Abstracts Asociación Mexicana de Hepatología (AMH) 2024
More infoDrug-induced liver injury (DILI) refers to hepatic function alterations associated with drugs. The idiosyncratic form can progress from remission to acute liver failure (ALF).
The objective is to present the case of a patient with ALF secondary to idiosyncratic DILI due to ibuprofen consumption.
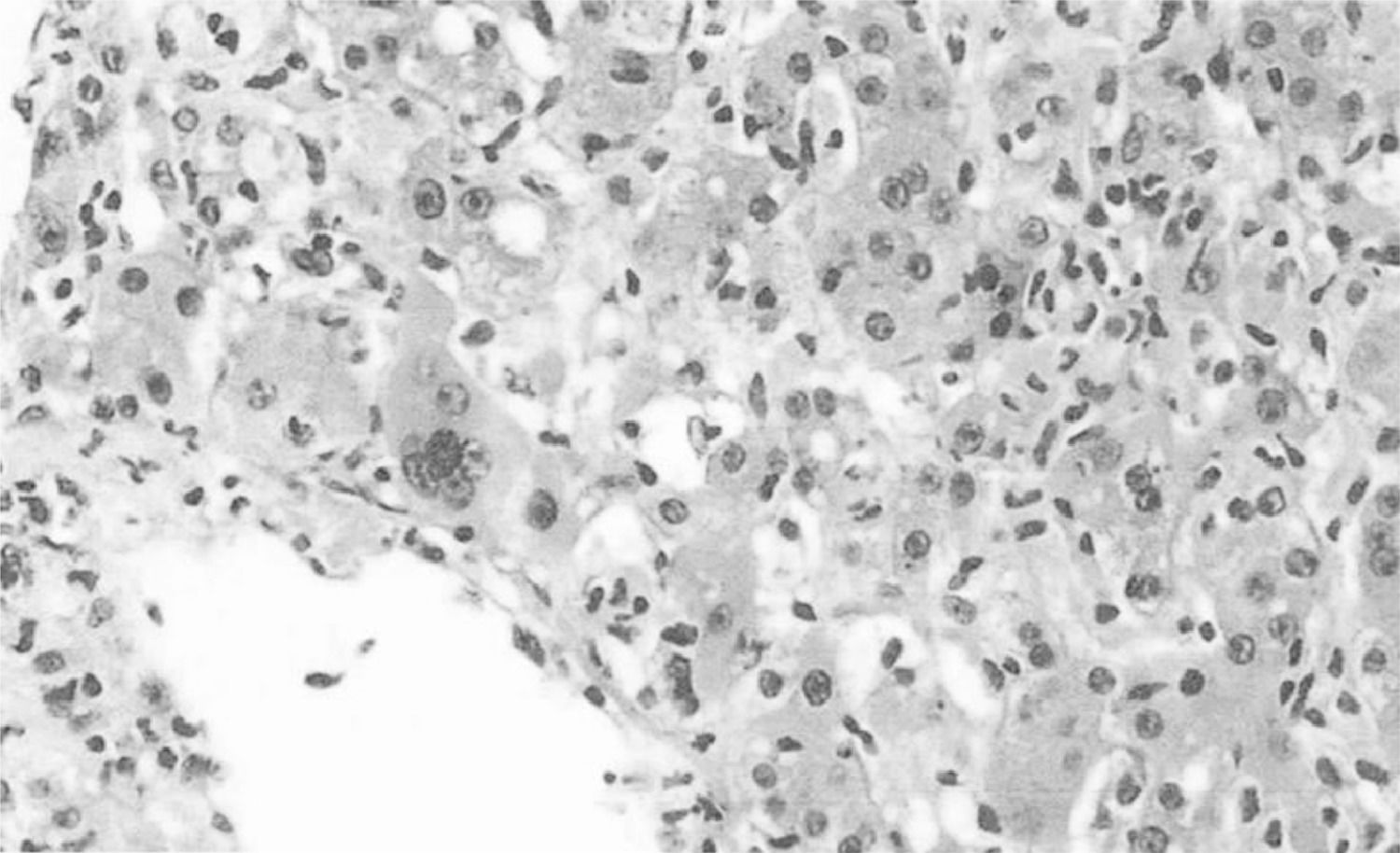
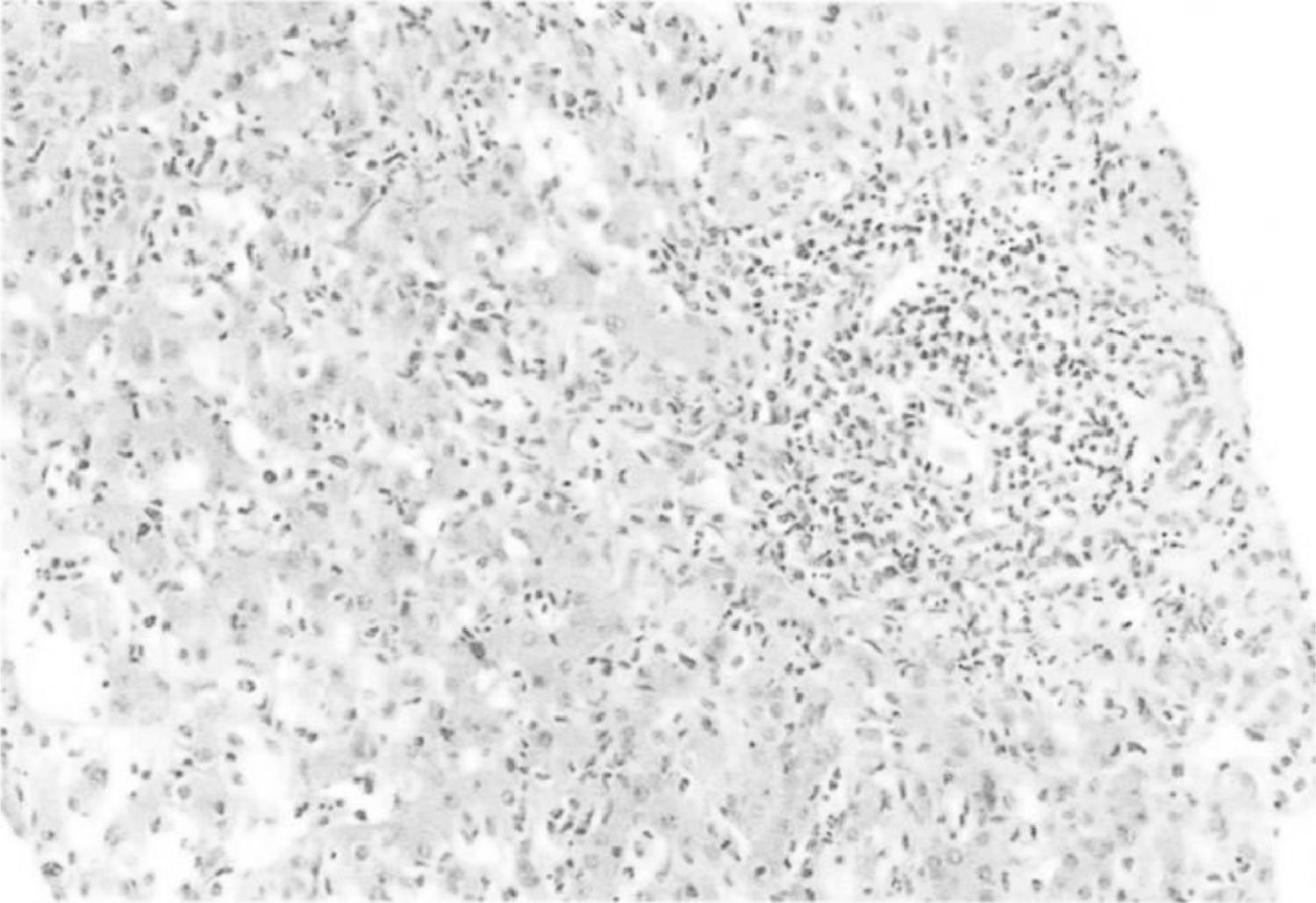
Materials and PatientsA 43-year-old woman with no history of alcohol, herbal, or drug consumption. She presented with asthenia, adynamia, and unquantified fever, self-medicating with ibuprofen 1.2 g/day. Subsequently, she developed right hypochondrium pain and generalized jaundice without discontinuing ibuprofen. Four weeks after the onset of symptoms, she developed choluria, acholia, and hyporexia, with laboratory findings showing mild thrombocytopenia (platelets 109,000 u/L), transaminasemia (aspartate aminotransferase 890 U/l, alanine aminotransferase 1183 U/l, alkaline phosphatase 311 U/l), direct hyperbilirubinemia (total bilirubin: 7.8 mg/dl, direct: 6.8 mg/dl), and prolonged prothrombin time. Hepatotropic virus and HIV infections were ruled out, as well as autoimmune liver diseases. Hepatic ultrasound showed a starry sky pattern and splenomegaly. Magnetic resonance cholangiopancreatography revealed only hepatosplenomegaly. Liver biopsy showed intense inflammation with polymorphonuclear and lymphocytic infiltrate, total acinar involvement, cholestasis, and hepatocellular necrosis, compatible with acute severe hepatitis and accentuated cholestasis probably secondary to DILI. Management with ursodeoxycholic acid and prednisone (50 mg/day) was initiated without improvement, with a torpid evolution due to the development of hepatic encephalopathy, coagulopathy, and upper gastrointestinal bleeding.
ResultsDILI has an estimated annual incidence of 2.5 cases/100,000 inhabitants, considered a diagnosis of exclusion, with complementary studies useful to increase diagnostic suspicion. In this context, the R factor should be calculated to characterize the type of liver injury. Liver biopsy is useful and shows three patterns: necroinflammatory, cholestatic, and mixed. Idiosyncratic reactions occur in susceptible individuals, are dose-independent, and mostly occur 5-90 days after drug intake. Ibuprofen is associated with a mixed pattern in this presentation. DILI is one of the main causes of ALF, defined by the appearance of hepatic encephalopathy between 7-28 days after the onset of jaundice, with coagulopathy and moderate elevation of transaminases and bilirubin. In this case, a woman with no history of liver disease, recent ibuprofen intake, and acute liver damage was observed. During her evaluation, alcoholic, infectious, and autoimmune pathologies were ruled out, revealing a mixed pattern of liver injury (necroinflammatory and cholestatic) on imaging and histopathological studies. In patients with ALF secondary to DILI, early liver transplantation should be considered due to the high risk of irreversible damage and complications.
ConclusionsIn patients with recent-onset liver failure, it is essential to rule out recent drug intake that may cause DILI to detect it early and initiate timely supportive management, considering liver transplantation due to the high risk of associated complications.
Ethical Statement: This case report has been prepared following the highest ethical standards and respecting the principles of integrity and transparency. All relevant ethical guidelines have been followed, ensuring the privacy and confidentiality of the individuals involved.
Conflict of Interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Figure 1. Liver biopsy from the patient
Giant cells, cholestasis, Kupffer cells and hepatocelular necrosis were identified