Here, we present the first description of the Andalusian Bipolar Family (ABiF) Study. This longitudinal investigation of families from Andalusia, Spain commenced in 1997 with the aim of elucidating the molecular genetic causes of bipolar affective disorder. The cohort has since contributed to a number of key genetic findings, as reported in international journals. However, insight into the genetic underpinnings of the disorder in these families remains limited.
MethodIn the initial 1997–2003 study phase, 100 multiplex bipolar disorder and other mood disorder families were recruited. The ongoing second phase of the project commenced in 2013, and involves follow-up of a subgroup of the originally recruited families. The aim of the follow-up investigation is to generate: (i) longitudinal clinical data; (ii) results from detailed neuropsychological assessments; and (iii) a more extensive collection of biomaterials for future molecular biological studies.
ResultsThe ABiF Study will thus generate a valuable resource for future investigations into the etiology of bipolar affective disorder; in particular the causes of high disease loading within multiply affected families.
DiscussionWe discuss the value of this approach in terms of new technologies for the identification of high-penetrance genetic factors. These new technologies include exome and whole genome sequencing, and the use of induced pluripotent stem cells or model organisms to determine functional consequences.
Se presenta la primera descripción del estudio denominado Andalusian Bipolar Family (ABiF). Se trata de una investigación longitudinal con familias procedentes de Andalucía (España), que comenzó en 1997, con el objetivo de dilucidar las causas geneticomoleculares del trastorno afectivo bipolar. Desde entonces, esta cohorte ha contribuido a una serie de hallazgos clave, que han sido publicados en revistas internacionales. Sin embargo, el conocimiento sobre las bases genéticas del trastorno en estas familias sigue siendo limitado.
MétodoEl estudio consta de dos fases: en la fase inicial se reclutaron 100 familias con múltiples afectados de trastorno bipolar y otros trastornos del ánimo. La segunda fase del proyecto, actualmente en curso, comenzó en 2013 con el objetivo de realizar un seguimiento de la cohorte de familias reclutadas originalmente. Los objetivos del estudio de seguimiento son: i) recoger nuevos datos clínicos longitudinales; ii) realizar una evaluación neuropsicológica detallada, y iii) obtener una extensa colección de biomateriales para futuros estudios moleculares.
ResultadosEl estudio ABiF, por tanto, generará unos recursos valiosos para futuras investigaciones sobre la etiología del trastorno afectivo bipolar; particularmente con respecto a las causas de la alta carga genética del trastorno en las familias con múltiples afectados.
DiscusiónSe discute el valor de este enfoque en relación con las nuevas tecnologías para la identificación de factores genéticos de alta penetrancia. Estas nuevas tecnologías incluyen la secuenciación del exoma y del genoma completo, y el uso de células madre pluripotentes inducidas u organismos modelo para la determinación de consecuencias funcionales.
Bipolar disorder (BD) is characterized by recurrent episodes of mania and depression,1 and ranks among the major contributors to the global burden of disease.2,3 BD has a life-time prevalence of 0.5–1%,4 and an estimated heritability of around 70%.5–7 Molecular genetic candidate studies and recent genome wide association studies (GWAS), have identified the first BD susceptibility genes.6,8–13 All genetic risk variants identified to date are common in the general population, and confer only a small individual risk. Although the identification of the first susceptibility genes is a major achievement, researchers assume that a larger disease risk is conferred by, as yet unidentified rare variants.6,14 Such variants may explain, at least in part, the high familial loading observed in some families. Detection of these variants may be of particular importance for investigations of the functional consequences of genetic variants. Such studies will in turn elucidate the biological pathways that are perturbed in BD. On the clinical level, families segregating high-risk variants are particularly suited for the study of pleiotropic effects, i.e., examination of the diversity of clinical symptoms and endophenotypes that are associated with a given variant, and analyses of gene–environment interactions. However, due to ever decreasing family sizes and the increasing mobility of family members, the recruitment of multiplex families is problematic, particularly in Europe and North America.
In 1997, our group initiated a study of BD multiplex families from Andalusia, Spain, involving the collection of detailed phenotypic data and biomaterials. The present report describes the study, and how it has already contributed to key molecular genetic findings in the field. We discuss the potential value of this family cohort for future new technology studies of BD etiology.
MethodsInitiation of the studyThe project was initiated by the identification of a BD inpatient of Andalusian origin at the Department of Psychiatry, University of Düsseldorf, Germany, who reported multiple affected family members. In view of the potential value of this pedigree to BD research, the responsible Spanish speaking neurologist (GA) confirmed the family history, and contacted psychiatric geneticists PP and MMN at the Institute of Human Genetics in Bonn, Germany. Together with Spanish speaking German psychiatrist MR from the Department of Psychiatry, University of Bonn (now at the Central Institute of Mental Health, University of Heidelberg, Germany), a collaboration was initiated with psychiatrists FR and FM from the Regional University Hospital of Malaga, Spain. To identify further multiplex BD families, this collaboration was subsequently extended to include the mental health departments of the following six centers in Andalusia: University Hospital Reina Sofia of Córdoba, Provincial Hospital of Jaen; Hospital of Jerez de la Frontera (Cádiz); Hospital of Puerto Real (Cádiz); Hospital Punta Europa of Algeciras (Cádiz); and Hospital Universitario San Cecilio (Granada).
Original cohort (initial study phase, 1997–2003)AssessmentRecruitment commenced with in- and outpatient index cases, and was then extended to family members. Diagnostic assessment was performed using the Schedule for Affective Disorders and Schizophrenia (SADS)15; the Operational Criteria Checklist for Psychotic Illness (OPCRIT)16; a review of medical records; and interviews with first and/or second degree family members using the Family Informant Schedule and Criteria (FISC).17 Consensus best estimate diagnoses were assigned by two or more independent senior psychiatrists and/or psychologists, and according to the Research Diagnostic Criteria,18 and the Diagnostic and Statistical Manual of Mental Disorders IV.19 Both affected individuals and healthy relatives were evaluated. Over the initial six year study period, recruitment was carried out by the same neurologist (GOD) from the Regional University Hospital of Malaga. GOD was trained and supervised by three senior psychiatrists (MR, FR, and FM), and a human geneticist (MMN). Blood samples were collected from all participants and sent to the Institute of Human Genetics in Bonn for whole blood DNA extraction.
The study protocols for clinical assessment and genetic investigation were approved by the local ethics committees of all participating centers.
Between 1997 and 2003, a total of 1174 individuals from 100 multiplex families were recruited. Face-to-face SADS interviews were conducted with 758 individuals. For 655 of these 758 individuals, information was also obtained from up to seven best informants using the FISC. For 14 individuals only OPCRIT data were available based on different sources of information. For 402 additional individuals, information was obtained from best informants only: from 320 individuals with the FISC and from 76 individuals the obtained information was so sparse that no valid FISC could be completed. From six further family members, no information could be obtained except for sex, relationship to the family, and disease status. Thus valid FISC information was available for 975 individuals. Blood samples were collected from a total of 732 individuals. Supplementary Figure 1 displays the multiplex family recruitment procedure in flow-chart form.
Follow-up study (2013–ongoing)For the follow-up investigations, the study protocol has been extended to include neuropsychological phenotypes and the collection of additional biomaterials.
Sample re-assessmentFamilies who had previously given their written informed consent to being re-contacted are now being asked whether they are willing to participate in a follow-up assessment. Written informed consent to the extended study protocol is then obtained. At the time of writing, four families (4–28 family members) have already been re-contacted, and are now undergoing assessment.
Clinical re-assessmentDetailed phenotype characterization is performed for each family member. This involves: (i) the Interview for Psychiatric Genetic Studies (IPGS; Fangerau et al., 2004); (ii) a comprehensive inventory for phenotype characterization, including the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I),20 and the OPCRIT; (iii) a review of all medical records; and (iv) interviews with family members. Clinical diagnoses are assigned by two trained clinicians using a best estimate approach. The IPGS enables a highly structured and reproducible assessment of the DSM-IV diagnoses; sociodemographic characteristics; life-time psychopathological symptoms; current depressivity; level of functioning prior to disease onset, in the life-time worst disease episode, and after remission from the last disease episode; alcohol and nicotine abuse and dependence; premorbid adjustment; history of medical and obstetric complications; medical treatment and side effects; personality; and environmental factors, such as parental bonding, trauma and stressful life-events. The phenotypes of interest and the clinical assessment instruments are shown in Table 1.
Phenotypes and clinical assessment instruments used in the follow-up study.
| Phenotype | Clinical assessment instrument | Details of assessment instruments |
|---|---|---|
| Interview | ||
| Clinical DSM-IV diagnosis | Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)20 | Research version 2.0 |
| Family interview | Family Informant Schedule and Criteria (FISC)17 | Interview with best informants from the family |
| Impairment in social, occupational, or other important areas of functioning | Global Assessment of Functioning Scale (GAF)45 Functioning Assessment Short Test (FAST)46 | Overall rating of functioning between 0 and 100% 24 items (3-point rating scale) to assess functioning in the worst, best, and current period |
| Affective and/or psychotic symptoms | Operational Criteria in Psychotic Illness OPCRIT16 | 90-Item scale assessing affective and psychotic core psychopathology |
| Circadian rhythms | Reduced Horne and Ostberg Questionnaire47 | 5-Item scale to assess morningness and eveningness |
| History of somatic illnesses and obstetric complications | Interview for Psychiatric Genetic Studies (IPGS)48 | |
| Past/current treatment with psychotropic medication | Interview for Psychiatric Genetic Studies (IPGS)48 | Assessment of dosage, treatment response, and side effects |
| Lithium response | Alda lithium response scale49 | Brief 11-item scale |
| Aggressiveness | Life history of aggression50 | 11 items with a 6-point scale assessing aggression during, and independently of, disease episodes |
| Parental bonding | Parental bonding instrument51 | Fundamental parental dimensions of care and protection |
| Premorbid adjustment | Premorbid Adjustment Scale (PAS)52 | Degree of achievement of developmental goals at several periods of life before the onset of schizophrenia |
| Life-events | List of life-events based on various life-event scales53–56 | 79 life-events (LE) to assess if LE occurred 6 months prior to onset of illness, and is perceived as having caused the disorder and as pleasant, neutral or unpleasant |
| Self-rating | ||
| Psychological symptoms | Brief Symptom Inventory (BSI)57 | 53 items (5-point rating scale) to assess the burden of mental symptoms |
| Depressivity within previous week | The Center for Epidemiologic Studies Depression Scale (CES-D)58 | 20 items (4-point rating scale) |
| Childhood trauma | Childhood Trauma Screener (CTS)59 | 5-Item brief screening version of the Childhood Trauma Questionnaire (CTQ) |
| Personality | Short version of the Big Five Inventory (BFI-10)60; Neuroticism scale of the NEO Five-Factor Inventory (NEO-FFI)61 | 10 items (5-point rating scale) to assess the Big Five personality dimensions; 12 items (5-point rating scale) |
| Impulsiveness | Barratt Impulsiveness Scale (BIS)62 | 34 items (4-point rating scale) |
| Alcohol consumption and dependence | Alcohol Use Disorders Identification Test (AUDIT)63 | 10-Item questionnaire developed by the WHO |
| Nicotine consumption and dependence | Fagerström Test of Nicotine Dependence (FTND)64 | 10-Item questionnaire to assess the intensity of physical addiction to nicotine |
| Self-efficacy | General Self-Efficacy Scale (GSE)65 | 10 items (4-point rating scale) to assess perceived self-efficacy |
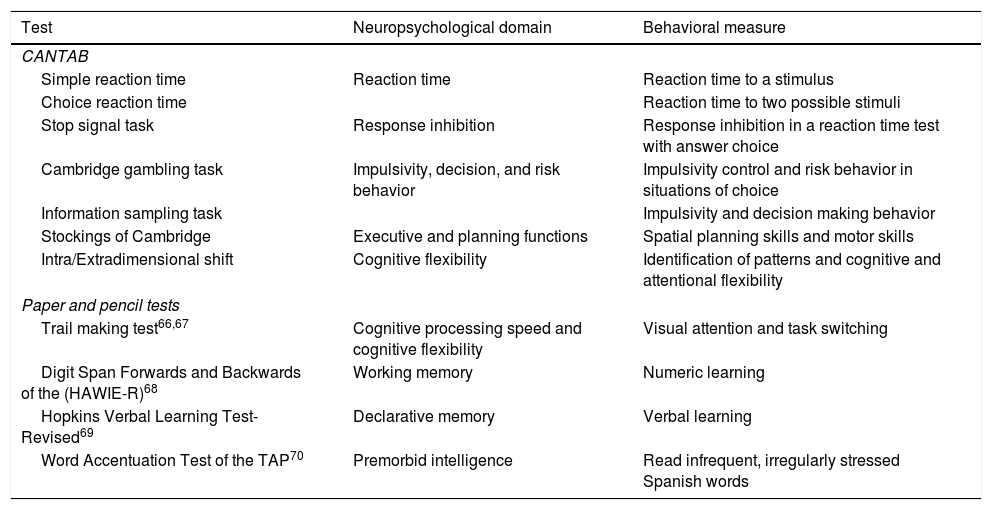
The neuropsychological assessment comprises tests selected from the Cambridge Neuropsychological Test Automated Battery (CANTAB),21 as well as paper and pencil tests. The neuropsychological tests are listed in Table 2.
Neuropsychological assessment at follow-up.
| Test | Neuropsychological domain | Behavioral measure |
|---|---|---|
| CANTAB | ||
| Simple reaction time | Reaction time | Reaction time to a stimulus |
| Choice reaction time | Reaction time to two possible stimuli | |
| Stop signal task | Response inhibition | Response inhibition in a reaction time test with answer choice |
| Cambridge gambling task | Impulsivity, decision, and risk behavior | Impulsivity control and risk behavior in situations of choice |
| Information sampling task | Impulsivity and decision making behavior | |
| Stockings of Cambridge | Executive and planning functions | Spatial planning skills and motor skills |
| Intra/Extradimensional shift | Cognitive flexibility | Identification of patterns and cognitive and attentional flexibility |
| Paper and pencil tests | ||
| Trail making test66,67 | Cognitive processing speed and cognitive flexibility | Visual attention and task switching |
| Digit Span Forwards and Backwards of the (HAWIE-R)68 | Working memory | Numeric learning |
| Hopkins Verbal Learning Test-Revised69 | Declarative memory | Verbal learning |
| Word Accentuation Test of the TAP70 | Premorbid intelligence | Read infrequent, irregularly stressed Spanish words |
A 32.5ml blood sample is collected for DNA extraction (20ml); permanent cell lines as a basis for induced Pluripotent Stem Cells (iPSCs; 8ml); mRNA (2.5ml); and protein expression and metabolomics (2ml). Hair samples are collected for measurement of hair cortisol and the hormones testosterone, progesterone, and dehydroepiandrosteron. The blood and hair samples are sent to the laboratory of the Regional University Hospital of Málaga where the samples are processed and stored according to biobank guidelines. Depending on the planned experiments, samples are sent to collaborating laboratories such as the laboratories of the Central Institute of Mental Health in Mannheim, and the Institute of Human Genetics in Bonn.
Informed consent and ethical approvalThe follow-up study protocol was approved by the ethics committee of the University Regional Hospital of Malaga (Málaga Nordeste) as re-assessment commenced at this institution. When ready to commence re-assessment, the other centers will obtain ethical approval from their local ethics committees. The updated informed consent addresses ethical issues that have arisen through new developments and advances in technology in more detail than was the case in the original informed consent procedure. Among others, these developments enable genome-wide sequencing to be performed in a research context. Important issues in terms of informed consent are data protection, the disclosure of incidental and secondary findings, and confidentiality. Confidentiality and the sharing of genetic information with relatives can become particularly problematic in cases where large-scale sequencing generates secondary findings.22 For example, if a dominantly inherited cancer-causing mutation is identified as a secondary finding in one family member, the mutation carrier may not wish to share this information with his children, even though the cancer is preventable. We therefore follow up-to-date guidelines and recommendations for informed consent, such as those from EURAT (Ethical and Legal Aspects of Whole Genome Sequencing), and engage in regular discussions of ethical issues with representatives from European ethics commissions, as well as international philosophers and legal experts. During the informed consent procedure and thus prior to inclusion, ABiF participants must indicate whether or not they wish to be informed of potential incidental and secondary findings. Participants must also indicate whether they are willing to be re-contacted for further research investigations, e.g., neuro-imaging or more extensive neuropsychological assessment.
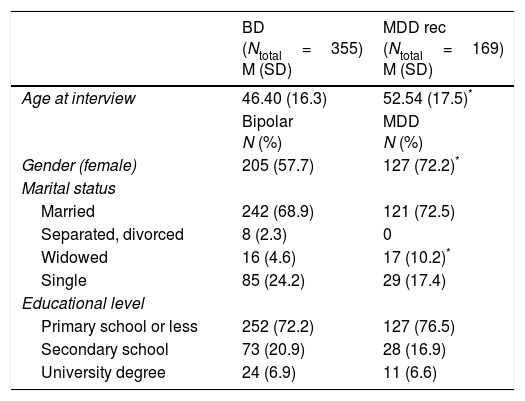
ResultsSociodemographic and clinical characteristicsAnalysis of sociodemographic and clinical characteristics was performed for all multiplex family members using: (i) both SADS and OPCRIT data, n=426; (ii) SADS data only, n=332; (iii) OPCRIT data only, n=14; (iv) or best informant information only, n=402. Supplementary Table 1 provides an overview of the total number of family members and affected family members per multiplex family and per assessment center. Table 3 shows the sociodemographic and clinical characteristics of the family members, as well as for the diagnostic groups MDD rec (Major Depressive Disorder recurrent, n=169); and BD (Bipolar Disorder I, n=258; Bipolar Disorder II, n=78; Bipolar Disorder not otherwise specified, n=18; Schizoaffective Disorder Bipolar Type; n=1).
Sociodemographic characteristics of individuals from the 100 multiplex families of the original cohort (1997–2003) presenting with BD (BD-I, BD-II, BP-NOS, SCA-B) and MDD rec.
| BD (Ntotal=355) M (SD) | MDD rec (Ntotal=169) M (SD) | |
|---|---|---|
| Age at interview | 46.40 (16.3) | 52.54 (17.5)* |
| Bipolar N (%) | MDD N (%) | |
| Gender (female) | 205 (57.7) | 127 (72.2)* |
| Marital status | ||
| Married | 242 (68.9) | 121 (72.5) |
| Separated, divorced | 8 (2.3) | 0 |
| Widowed | 16 (4.6) | 17 (10.2)* |
| Single | 85 (24.2) | 29 (17.4) |
| Educational level | ||
| Primary school or less | 252 (72.2) | 127 (76.5) |
| Secondary school | 73 (20.9) | 28 (16.9) |
| University degree | 24 (6.9) | 11 (6.6) |
Note. BD: bipolar disorder; MDD rec: Major Depressive Disorder recurrent; M: mean; SD: standard deviation.
Note. In Marital status the category of reference for comparisons is Married and in Educational level is Primary School or Less.
The multiplex family cohort collected between 1997 and 2003 comprised 355 BD cases, 169 MDD rec cases, and 650 individuals in the category others (Major Depressive Disorder single episode, n=35; Depressive Disorder not otherwise specified, n=12; Drug or Alcohol Abuse or Dependence, n=31; Schizophrenia, n=2; Agoraphobia, n=1; and Healthy, n=569). Compared to MDD rec patients, BD patients showed: a significantly higher duration of continuous outpatient treatment; a lower age at onset of the psychiatric disorder, first treatment, and first depressive episode; more hospitalizations over their lifetime and during depressive episodes; more suicide attempts over their lifetime and during depressive episodes; and more delusions during depressive episodes (for details see Supplementary Table 2). BD patients were also more likely to have been unable to work within the preceding five years due to psychological problems as compared to MDD rec patients.
Analysis of the clinical symptom dimensions Mania/Excitement, Depression, Disorganization, Positive Symptoms, and Negative Symptoms showed that BD patients scored higher than MDD rec patients on the dimensions Mania/Excitement, Depression, and Positive and Negative Symptoms (for details see Supplementary Table 3). For the depression-dimensions Suicidal Ideation, and Early Morning Wakening were more frequent in BD than in MDD rec patients, while Loss of Energy and Slowed Activity were more frequent in MDD rec than in BD.
Molecular genetic findingsFor the investigation of risk genes for BD, a plausible hypothesis is that multiplex families carry high penetrance genetic variants, and that these are shared across affected family members. To investigate this, we initially performed linkage analyses of the most promising Andalusian families. These linkage data were then analyzed in combination with data collected from non-Andalusian multiplex families.
For the Andalusian families, linkage analysis generated evidence for a new susceptibility locus on chromosome 1p35–p36, and provided support for an established locus on chromosome 6q21–q24.23 In pooled analyses with family samples from other countries, evidence for linkage was obtained for the pseudoautosomal region 1 Xp22.3/Yp11.3.24 Using a covariate approach, suggestive evidence was generated for linkage of mood incongruent psychotic symptoms in BD to 1q32.3, 7p13, and 20q13.31.25 In the first genome-wide linkage study of BD, testing for interaction between genomic loci in families from different countries – including pedigrees from the Andalusian Bipolar Family Study – generated evidence of genetic epistasis between regions of chromosomes 6q and 2q.26 Unfortunately, the linkage paradigm has not led to the unequivocal identification of chromosomal loci for BD. Even if a high penetrance gene effect does exist in individual families, the sharing of such loci across families is probably too low to allow consistent replication of linkage findings across samples.
The first consistent molecular genetic findings for BD were generated by GWAS. Index patients from the Andalusian families underwent genome-wide genotyping of single-nucleotide polymorphisms and these data were subsequently included in larger, international analyses. These GWAS generated evidence for the involvement of chromosomal regions harboring genes such as NCAN, ANK3, ODZ4, TRANK1, ADCY2, and a region between MIR2113 and POU3F2.9,11
DiscussionIn this work, we present the first description of the Andalusian Bipolar Family (ABiF) Study which provides a sociodemographic and clinical analysis of the original cohort of multiplex families, a summary of genetic studies and relevant findings to which the cohort has contributed, and a new extensive protocol for the re-assessment of the multiplex families.
Regarding sociodemographic and clinical differences between BD and MDD rec, previous authors have also reported similar results. The respective studies found that the following features were more frequent in BD compared to MDD: (i) lower age of onset27–32; (ii) suicidal behavior31,33; (iii) psychotic symptoms30,32,34,35; (iii) hospitalization27,28,30,35; (iv) impaired social functioning28; (v) alcohol abuse27,28,31; and (vi) depressive symptoms.28 The present findings are therefore in line with data reported from investigations of non-familial BD and MDD cases. However, the present analyses could not replicate the following differences observed in previous studies: (i) more depressive episodes in BD27,30–32,34,36; (ii) shorter depressive episodes in BD,27,29,34 and (iii) more cyclothymia in BD.31
With respect of genetic findings, despite the success of GWAS, the majority of BD genetic variants still await identification, and the investigation of extended pedigrees with multiple affected family members is experiencing a true Renaissance in research into rare mutations.37–40 Sequencing efforts are currently underway to identify such rare high penetrance variants, and these efforts are being facilitated by the use of new DNA sequencing technologies. Since the completion of the human genome project in 2003, extraordinary progress in developing new sequencing technologies has been made and the availability of so-called next generation sequencing (NGS) technologies render large-scale exome (containing all protein coding sequences) or genome-wide sequencing more efficient and affordable.41 However, due to the rarity of individual mutations and the overall abundance of neutral rare variation in the genome,42 confirming a definite association with the illness is a challenging task. Besides the identification of several mutations in the same gene, the investigation of familial segregation will be an important aspect of such research attempts, and the ABiF sample will provide a highly valuable resource for this. The observation of implicated mutations in a number of relatives also opens the possibility to explore the phenotypic spectrum associated with a particular mutation. The assessment of neuropsychological functions may allow insights into underlying functional processes, such as those conceptualized in the Research Domain Criteria (RDoC),43 and may, therefore, generate hypotheses for future functional studies.
Once promising rare variants have been identified, their function can be tested in various ways, including bioinformatic analyses and the use of animal model systems such as the mouse. However, complete replication in the mouse of the complex genetic make-up of a particular patient is impossible. Here, iPSCs from these patients offer great potential.44 In a recent study, iPSCs were generated from family members of an Old Order Amish pedigree with- and without BD, and functional effects and expression changes were demonstrated.39 The acquisition of blood cells, which may later be transformed in iPSCs, is therefore an important aspect of the ABiF follow-up study. Moreover, the broad collection of biomaterials, including mRNA, proteins, and hairs will allow the investigation of functional changes at various biological levels in vivo and the exploration of such changes as potential biomarkers.
In summary, the phenotype data and the biomaterials obtained within the ABiF study represent a promising resource for future investigations into the etiology of BD.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have adhered to the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestsThe authors declare no conflict of interest.
Share the first and last author.
Please cite this article as: Guzman-Parra J, Rivas F, Strohmaier J, Forstner A, Streit F, Auburger G, et al. El estudio Andalusian Bipolar Family (ABiF): protocolo y descripción de la muestra. Rev Psiquiatr Salud Ment (Barc.). 2018;11:199–207.