Transpulmonary bubble transit (TPBT) detected with contrast echocardiography is reported as a sign of intrapulmonary arteriovenous shunt. However, its pathological meaning is not clear during coronavirus-2019 disease (COVID-19) related acute respiratory distress syndrome (ARDS). Our aim was to determine the prevalence and clinical significance of TPBT detection during COVID-19 related ARDS.
MethodsWe carried out a prospective observational study performed in a high complexity intensive care unit from Argentina. Patients with COVID-19 related ARDS underwent transthoracic echocardiography with saline contrast. Moderate-to-large TPBT was defined as right-to-left passage of at least twelve bubbles to left chambers after at least three cardiac cycles and complete opacification of the right atrium.
ResultsWe analyzed the results of 28 patients (24 men and 4 women). Seventy-five percent of the patients received invasive mechanical ventilation. Moderate-to-large TPBT was detected in 1 patient (3.5%). Among the 27 patients without significant TPBT, 23 had no TPBT and 4 had a minor TPBT. TPBT was not associated with invasive mechanical ventilation requirement (p=0.5737) nor in-hospital mortality (p=1).
ConclusionsTPBT was not associated with severe hypoxemia or invasive mechanical ventilation requirement, although more studies are needed to further clarify its contributing role in COVID-19 hypoxemia.
El tránsito transpulmonar de burbujas (TTPB) detectado con ecocardiografía de contraste se reporta como un signo de cortocircuito arteriovenoso intrapulmonar. Sin embargo, su significado patológico no está claro durante el síndrome de dificultad respiratoria aguda (SDRA) secundario a enfermedad por coronavirus-2019 (COVID-19). Nuestro objetivo fue determinar la prevalencia y la importancia clínica de la detección de TTPB durante el SDRA por COVID-19.
MétodosEstudio observacional prospectivo realizado en una unidad de cuidados intensivos de alta complejidad de Argentina. Los pacientes con SDRA por COVID-19 se sometieron a una ecocardiografía transtorácica con contraste salino. El TTPB moderado a grande se definió como el paso de derecha a izquierda de al menos doce burbujas aéreas a las cavidades cardíacas izquierdas después de al menos tres ciclos cardíacos con opacificación completa de la aurícula derecha.
ResultadosSe analizaron los resultados de 28 pacientes (24 hombres y 4 mujeres). El 75% de los pacientes recibieron ventilación mecánica invasiva. Se detectó TTPB moderado a grande en un paciente (3,5%). Entre los 27 pacientes sin TTPB significativo, 23 no tenían TTPB y 4 tenían un TTPB menor. El TTPB no se asoció con la necesidad de ventilación mecánica invasiva (p=0,5737) ni con la mortalidad hospitalaria (p=1).
ConclusionesEl TTPB no se asoció con hipoxemia grave o necesidad de ventilación mecánica invasiva, aunque se necesitan más estudios para aclarar el papel contributivo en la hipoxemia por COVID-19.
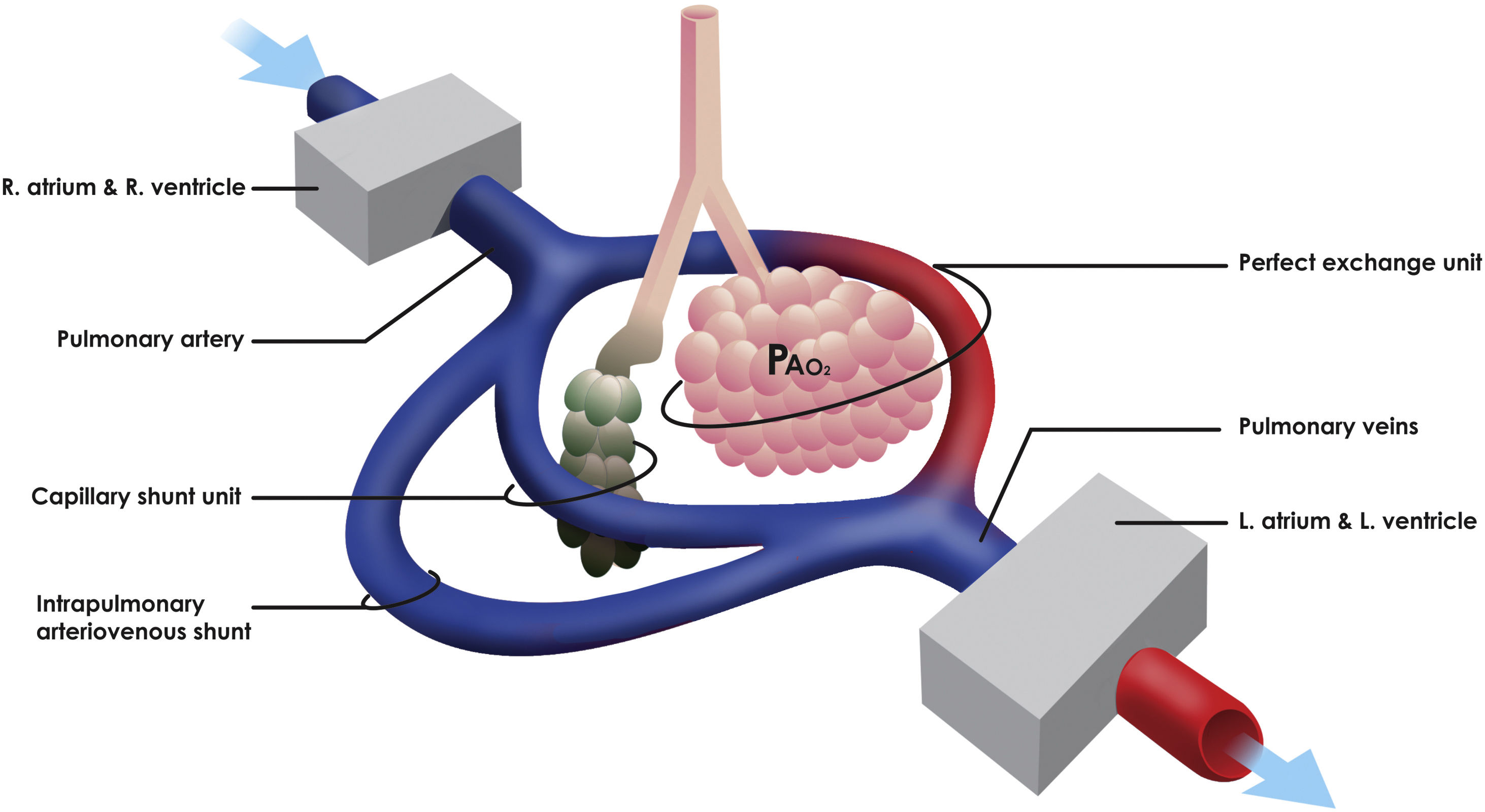
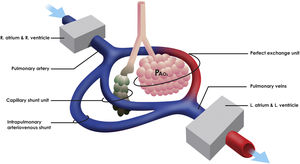
The pathophysiological mechanisms that generate hypoxemia in acute respiratory distress syndrome (ARDS) are fundamentally associated with the alteration alveolar ventilation–perfusion (V/Q) ratio, which requires an optimal correlation between its two components to obtain a suitable gas exchange. Also, different simultaneous states of alteration of the V/Q ratio such as capillary shunting and intrapulmonary arteriovenous shunt (IPshunt) may coexist (Fig. 1).1
Concept of intrapulmonary shunting. Capillary shunt is fundamentally associated with the alteration alveolar ventilation–perfusion (V/Q) ratio due to alveolar damage. Intrapulmonary arteriovenous shunt (IPshunt) promotes the passage of blood through intrapulmonary arteriovenous anastomoses directly to pulmonary veins without normal exposure to alveolo-capillary membrane. PaO2 is the alveolar oxygen tension.
One of the distinctive characteristics of the coronavirus-2019 disease (COVID-19) related ARDS is the dissociation between the severity of hypoxemia and the maintenance of relatively preserved respiratory mechanics.2 Likewise, it has been shown that there is an additive effect between the hypoxic stimulus and the elevated vascular pressure secondary to the hypoxic vasoconstriction to promote the passage of blood through the intrapulmonary arteriovenous anastomoses generating IPshunt.3 Intrapulmonary arteriovenous anastomoses are also recruited against other physiological or pathological stress stimuli such as intense exercise or increased cardiac output. However, the importance of blood flow through these anastomoses in terms of the efficiency of pulmonary gas exchange in normal and pathological conditions remains controversial.4
IPshunt, also called anatomical intrapulmonary shunt, has been studied in healthy individuals during exercise in normoxia, hypoxia and hyperoxia; in patients with hepato-pulmonary syndrome due to cirrhosis; and in ARDS in both prone and supine position).5–7 However, information on IPshunt among COVID-19 patients is still limited. The objective of our study was to determine the prevalence and clinical significance of IPshunt detected with transpulmonary bubble transit (TPBT) by contrast transthoracic echocardiography (TTE) during COVID-19 related ARDS.
MethodsFor this prospective, single-center study, patients were recruited between June 1st, 2020 and July 1st, 2020. Data were obtained from medical records of adult patients (18 years of age or older) with laboratory-confirmed COVID-19 hospitalized in the intensive care unit (ICU) of a high complexity hospital from Buenos Aires, Argentina. Data registration included demographic, clinical and laboratory information, severity scores, the radiographic assessment of lung edema (RALE) score,8 and mechanical ventilation measurements. The number of patients who died or been discharged, and those that stayed in ICU until August 31st, 2020 was recorded. Additionally, ICU length of stay was determined.
TTE was performed within the three days after ICU admission. Non-inclusion criteria were therapeutic effort adaptation, extracorporeal circulation membrane or inhaled nitric oxide requirement, obesity (body mass index>30kg/m2), history of chronic lung disease defined by spirometry as forced expiratory volume in the first second/forced vital capacity <0.75 or pulmonary hypertension defined as pulmonary systolic blood pressure >35mmHg by any method of assessment, patent foramen ovale (PFO) or any defect in the cardiac interatrial or interventricular septum, history of Rendu Osler Weber Syndrome, and hepatic cirrhosis. Due to the fact that we routinely use TTE to assess the circulatory status of mechanically ventilated patients with COVID-19 in our ICU, TTE was considered a component of standard care. Nevertheless, contrast TTE is not routinely performed, therefore written patient's consent was solicited. Also written and oral information about the study was given to the families. The study was approved by the institutional ethics committee of our hospital under protocol number 5657. Our manuscript complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for observational cohort studies9 (Table E1 of Supplementary material).
EchocardiographyTTE was performed using a Philips Affiniti 50 ultrasound system (Philips Ultrasound, Bothell, WA, USA) equipped with QLAB cardiac analysis software and a 2–4MHz sector cardiac transducer. TTE operators were university cardiologists with certification in TTE and trained in advanced echocardiography of critical care patients. Our evaluation protocol included examining the apical four-chamber image or, failing that, the subxiphoid four-chamber image if the first does not achieve sufficient quality. Echocardiographic images were recorded at a minimum rate of 30 frames per second, and stored in digital format.
For the generation of the contrast bubbles, we used the technique described by Lovering et al.10 This technique requires a 20-gauge peripheral venous catheter or a central venous access and a three-way stopcock to which two 10mL syringes are connected. One syringe contained 10mL of saline and the other 1mL of room air. Contrast bubbles were created by rapidly passing the solution from one syringe to another for at least 15s, removing any residual macroscopic air prior to infusion through the patient's vein. These contrast bubbles are highly echogenic and are easily visualized in the right chambers after venous injection. The injection was considered successful if the entire right atrium was opacified with microbubble-induced contrast. Up to two successful contrast studies were performed on each patient.
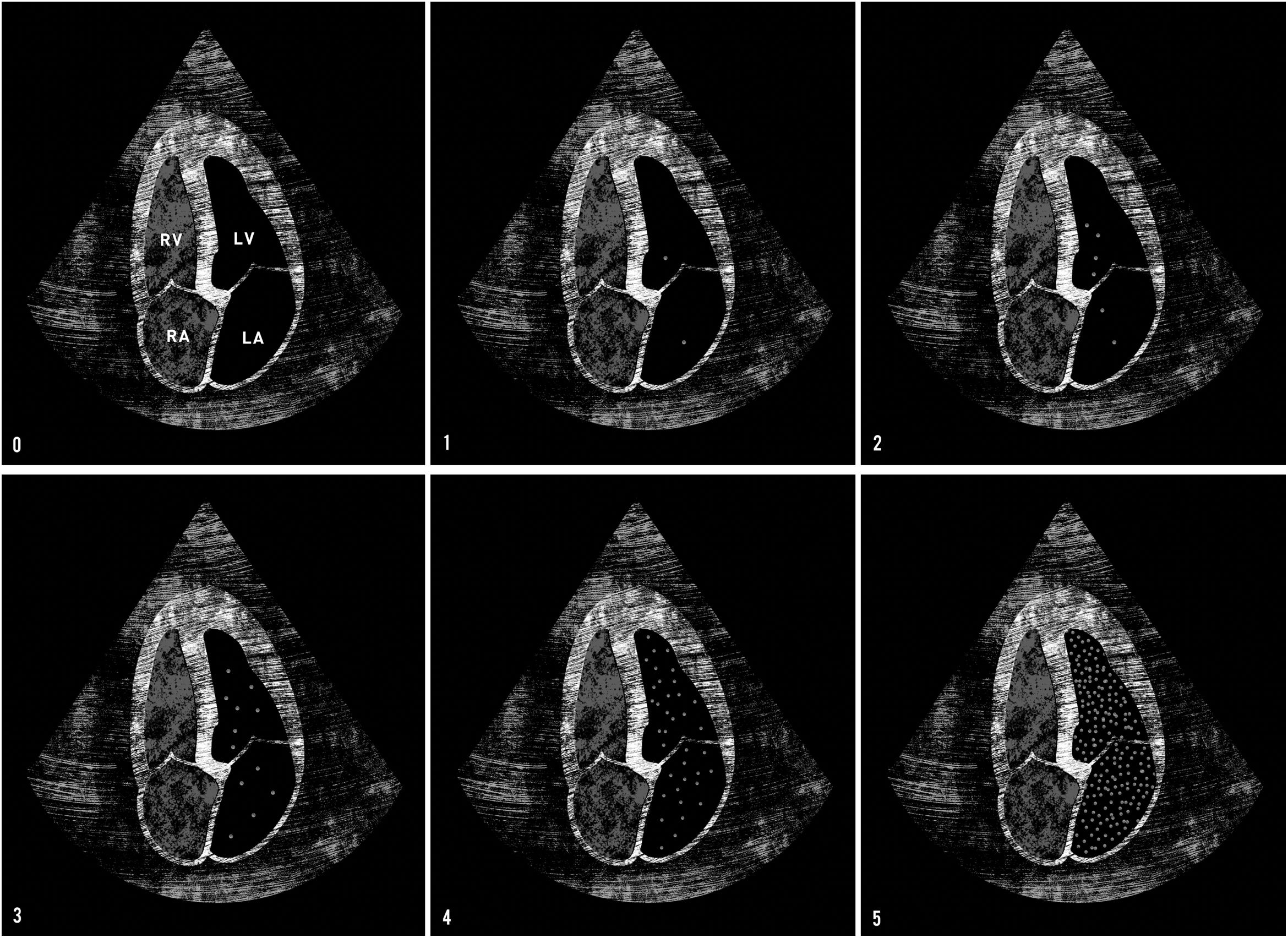
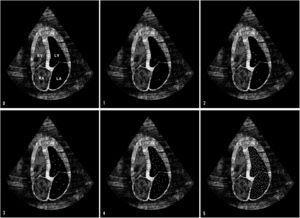
Bubble scoreTo obtain a qualitative evaluation of the degree of TPBT, the Bubble score tool described by Lovering et al.10 was used (Table E2 of Supplementary material). This score is based on both the density and the spatial distribution of the microbubbles in the left chambers (Fig. 2). If there was no right-to-left shunt, the infused contrast bubbles appeared as a cloud of echoes in the right chambers and then gradually disappeared as the bubbles became trapped and eliminated into pulmonary microcirculation. On the other hand, if there was an intracardiac shunt at the atrial or ventricular level, the contrast bubbles rapidly filled the left chambers, in less than three cardiac cycles. If the contrast bubbles passed through the lungs in the presence of TPBT, they appeared in the left chambers after a delay of at least three cardiac cycles. The late appearance of bubbles in the left heart indicated the transpulmonary passage of contrast bubbles through IPshunt. Therefore, the presence of IPshunt was defined as the appearance of more than three bubbles in the left chambers after at least three cardiac cycles (Bubble score of 2 or more).
Bubble score tool. Bubble score 0: no bubbles transit. Bubble score 1: 1–3 bubbles in left chambers. Bubble score 2: 4–12 bubbles in left chambers. Bubble score 3: >12 isolated bubbles in left chambers. Bubble score 4: >12 bubbles distributed heterogeneously in left chambers. Bubble score 5: >12 bubbles distributed homogeneously in left chambers. Late appearance of bubbles in the left heart indicates a transpulmonary passage of contrast bubbles through intrapulmonary arteriovenous shunt (IPshunt). Therefore, the presence of IPshunt was defined as the appearance of more than three bubbles in the left chambers after at least three cardiac cycles (Bubble score of 2 or more). Abbreviations: RV: right ventricle; LV: left ventricle; RA: right auricle; LA: left auricle.
Among mechanically ventilated patients, TTE was performed in volume-assist control mode, with a target tidal volume (VT) of 6mL/kg of predicted body weight. In patients with severe hypoxemia (PaO2/FiO2 ratio<100), PEEP was titrated to avoid exceeding plateau pressure values (Pplat) greater than 30cm H2O and obtaining a driving pressure less than 15cm H2O. If Pplat exceeded this maximum threshold, VT was lowered until Pplat was less than 30cm H2O. On the other hand, to counteract the effect of the reduction in VT on alveolar ventilation, the respiratory rate was increased. Fraction of inspired oxygen (FiO2) was adjusted to obtain a minimum saturation of 92%.
Spontaneously breathing patients who required oxygen were supported with nasal cannula or non-rebreathing mask oxygenator delivering a minimum oxygen flow to achieve SpO2 greater than 92%. Setting primary focus on health-care personnel security, no patient in our ICU received non-invasive mechanical ventilation (e.g. continuous positive airway pressure, non-invasive positive pressure ventilation, or high-flow nasal cannula) due to the risk of aerosol dispersion.11
Statistical analysisNo statistical sample size calculation was performed in advance and the sample size was equal to the number of patients treated during the study period. Continuous variables were expressed as medians and interquartile ranges or simple ranges, as appropriate. Categorical variables were summarized as counts and percentages.
No imputation was made for missing data. Mann–Whitney rank-sum test was used to compare nonparametric continuous variables. χ2 or Fisher exact test was used for categorical variables as appropriate. All statistical tests were 2-tailed, and statistical significance was defined as p<.05. The analysis has not been adjusted for multiple comparisons, and given the possibility of a type I error, the findings should be interpreted as exploratory and descriptive. All the analyses were performed with the use of R Software, version 3.6.2 (R Foundation for Statistical Computing).
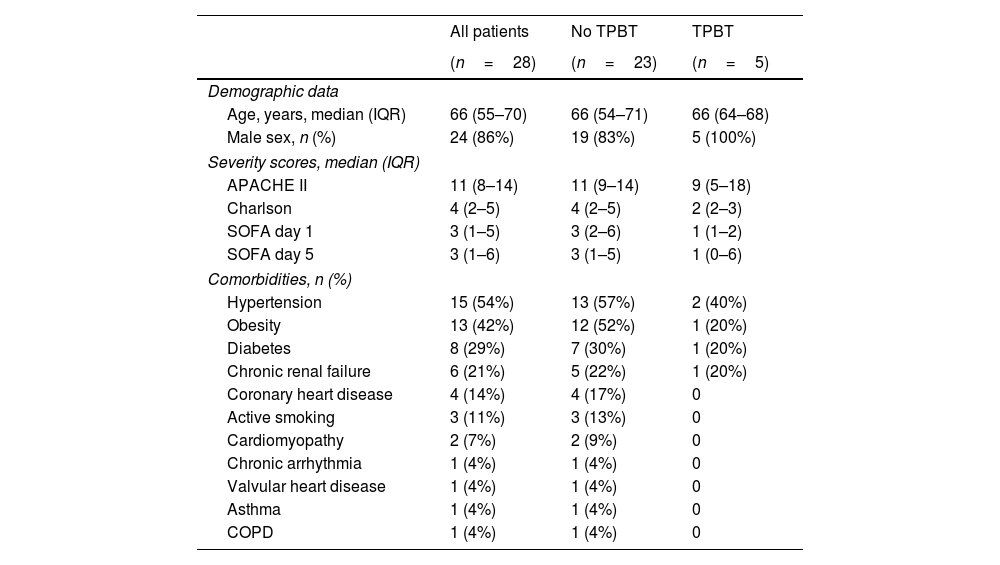
ResultsDuring the study period, a total of 32 patients with laboratory-confirmed COVID-19 met inclusion criteria and underwent contrast TTE. Four patients were excluded from the analysis: 2 due to a poor acoustic window, and 2 patients due to PFO. Thus, we analyzed the results of 28 patients (24 men and 4 women), with a median age of 66 (interquartile range [IQR] 55–70) years and a median APACHE II score 11 (IQR 8–14). Seventy-five percent (21) of the patients received invasive mechanical ventilation (iMV) and 38% (8) of them required prone position ventilation. Median ICU length of stay was 19 days (IQR 9–29) presenting an inhospital mortality rate of 25% (7). Patient characteristics are listed in Table 1.
Patient characteristics.
| All patients | No TPBT | TPBT | |
|---|---|---|---|
| (n=28) | (n=23) | (n=5) | |
| Demographic data | |||
| Age, years, median (IQR) | 66 (55–70) | 66 (54–71) | 66 (64–68) |
| Male sex, n (%) | 24 (86%) | 19 (83%) | 5 (100%) |
| Severity scores, median (IQR) | |||
| APACHE II | 11 (8–14) | 11 (9–14) | 9 (5–18) |
| Charlson | 4 (2–5) | 4 (2–5) | 2 (2–3) |
| SOFA day 1 | 3 (1–5) | 3 (2–6) | 1 (1–2) |
| SOFA day 5 | 3 (1–6) | 3 (1–5) | 1 (0–6) |
| Comorbidities, n (%) | |||
| Hypertension | 15 (54%) | 13 (57%) | 2 (40%) |
| Obesity | 13 (42%) | 12 (52%) | 1 (20%) |
| Diabetes | 8 (29%) | 7 (30%) | 1 (20%) |
| Chronic renal failure | 6 (21%) | 5 (22%) | 1 (20%) |
| Coronary heart disease | 4 (14%) | 4 (17%) | 0 |
| Active smoking | 3 (11%) | 3 (13%) | 0 |
| Cardiomyopathy | 2 (7%) | 2 (9%) | 0 |
| Chronic arrhythmia | 1 (4%) | 1 (4%) | 0 |
| Valvular heart disease | 1 (4%) | 1 (4%) | 0 |
| Asthma | 1 (4%) | 1 (4%) | 0 |
| COPD | 1 (4%) | 1 (4%) | 0 |
Continuous variables are presented as median (and interquartile range), and categorical variables as count (%).
TPBT: transpulmonary bubble transit; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment score; COPD: chronic obstructive pulmonary disease.
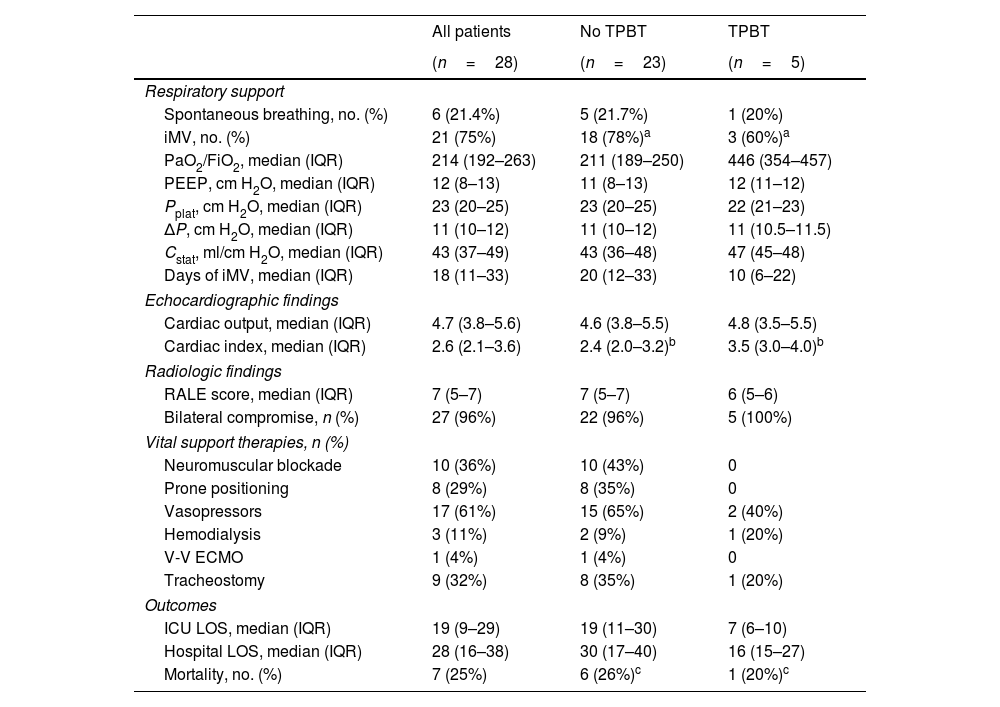
Moderate-to-large TPBT (Bubble score 4) was detected in 1 patient (3.5%). Among the 27 patients without significant TPBT, 23 had no TPBT and 4 presented minor TPBT (Bubble score 1). TPBT was not associated with iMV requirement (p=0.57), nor in-hospital mortality (p=0.99). Respiratory support requirement, imaging findings and ICU outcomes are listed in Table 2.
Respiratory support, imaging findings and ICU outcomes.
| All patients | No TPBT | TPBT | |
|---|---|---|---|
| (n=28) | (n=23) | (n=5) | |
| Respiratory support | |||
| Spontaneous breathing, no. (%) | 6 (21.4%) | 5 (21.7%) | 1 (20%) |
| iMV, no. (%) | 21 (75%) | 18 (78%)a | 3 (60%)a |
| PaO2/FiO2, median (IQR) | 214 (192–263) | 211 (189–250) | 446 (354–457) |
| PEEP, cm H2O, median (IQR) | 12 (8–13) | 11 (8–13) | 12 (11–12) |
| Pplat, cm H2O, median (IQR) | 23 (20–25) | 23 (20–25) | 22 (21–23) |
| ΔP, cm H2O, median (IQR) | 11 (10–12) | 11 (10–12) | 11 (10.5–11.5) |
| Cstat, ml/cm H2O, median (IQR) | 43 (37–49) | 43 (36–48) | 47 (45–48) |
| Days of iMV, median (IQR) | 18 (11–33) | 20 (12–33) | 10 (6–22) |
| Echocardiographic findings | |||
| Cardiac output, median (IQR) | 4.7 (3.8–5.6) | 4.6 (3.8–5.5) | 4.8 (3.5–5.5) |
| Cardiac index, median (IQR) | 2.6 (2.1–3.6) | 2.4 (2.0–3.2)b | 3.5 (3.0–4.0)b |
| Radiologic findings | |||
| RALE score, median (IQR) | 7 (5–7) | 7 (5–7) | 6 (5–6) |
| Bilateral compromise, n (%) | 27 (96%) | 22 (96%) | 5 (100%) |
| Vital support therapies, n (%) | |||
| Neuromuscular blockade | 10 (36%) | 10 (43%) | 0 |
| Prone positioning | 8 (29%) | 8 (35%) | 0 |
| Vasopressors | 17 (61%) | 15 (65%) | 2 (40%) |
| Hemodialysis | 3 (11%) | 2 (9%) | 1 (20%) |
| V-V ECMO | 1 (4%) | 1 (4%) | 0 |
| Tracheostomy | 9 (32%) | 8 (35%) | 1 (20%) |
| Outcomes | |||
| ICU LOS, median (IQR) | 19 (9–29) | 19 (11–30) | 7 (6–10) |
| Hospital LOS, median (IQR) | 28 (16–38) | 30 (17–40) | 16 (15–27) |
| Mortality, no. (%) | 7 (25%) | 6 (26%)c | 1 (20%)c |
Continuous variables are presented as median (and interquartile range), and categorical variables as count (%).
TPBT: transpulmonary bubble transit; iMV: invasive mechanical ventilation; PaO2: partial pressure of oxygen; FiO2: fraction of inspired oxygen; PEEP: positive end-expiratory pressure; Pplat: plateau pressure; ΔP: driving pressure; Cstat: static pulmonary compliance; RALE: radiographic assessment of lung edema; V-V ECMO: veno-venous extracorporeal membrane oxygenation; LOS: length of stay.
Statistical analysis: ap=0.5737; bp=0.928; cp=1.
In this prospective study conducted in a single-center, we found no association between the presence of TPBT and the development of hypoxemia in patients with COVID-19 hospitalized in the ICU. Nor did we certify an association between the presence of TPBT and hospital mortality or the requirement for iMV assistance. The prevalence of TPBT was 17.8%, and moderate-to-large TPBT (Bubble score 4) was recorded in only one patient.
IPshunt has been described over the years as a pathologic pathway that could cause hypoxemia in different respiratory conditions. However, its clinical implication in critical pulmonary disease remains controversial. Previous to the current COVID-19 pandemic, Boissier and colleagues7 studied the impact of TPBT during ARDS in 219 patients. Forty-four percent of the patients presented with TPBP but only 26% were classified as moderate-to-large TPBT. The PaO2/FiO2 ratio did not differ between groups with or without TPBT.
Closer in time, during the beginning of the SARS-CoV-2 outbreak, the discussion on IPshunt as a contributory mechanism of hypoxemia in COVID-19 related ARDS was again fueled.12,13 In this sense, series of contrast echocardiography were reported to determine the prevalence of IPshunt with disparate results. Masi and colleagues14 informed that 12 of 60 (20%) critically ill COVID-19 patients achieving Berlin ARDS criteria presented TPBT. Furthermore, they conclude TPBT did not seem to be the main driver of hypoxemia in this population, especially in patients with lower respiratory system elastance. As for the contrary, Brito-Azevedo et al.15 in a study of 9 patients, detected a TPBT prevalence of 78%, and hypothesized the probability that SARS-CoV-2 causes an important intrapulmonary vascular dilatation, with a shunt mechanism.
There are several limitations to the present study. First, it was conducted in a single clinical center, which weakens the external validation. Second, it was performed on a small number of patients, and the lack of preliminary sample size calculation conditioned to interpret findings as exploratory and descriptive. Third, screening for TPBT by TTE in comparison with contrasted transesophageal echocardiography may present lower sensitivity for bubble transit detection. In conclusion, we did not observe an association between TPBT and severe hypoxemia, nor that IPshunt has implications in presenting a higher risk of iMV requirement. Nevertheless, further studies are required to entirely comprehend IPshunt and hypoxemia relation among COVID-19 patients, and test whether TPBT can be used in clinical settings for this purpose.
FundingThis study did not receive any funding sources.
Conflicts of interestWe have no conflict of interest to declare.
The research team wants to thank Maria de los Ángeles Magaz for the English translation of this work. Also, we would like to thank Lisandro Ziperovich (Ziperart) for the artwork of this study.