the leading causes of death after the first year of LT.105 Studies suggest that patients transplanted for metabolic dysfunction-associated steatotic liver disease (MASLD) have a worse post-LT outcome than other etiologies.105,106
Recurrence of steatosis in patients with MASLD is almost the rule after LT, reaching 82% at 5 years after LT.105 “De novo steatosis” also occurs in LT recipients of other etiologies after LT.107 It is multifactorial and depends on donor characteristics, immunosuppressors and its relationship with MetS.
Obesity is associated with an increased risk of de novo MASLD.105 Patients regain their appetite and recover their nutritional status, but up to 42% of patients become obese, with a higher long-term all-cause mortality.108
MASLD transplant patients have a higher incidence of obesity and diabetes compared to patients transplanted for other etiologies,106 with 30% of them being obese within 3 years of LT.109 Glucagon-like peptide-1 (GLP1) receptor agonists (semaglutide, liraglutide, tirzepatide), may have significant benefits, but to date, there are no studies to support their routine use in this setting.105
Bariatric surgery has also been offered to LT patients with obesity. The techniques have included sleeve gastrectomy, gastric bypass surgery, gastric banding, and biliopancreatic diversion. It appears to be a promising strategy, however, there is a lack of solid evidence-based data on the optimal management for patients with obesity and LT. It should be performed in centers with expertise and with multidisciplinary teams.110
Post-transplant diabetes mellitus (PTDM) develops in up to 30% of recipients and is associated with shorter survival, reduced graft function, increased risk of infections, cardiovascular events, and lower quality of life.111 Risk factors associated with PTDM include obesity, etiology of liver disease (hepatitis C, MASLD), immunosuppressors, and donor factors (steatosis).111,112 Steroids and tacrolimus contribute to the development of PTDM.113
Major adverse cardiovascular events (MACE) represent a significant cause of morbimortality following LT. The transplantation procedure and postoperative care can be regarded as significant stressors that can affect preexisting cardiovascular (CV) dysfunction, mostly in the early LT period.
Observational studies reporting CV outcomes in LT recipients have shown a 10-year risk of developing CV events of 13,6%, with an early CV death of 2.9% within the first 30 days of LT. Those with MetS were approximately 4 times more likely to have a CV event.114,115 Studies demonstrate an increased risk of CV events among MASLD recipients, even after controlling preexisting comorbidities after extensive preoperative workup.116,117
Recommendations
- A.
Bone mineral density screening should be performed yearly for patients with pre-existing osteoporosis or osteopenia, and every 2–3 years in those with normal bone mineral density. Supplementation with calcium/vitamin D should be always considered.
- B.
Bisphosphonates could be indicated in patients with T scores ≤-2.5 or following pathological fractures and may be appropriate in patients with T scores ≤-1.5.
- C.
A healthy lifestyle with a balanced diet and regular physical activity should be encouraged after LT.
- D.
A continuous cardiovascular risk stratification and aggressive management of the MetS, as well as modification of risk factors including tailoring the immunosuppressive regimen, are mandatory to avoid cardiovascular morbidity and mortality after LT. Statins are safe and beneficial for lipid management after liver transplantation. Patients on mTOR-inhibitor are at increased risk of hyperlipidemia and should be monitored closely.
Studies have shown a prevalence of AKI ranging from 45% to 60% after LT in LATAM.1-7 Up to 15% of patients will require renal replacement therapy (RRT) in the immediate post-transplant period,118-121 which is associated with a higher risk of CKD usually seen in 4% to 18% of the patients after solid organ transplantation122.
There are several recipient-associated factors leading to a higher risk of CKD after LT, including mainly pre-LT renal injury, age, co-morbidities (type 2 diabetes and arterial hypertension), as well as donor-associated factors, including graft steatosis and cold ischemia time. Surgical factors may also be involved, particularly intra-operative bleeding and hypotension.123
Pre-transplant AKI is a significant predisposing factor for the development of CKD after LT,124 with higher late mortality and cardiovascular events. Stage 2/3 CKD develops in 50–60% of LT recipients.122 Post-LT CKD is mainly attributed to the chronic use of CNI.18,122,123 The nephrotoxic potential of CNIs is largely attributed to their vasoconstrictive effect on the afferent and efferent glomerular arterioles, with a reduction of renal blood flow and estimated glomerular filtration rate (e-GFR). Approximately one-third of these patients eventually progress to end-stage renal disease requiring RRT.123,124
Early identification of patients at risk for CKD may prevent renal deterioration. Biomarkers such as urinary neutrophil-gelatinase-associated-lipocalin (uNGAL) might be helpful in the early identification of patients who are prone to developing CKD.125,126 mTOR-inhibitors appear to have no intrinsic nephrotoxic potential, and for that reason, they are used when renal impairment develops. Nevertheless, data suggest that sirolimus has been implicated as a cause of cast nephropathy, proteinuria, hyperlipidemia, and focal segmental glomerulosclerosis. Hence, conversion to sirolimus- or everolimus-regimens should not be considered if there is significant proteinuria.44
Recommendations
- A.
The use of renal-sparing immunosuppressive regimens as early as possible reduces the incidence of AKI and offers some benefits regarding the progression to CKD.
- B.
Appropriate management of immunosuppression and co-morbidities such as arterial hypertension, type 2 diabetes, and obesity is crucial for the prevention of CKD.
- C.
Non-steroidal-anti-inflammatory-drugs and nephrotoxic antibiotics such as aminoglycosides should be avoided in LT recipients and contrast-enhanced imaging studies should be employed with caution to prevent further deterioration of renal function.
Solid organ transplantation recipients have a high risk of developing DNMs.127 The main factors are baseline IS, viral infection with EBV, CMV, HHV8, and HBV, smoking, alcohol, and premalignant conditions.128 The overall incidence of DNMs ranges from 10 to 14.5% at 5 years to 20–32% at 10 years.127,128 LT recipients carry a 2–3-fold increased risk of solid-organ malignancies and a 30-fold increased risk of hematologic and skin cancers.127
DNMs must be distinguished from recurrent cancers and donor-derived cancers present in the graft (<0.1%). Malignancies developing within the first 6 months of LT may have pre-existed and are thus not considered DNMs, except for post-transplant lymphoproliferative disorders (PTLDs).
Skin cancers are the most common DNMs (40% of malignancies).127 Cutaneous squamous cell carcinoma (SCC) is the most common skin cancer, occurring more commonly in sun-exposed areas and being more aggressive than in the nontransplant population.129
The incidence of PTLD ranges from 1%–3%, with a SIR of 3.9–21.130,131 Immunosuppression and EBV infection have a critical role.130,132
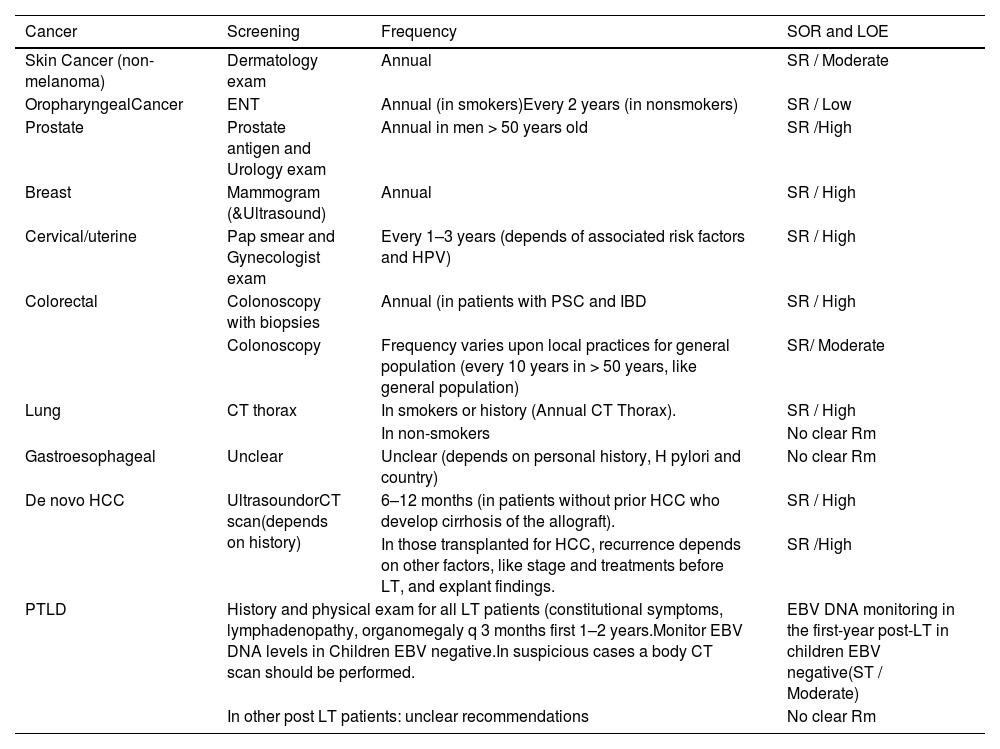
Screening is important to reduce the burden of malignancy in post-LT132 (Table 6).
Table 6.General screening recommendations for adult LT patients.
HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; SOR, strength of recommendation; LOE, level of evidence; SR, strong; Rm: recommendations.
Recommendations:
- A.
Cancer screening protocols are warranted after LT to detect DNMs and recurrent cancers at an early stage. LT recipients should have an annual dermatologic and oropharynx examination should avoid sun exposure and participate in regular screening programs (cervical, breast, prostate, and colorectal cancer).
- B.
Patients with PSC and inflammatory bowel disease (IBD) should undergo an annual colonoscopy screening.
- C.
For patients who develop recurrent cirrhosis of the allograft, surveillance for de-novo HCC should be undertaken.Patients with prior HCC should be monitored for recurrence.
Sexual dysfunction is very common in LT candidates. It is usually restored 3 to 6 months after surgery. Pregnancy outcomes post-LT are favorable, with maternal/fetal death rates like the general population. However, there is an increased risk of pre-term delivery, low birth weight, pre-eclampsia, gestational diabetes, and intrahepatic cholestasis of pregnancy. Monitoring of graft function and immunosuppressive drug levels is essential, as the increase in serum volume during pregnancy may reduce the area under the curve of drugs used for immunosuppression.133,134
Recommendations:
- A.
Pregnancy should be delayed to one year after transplantation due to unstable immunosuppression and risk of CMV infection.
- B.
Tacrolimus is the CNI of choice during pregnancy. Mycophenolate is contraindicated and should be discontinued due to the risk of congenital malformations. Given the limited safety data, mTOR-inhibitors are not recommended. Azathioprine can be considered an alternative to mycophenolate for women of childbearing age who wish to conceive.
- C.
The mode of delivery should be guided by obstetric indications.
- D.
Breastfeeding is not contraindicated after LT.
- E.
In cases of infertility, assisted reproduction can lead to successful pregnancies.
ALEH advocates strategies to address organ shortages and calls for increased funding to support programs, expand treatment options, and promote the adoption of advanced technologies aimed at improving the long-term outcomes of liver transplantation in Latin America.
Author contributionsLC, RZ, MM, PLB: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript, study supervision. AMFJ, JCR, LLS, LMM, WA, AG, PMPM, AV, RSBS, GECN, JP, DRBT, AU, MGP, VM, RP, OI, SG, RW, EA, LT, ECR, FC, MU: drafting and critical revision of the manuscript.
Declaration of use of artificial intelligenceNone.
Expert review panelThe guidelines have been meticulously reviewed by the distinguished Dr. Carmen Vinaixa, MD, PhD, from Hospital Universitarii Politecnic La Fe (Spain), and the esteemed Dr. Daniel R. Ganger, MD, from the Department of Medicine at Northwestern University (Chicago, IL, United States), whose invaluable insights and clinical expertise have significantly enhanced the quality and accuracy of the recommendations presented herein.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.