Abstracts of the 2025 Annual Meeting of the ALEH
More infoDrug-induced liver injury (DILI) and herb-induced liver injury (HILI) represent diagnostic and prognostic challenges in hepatology. Objective: To evaluate histological, epidemiological, biochemical variables and the prevalence of cytochrome P450 (CYP450) genotypes in individuals with DILI/HILI at a hepatotoxicity clinic.
Materials and MethodsCross-sectional study with individuals who developed DILI/HILI and underwent liver biopsy.
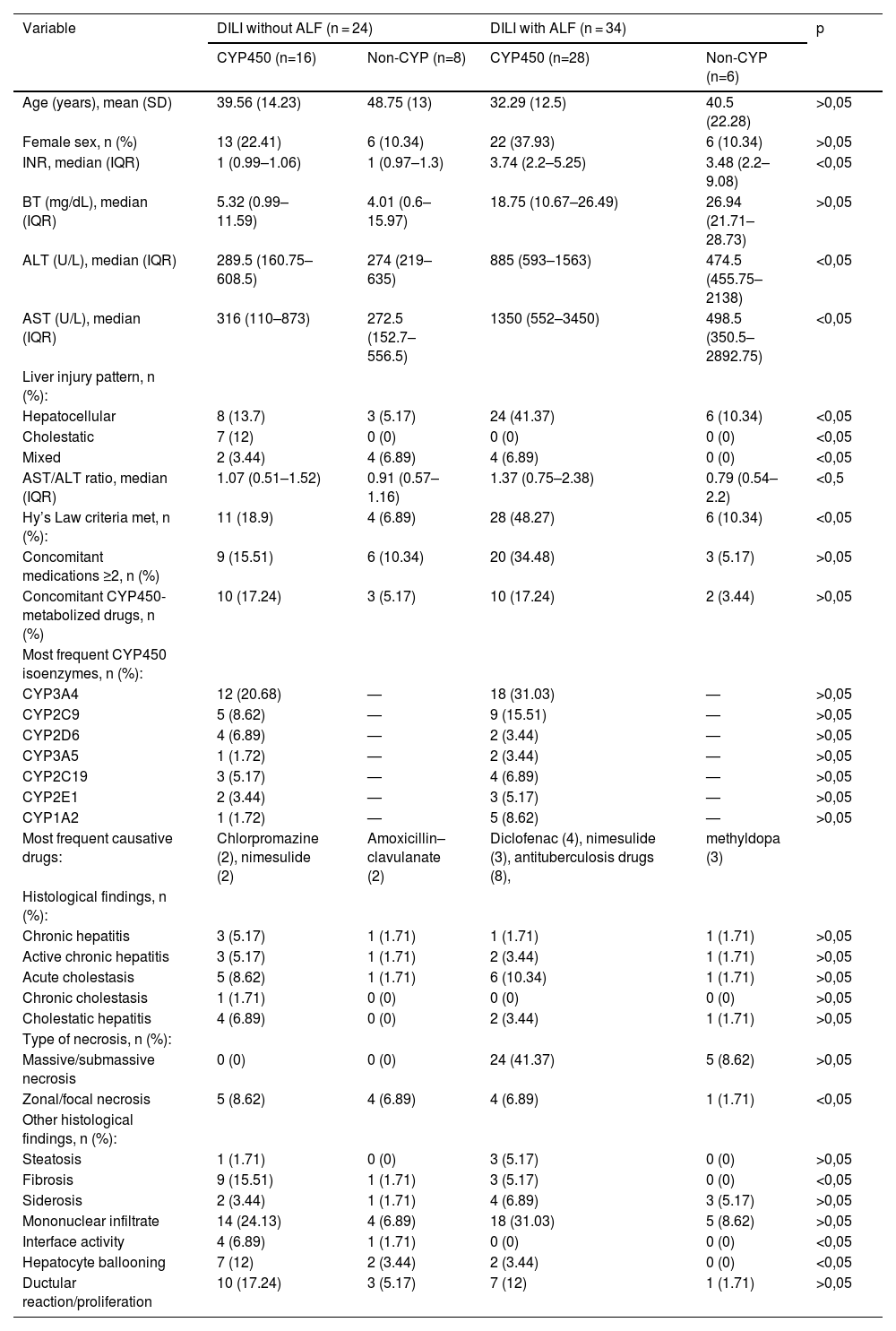
ResultsSample comprised 58 individuals, divided into 2 groups: those who developed ALF (34) and those who did not (24). Mean age 38.71 (SD 14.58), 79.3% female. Hepatocellular biochemical pattern: 70.7%, mixed 12.1%, cholestatic 17.2%. The ALF group did not present a cholestatic pattern. Most frequent drugs: antituberculosis (8), nimesulide (5), diclofenac (4). Regarding biochemistry, in the group without ALF, comparing presence of the main drug with or without CYP450 metabolism: BT 5.32 mg/dL vs 4.01; ALT 289.5 U/L vs 274; AST 316 U/L vs 272.5; INR 1 vs 1; AST/ALT 1.07 vs 0.91. In the ALF group: BT 18.75 vs 29.94 mg/dL; ALT 885 vs 474.5 U/L; AST 1350 vs 498.5 U/L; AST/ALT 1.37 vs 0.79. Hy's law applied to 100% of ALF cases. Patients using two or more concomitant drugs showed worse biochemical and histological findings. Most frequent CYPs among non-ALF: CYP3A4 20.68%, CYP2C9 8.62%, CYP2D6 6.89%; among ALF: CYP3A4 31.03%, CYP2C9 15.51%, CYP2A2 8.62%. Histological findings: massive/submassive necrosis was present in 85.29% of ALF patients, mainly in those with CYP metabolism. Fibrosis was more frequent in the group without progression to ALF (9 vs 3).
ConclusionsHepatic metabolism by CYP450 is associated with more severe DILI/HILI, including higher frequency of hepatic necrosis and elevated biochemical values. Recognizing the metabolic profile of implicated drugs may help predict injury severity and guide earlier, individualized treatment strategies.
Conflict of interest: None