Patients with cirrhosis are at risk of malnutrition and this has been recognized as a predictor of morbidity and mortality. The aim of this study was to compare the frequency of serious complications (variceal bleeding, ascites, hepatic encephalopathy and infections) between cirrhotic patients with and without malnutrition.
Subjects and methodsAn observational, analytic, cross-sectional study. The nutritional status of cirrhotic patients was evaluated according to Subjective Global Assessment (SGA). Characteristics of cirrhotic patients with and without malnutrition were compared.
Results103 cirrhotic patients were included, 58 (56.3%) were women; the media of age was 55±12.2 years. According to SGA, 45 patients (43.7%) were classified as well-nourished, and 58 (56.3%) as malnourished. The development of serious complications was related to nutritional status. Malnourished patients had higher frequency of development of ascites (67.2% vs. 42.2%, P=0.01, OR=2.8; 95% CI: 1.3–6.3) and infections (21.4% vs. 6.7%, P=0.03, OR=4.5; 95% CI: 1.2–16.6).
ConclusionsThe presence of serious complications, such as, ascites and development of bacterial and fungal infections, was more frequent between cirrhotic patients with malnutrition than in those well-nourished.
Los pacientes con cirrosis están en riesgo de malnutrición, y esta ha sido reconocida como un predictor de morbi-mortalidad. El objetivo de este estudio fue comparar la frecuencia de complicaciones serias (hemorragia variceal, ascitis, encefalopatía hepática e infecciones) entre pacientes cirróticos con y sin malnutrición.
Sujetos y MétodosEstudio observacional, analítico y transversal. El estado nutricional de los pacientes cirróticos fue evaluado de acuerdo con la Evaluación Global Subjetiva (EGS). Se compararon las características de los pacientes cirróticos con y sin malnutrición.
Resultados103 pacientes cirróticos fueron incluidos, 58 (56.3%) fueron mujeres, la media de edad fue de 55±12.2 años. De acuerdo con la EGS, 45 pacientes (43.7%) se clasificaron como bien nutridos, y 58 (56.3%) como malnutridos. El desarrollo de complicaciones serias estuvo relacionado al estado nutricional. Los pacientes malnutridos tuvieron mayor frecuencia en cuanto al desarrollo de ascitis (67.2% vs. 42.2%, P=0.01, RM=2.8; IC al 95% 1.3 a 6.3) e infecciones (21.4 vs. 6.7%, P=0.03, RM=4.5; IC al 95% 1.2 a 16.6).
ConclusionesLa presencia de complicaciones serias, como, ascitis y desarrollo de infecciones bacterianas y fúngicas fueron más frecuentes en aquellos pacientes malnutridos en comparación con los pacientes bien nutridos.
According to the first-ever World Health Organization (WHO) study of liver disease mortality, total deaths worldwide from cirrhosis and liver cancer rose by 50 million per year over 2 decades; therefore, this is a substantial contributor to global mortality.1
Compared with the general population, patients with compensated cirrhosis have a near 5-fold increased risk of death, while those with decompensated cirrhosis have a near 10-fold increased risk of death.2
Patients with chronic liver disease (CLD) are susceptible of malnutrition because of the liver plays an important role to regulate the caloric homeostasis.3 Patients with CLD are at risk of malnutrition for several causes, such as: poor dietary intake, fat malabsorption from reduced bile acid synthesis, increased intestinal protein losses, decreased hepatic protein synthesis and storage capacity, poor substrate utilization and hypermetabolism. Carbohydrate and fat metabolism are altered in cirrhosis, and there is evidence of insulin resistance. Nausea and early satiety, which may be secondary to gastroparesis, ascites, altered gut motility or bacterial overgrowth also can contribute.4
The loss of appetite can be related to the up-regulation of inflammation and appetite mediators. Tumor necrosis factor-alpha (TNF-α) and leptin correlate with satiety and energy expenditure, and these mediators are increased in patients with cirrhosis.5 TNF-α is a cytokine that may affect appetite and metabolism by acting on the central nervous system, altering the release and function of several neurotransmitters. Also, leptin is increased in patients with cirrhosis.5,6 Furthermore, patients with cirrhosis have abnormal fasting levels of ghrelin, but its relation with anorexia is unclear.
The loss of muscle mass or sarcopenia is a common complication of cirrhosis and it becomes worse with the advance of the disease, which is associated with poor quality of life.7,8 The prevalence of malnutrition in patients with CLD has been reported with variable frequencies, but as high as 90%.3,5,6,8 Malnutrition has been recognized as a predictor of morbidity and mortality in patients with CLD.5
The aim of this study was to compare the frequency of serious complications: variceal bleeding (VB), ascites, hepatic encephalopathy (HE) and infections, between cirrhotic patients with and without malnutrition.
Subjects and methodsDesign of the studyAn observational, analytic, cross-sectional study was conducted. The sample size was taken as a convenience sample size, including all those cirrhotic patients who accepted to participate in this study and who were attended between January and June 2014 at Liver Clinic from Hospital General de Mexico “Dr. Eduardo Liceaga”, Mexico City. The nutritional status of cirrhotic patients was evaluated according to Subjective Global Assessment (SGA). Characteristics of cirrhotic patients with and without malnutrition were compared. Patients with other chronic comorbidities which could affect the nutritional status, such as diabetes, chronic kidney disease, cardiac disease, neoplasms, acquired immunodeficiency syndrome, and those who did not accept to participate were excluded.
ProcedurePatients were evaluated through a complete clinical history. We collected demographic data such as, age and gender, etiology of cirrhosis, laboratory and clinical data to calculate Child-Pugh, development of manifestations of decompensated cirrhosis (ascites, VB, HE, infections). Anthropometric parameters such as, weight, height, mid-arm circumference (MAC), triceps skinfold thickness (TST), were measure according to Lohman technic,9 with these data were calculated body mass index (BMI), and ideal mid-arm muscle circumference (IMAMC) as follows:
BMI (kg/m2)=weight (kg)/height (m).2
IMAMC=MAC (cm)−(3,14×TST (cm)).10
The obtained results were compared with Frisancho's tables.10
Hand grip strength was evaluated with a dynamometer with patient seated and instructed for its use and realizing the test with the dominant hand.
SGA was performed according to the Royal Free Hospital Assessment,11,12 in order to determine the nutritional status of the patients. SGA evaluates BMI, IMAMC, and the intake of the patient, this last one is graded according to the estimated requirements as follow: adequate if cover the requirements, inadequate if not covered it but is greater than 500kcal/day, and insignificant if it is less than 500kcal/day. Patients were classified as well nourished, moderately malnourished or severely malnourished.
Cirrhosis was defined according to García-Tsao13 as compensated if there are no clinical manifestations such as VB, HE, jaundice and ascites; and as decompensated if there is development of any of the previous mentioned.
Statistical analysisThe distribution of the variables was analyzed through asymmetry, kurtosis and the Kolmogorov–Smirnov test, in case of numeric variables with a non-normal distribution, logarithmic base 10 transformation was performed in order to normalize their distribution and can analyze them by parametric tests. The numeric variables were expressed as media and standard deviation (SD). The qualitative variables were expressed as proportion and percent. To compare between groups, Student's t test or chi square test were employed as appropriate. To evaluate risk factors, odds ratio and 95% confidence intervals were calculated. Logistic regression was used for multivariate analysis. A P value≤0.05 was considered significant.
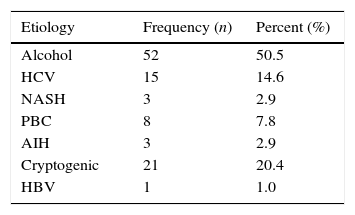
ResultsData from 103 cirrhotic patients who met the inclusion criteria were analyzed; of them, 58 (56.3%) were women and 45 (43.7%) were men. The median of age was 55±12.2 years. Regard to the etiology of cirrhosis, 52 patients (50.5%) had history of chronic alcohol intake, 21 patients (20.4%) were classified as cryptogenic cirrhosis, and 15 patients (14.6%) had cirrhosis due to chronic hepatitis C. Other etiologies are shown in Table 1.
Most frequent causes of cirrhosis in the evaluated patients.
| Etiology | Frequency (n) | Percent (%) |
|---|---|---|
| Alcohol | 52 | 50.5 |
| HCV | 15 | 14.6 |
| NASH | 3 | 2.9 |
| PBC | 8 | 7.8 |
| AIH | 3 | 2.9 |
| Cryptogenic | 21 | 20.4 |
| HBV | 1 | 1.0 |
HCV, hepatitis C virus; NASH, non-alcoholic esteatohepatitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; HBV, hepatitis B virus.
According to Child-Pugh, 59 patients (57.3%) were classified as Child-Pugh B, 24 (23.3%) as Child-Pugh C, and 20 (19.4%) as Child Pugh A.
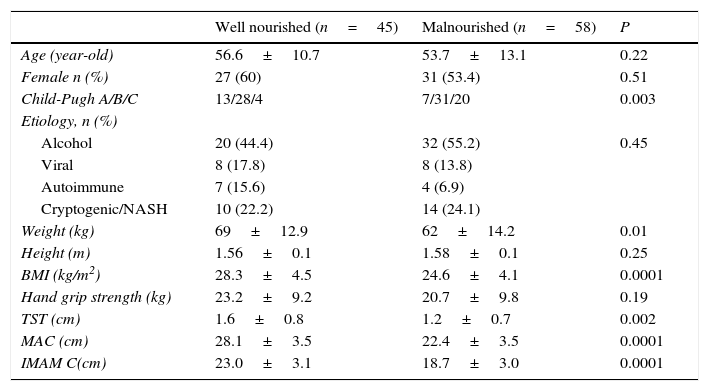
According to SGA, 56 patients (54%) had moderate malnutrition, and only two patients (1.9%) met criteria for severe malnutrition. Therefore, for purposes of the statistical analysis, we divided our patients in two groups: 45 (43.7%) well-nourished cirrhotic patients, and 58 (56.3%) malnourished cirrhotic patients. When we compared the anthropometric parameters between groups, we found differences with regard to weight (P=0.01), TST (P=0.002), BMI, MAC and IMAMC (P<0.0001). There was no difference between groups with regard to height and hand grip strength. However, when we stratified the groups according to gender, malnourished cirrhotic women had comparative lower hand grip strength than well-nourished cirrhotic women (14.5±6.6 vs. 18.3±6.5, P=0.04). There was no difference in hand grip strength between well-nourished and malnourished cirrhotic men (27.8±7.8 vs. 30.6±7.6, P=0.2).
Comparatively, in the group of malnourished patients there was greater proportion of patients classified as Child-Pugh C (P=0.003). There was no difference between groups regard to age, gender, nor etiology of cirrhosis (see Table 2).
Comparison between well-nourished and malnourished cirrhotic patients.
| Well nourished (n=45) | Malnourished (n=58) | P | |
|---|---|---|---|
| Age (year-old) | 56.6±10.7 | 53.7±13.1 | 0.22 |
| Female n (%) | 27 (60) | 31 (53.4) | 0.51 |
| Child-Pugh A/B/C | 13/28/4 | 7/31/20 | 0.003 |
| Etiology, n (%) | |||
| Alcohol | 20 (44.4) | 32 (55.2) | 0.45 |
| Viral | 8 (17.8) | 8 (13.8) | |
| Autoimmune | 7 (15.6) | 4 (6.9) | |
| Cryptogenic/NASH | 10 (22.2) | 14 (24.1) | |
| Weight (kg) | 69±12.9 | 62±14.2 | 0.01 |
| Height (m) | 1.56±0.1 | 1.58±0.1 | 0.25 |
| BMI (kg/m2) | 28.3±4.5 | 24.6±4.1 | 0.0001 |
| Hand grip strength (kg) | 23.2±9.2 | 20.7±9.8 | 0.19 |
| TST (cm) | 1.6±0.8 | 1.2±0.7 | 0.002 |
| MAC (cm) | 28.1±3.5 | 22.4±3.5 | 0.0001 |
| IMAM C(cm) | 23.0±3.1 | 18.7±3.0 | 0.0001 |
Viral, hepatitis B and C viruses; Autoimmune, primary biliary cirrhosis and autoimmune hepatitis; NASH, non-alcoholic esteatohepatitis; BMI, body mass index; TST, tricipital skinfold thickness; MAC, mid-arm circumference; IMAMC, ideal mid-arm muscle circumference.
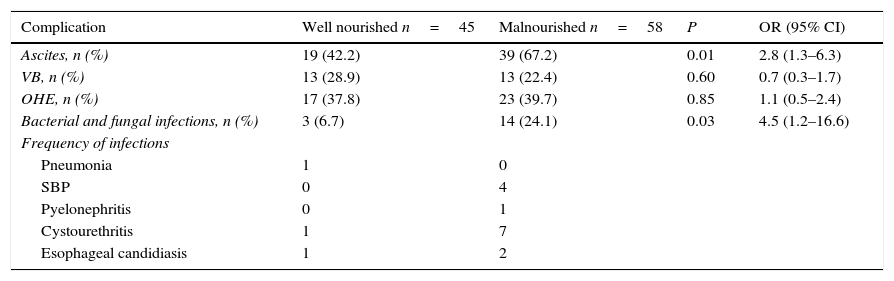
The development of serious manifestations of decompensated cirrhosis was related to nutritional status. Malnourished patients according to SGA, had higher frequency of ascites (67.2% vs. 42.2%, P=0.01, OR=2.8; 95% CI: 1.3–6.3) and infections (21.4 vs. 6.7%, P=0.03, OR=4.5; 95% CI: 1.2–16.6). There was no difference between groups regard to development of VB or HE (see Table 3).
Risk of developing complications according to nutritional status in cirrhotic patients.
| Complication | Well nourished n=45 | Malnourished n=58 | P | OR (95% CI) |
|---|---|---|---|---|
| Ascites, n (%) | 19 (42.2) | 39 (67.2) | 0.01 | 2.8 (1.3–6.3) |
| VB, n (%) | 13 (28.9) | 13 (22.4) | 0.60 | 0.7 (0.3–1.7) |
| OHE, n (%) | 17 (37.8) | 23 (39.7) | 0.85 | 1.1 (0.5–2.4) |
| Bacterial and fungal infections, n (%) | 3 (6.7) | 14 (24.1) | 0.03 | 4.5 (1.2–16.6) |
| Frequency of infections | ||||
| Pneumonia | 1 | 0 | ||
| SBP | 0 | 4 | ||
| Pyelonephritis | 0 | 1 | ||
| Cystourethritis | 1 | 7 | ||
| Esophageal candidiasis | 1 | 2 | ||
CI, confidence interval; OHE, overt hepatic encephalopathy; OR, odds ratio; SBP, spontaneous bacterial peritonitis; VB, variceal bleeding.
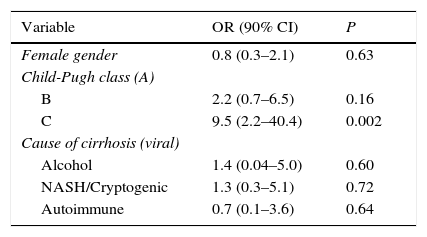
Child-Pugh class C was the only identified independent factor related to malnutrition in our patients (see Table 4).
Main factors contributing to malnutrition in cirrhotic patients: multivariate analysis.
| Variable | OR (90% CI) | P |
|---|---|---|
| Female gender | 0.8 (0.3–2.1) | 0.63 |
| Child-Pugh class (A) | ||
| B | 2.2 (0.7–6.5) | 0.16 |
| C | 9.5 (2.2–40.4) | 0.002 |
| Cause of cirrhosis (viral) | ||
| Alcohol | 1.4 (0.04–5.0) | 0.60 |
| NASH/Cryptogenic | 1.3 (0.3–5.1) | 0.72 |
| Autoimmune | 0.7 (0.1–3.6) | 0.64 |
CI, confidence interval; OR, odds ratio.
The guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN) recommend for the identification of malnutrition bedside methods, such as the SGA, or anthropometry, or measurement of handgrip strength are considered adequate; the use of composite scores has not proven to be better.14,15
Thai et al. studied the clinical utility of the SGA in Asian patients with cirrhosis. In this study the SGA was much more applicable in clinical practice and highly predictive of malnutrition in advanced cirrhosis than the standard anthropometry.16 Hasse et al. found that the SGA was an alternative test for assessing the nutritional status of adult liver-transplant candidates, and it has a good interobserver reproducibility.17 Gunsar et al. found also similar results regard to the SGA and its applicability in the clinical setting.18
Malnutrition status contributes to the decline of liver function and therefore favors the development of complications. In our study development of ascites was a serious complication associated with malnutrition, and this finding is consistent to that reported by Alvarés da Silva, who found that 65.6% of malnourished patients had greater frequency of major complications such as, ascites, spontaneous bacterial peritonitis (SBP), HE, VB or hepatorenal syndrome, compared with only 5.8% in those without malnutrition.19 Sam also found that 65% malnourished patients presented ascites.20 Similarly, Gunsar18 and Sasidharan21 reported greater frequency of ascites in those with malnutrition compared with those without it.
The presence of ascites is the most common complication of cirrhosis and also is the most frequent complication of cirrhosis that leads to hospital admission.22,23 Nearly 50% of patients with compensated cirrhosis will develop ascites during the next 10 years of follow-up.22 The development of ascites is associated with greater death risk, near to 15% of patients with ascites die in the next year and 44% die in the next 5 years.24,25 Even though our aim was not to evaluate mortality associated with malnutrition, we can infer that if malnutrition is associated to the development of ascites as manifestation of decompensated cirrhosis, then malnutrition could favor greater mortality risk in cirrhotic patients.
In our study, the development of bacterial and fungal infections was also more frequent in those with malnutrition. Malnutrition is the primary cause of immunodeficiency worldwide. There is a strong relationship between malnutrition and infection. WHO has suggested that the relationship between infection and malnutrition is a synergistic one; therefore, malnutrition can make a person more susceptible to infection, and infection by itself also contributes to malnutrition.26
The malnutrition causes severe abnormalities of the immune system. It affects both the innate and adaptive immunity.27 While cirrhosis by itself is a state of immunocompromise, cirrhotic patients are therefore susceptible to infections that increase 4-fold mortality rate in these patients.28
The relationship between immune dysfunction and infection in cirrhosis, known as cirrhosis-associated immune dysfunction syndrome, is a multifactorial state of systemic immune dysfunction, which decreases the ability to clear cytokines, bacteria, and endotoxins from circulation. The liver contains 90% of the reticuloendothelial cells, such as Kupffer and sinusoidal endothelial cells, which are central to clearing bacteria.29
The most common reported infections in cirrhotic patients are SBP (25% of infections), urinary tract infection (UTI) (20%), and pneumonia (15%), this is consistent with our findings, but we found as the most frequent UTI, followed by SBP and finally pneumonia, and also we found fungal infections as esophageal candidiasis.30
Mortality is high in cirrhotic patients complicated with bacterial infections. Baijal31 found that infected cirrhotic patients had a 23.5% mortality rate, compared with 2.2% in those non-infected at 30-day follow-up. In this study, Infections were also more frequent in those with decompensated cirrhosis, with a risk 3.04-fold greater for those classified as Child-Pugh B, and 4.17-fold greater risk for those classified as Child-Pugh C, compared with those classified as Child-Pugh A or with compensated cirrhosis. In a systematic review, Arvaniti28 reported that mortality in infected cirrhotic patients was 30.3% at 1-month, 44% at 3-month, and 63% at 1-year of follow-up; furthermore, mortality was high in those infected comparatively with those non-infected (40.4% vs. 19.5%). Although our study did not evaluate the association between mortality and malnutrition, yet it is well known that developing of ascites, and presence of infections, particularly bacterial infections, are factors related to greater mortality rate and impairment of quality of life.
The occurrence of fungal infections in cirrhotic patients is not an uncommon condition. Hassan et al. found that a history of prophylactic antibiotics, hepatorenal syndrome, low ascitic fluid protein (<1g/dL), and multiple risk factors (≥3) were independent risk factors for the development of fungal infection.32
In our study, we did not find any association between malnutrition and the development of VB or HE. However, other authors have referred higher VB as a complication of malnutrition in cirrhotic patients.18–21 Gunsar18 and Sasidharan,21 found higher prevalence of HE grades 1 and 2 in malnourished patients.
The main limitation of our study is its cross-sectional nature, which currently allows us to demonstrate a higher frequency of ascites and bacterial and fungal infections between those malnourished cirrhotic patients compared with those well-nourished; however, our study design does not allow us to establish a cause-effect relationship between the nutritional status of our patients and a higher frequency of complications. Therefore, future prospective cohort studies are necessary.
ConclusionsThe presence of serious complications of decompensated cirrhosis, such as, ascites and development of bacterial and fungal infections, was more frequent between cirrhotic patients with malnutrition than in those well-nourished.
Conflict of interestAll authors declare that they neither have any conflict of interests, nor have received any financial support for this study.