Fungal endocarditis is a low-frequency disease with a challenging diagnosis, as it can be mistaken with bacterial endocarditis. Fungal endocarditis causes higher mortality rates in immunocompromised patients. In the clinical practice, the endocarditis caused by fungi represents up to 10% of all infectious endocarditis cases and has a mortality rate of nearly 50%.
Case reportHere we present the case of a 53-year-old woman under corticosteroid therapy with a history of rheumatic heart disease, aortic valve replacement, and rheumatoid arthritis, who presented with fungal endocarditis caused by Candida albicans. Even though the patient received 3 years of antifungal prophylaxis with fluconazole, had valve replacement surgery, and received intensive care, the patient finally worsened and died.
ConclusionsComorbidities and corticosteroid therapy predisposed the patient to acquire fungal endocarditis. This case highlights the importance of implementing procedures for the isolation and identification of fungi, and for carrying out antifungal-susceptibility testing, as well as establishing surveillance programs to identify infection-causing species and drug resistance patterns in hospitals. Moreover, designing and upgrading the algorithm for infectious endocarditis is the key to future improvements in diagnosis.
La endocarditis fúngica es una enfermedad de baja incidencia cuyo diagnóstico puede ser complicado al confundirse con la endocarditis bacteriana. La endocarditis fúngica se asocia a mayor mortalidad en pacientes inmunocomprometidos. En la práctica clínica, la endocarditis fúngica representa hasta el 10% de las endocarditis infecciosas, con una mortalidad de aproximadamente el 50%.
Caso clínicoMujer de 53 años con endocarditis fúngica por Candida albicans en tratamiento con corticosteroides por antecedentes de fiebre reumática, prótesis de válvula aorta y artritis reumatoide. A pesar de 3 años de profilaxis antifúngica con fluconazol, un nuevo reemplazo valvular y cuidados intensivos, la paciente finalmente empeora y muere.
ConclusionesLas comorbilidades y la toma de corticosteroides predispusieron a la paciente a adquirir una endocarditis fúngica. Esto resalta la importancia de implementar procedimientos de aislamiento, identificación del hongo y pruebas de sensibilidad a los antifúngicos, así como establecer programas de vigilancia para identificar especies causantes de infecciones y patrones de resistencia en hospitales. Además, diseñar y actualizar el algoritmo para un mejor diagnóstico de las endocarditis infecciosas es una cuestión clave.
Fungal endocarditis is a low-frequency disease with a higher mortality rate in immunocompromised patients, and it is difficult to differentiate from bacterial endocarditis. This condition appears in patients who have valve replacement surgery, diabetes, malignancy, organ transplant, chronic kidney disease, HIV/AIDS, and immunosuppressive or long-term antifungal therapy.2,16 Also, it is well-known that the survival of patients is inversely correlated to time to diagnosis.
Among the more frequent fungal pathogens associated with nosocomial infections in this group of patients, we find Candida, Histoplasma capsulatum, or Aspergillus, among others, depending on the geographic region.16 In the clinical practice, endocarditis by fungi represents up to 10% of infectious endocarditis, with a mortality rate of ∼50%.1 Therefore, due to the wide variety of possible etiologic agents causing this infection, patients with this condition require careful management.
Case reportA 53-year-old woman was admitted to the emergency room in the 4th Area General Hospital of Instituto Mexicano del Seguro Social (IMSS) in Celaya, Guanajuato, complaining of severe ankle pain due to rheumatoid arthritis diagnosed in 2010. During the intake interview the patient mentioned having intermittent fever with a temperature of up to 38.2°C, as well as petechiae on the ankle, outer side, and sole of the left foot. However, the patient did not mention respiratory, urinary, or other symptoms related to potential infectious foci. Other than that, she looked in good health with normal skin color and hydrated mucous membranes; the blood pressure was 134/71mmHg, temperature of 37.6°C, heart rate 84bpm, respiratory rate 14 breaths per minute, and oxygen saturation 98% at room air. In addition, a rhythmic metallic click and a systolic murmur 3/6 in the aortic area, not diagnosed previously, were heard during the examination.
The patient denied suffering from diabetes or hypertension, but had been diagnosed with rheumatic heart disease at age 22. In 2005, she underwent a mitral valvuloplasty due to severe stenosis and moderate portal hypertension. An aortic valve replacement was necessary five years later. In November 2011, she was admitted to the cardiology service since there was a suspicion of post-vaccine myocarditis. The transthoracic echocardiogram showed a mass attached to the aortic prosthesis, causing severe stenosis, strongly suggestive of endocarditis. The patient started antibiotic therapy without improvement. Thus, a replacement of the aortic prosthetic valve was necessary. Candida albicans, identified by means of VITEK 2 compact system (bioMérieux, Marcy-l’Étoile, France), was isolated in a culture of the material obtained from the removed prosthetic valve, and in subsequent blood cultures as well (Fig. 1). An antifungal therapy with fluconazole was started, and the patient showed a favorable clinical response. She stayed in the Intensive Care Unit (ICU) under observation for two weeks and was discharged with ambulatory treatment including enoxaparin sodium, digoxin, omeprazole, and celecoxib. Fluconazole 100mg/day was prescribed as prophylaxis for 12 months, but the patient intermittently self-prescribed the antifungal therapy for additional 24 months. An annual medical check-up was scheduled, but no data from the following years were available.
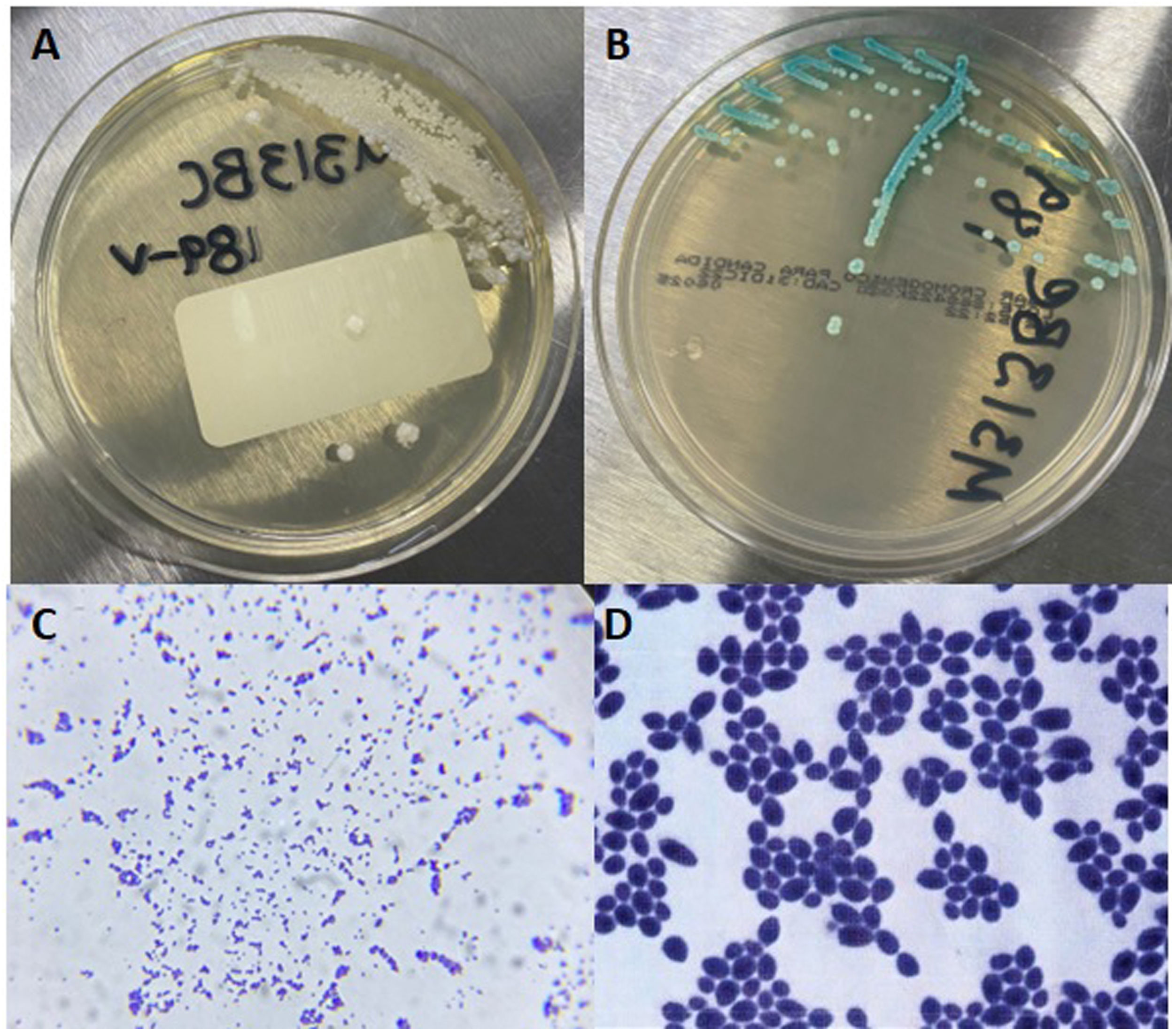
Isolation from blood culture. (A) White, creamy, smooth, glabrous, yeast-like colonies in agar Sabouraud, typical of Candida species. (B) Colonies with identical appearance showing green color in CHROMagar (Becton Dickinson; Franklin Lakes, NJ, USA), characteristic of C. albicans. (C) Gram stain observed at 100×. (D) 1000×.
In 2019 the patient was admitted in the condition explained at the beginning. Blood test results were the following: glucose 155mg/dL, sodium 135mEq/L, chloride 94mEq/L, C-reactive protein 7.3mg/dL, CK-MB 56.59ng/mL, troponin-T 2.11ng/mL, Hb 11.1g/dL, MCV 74fL, WBC 12.35×103/μL, neutrophils 8.24×103/μL, ESR 80mm/h, fibrinogen 487mg/dL. During the hospital stay, the patient had many episodes of arrhythmia due to auricular fibrillation, pain in the right hypochondrium and umbilical region, and fever (38.5°C). An abdominal ultrasound showed non-inflammatory cholelithiasis or choledocholithiasis. No hepatic or splenic abscesses were observed. Fluconazole was administered for one week without improvement.
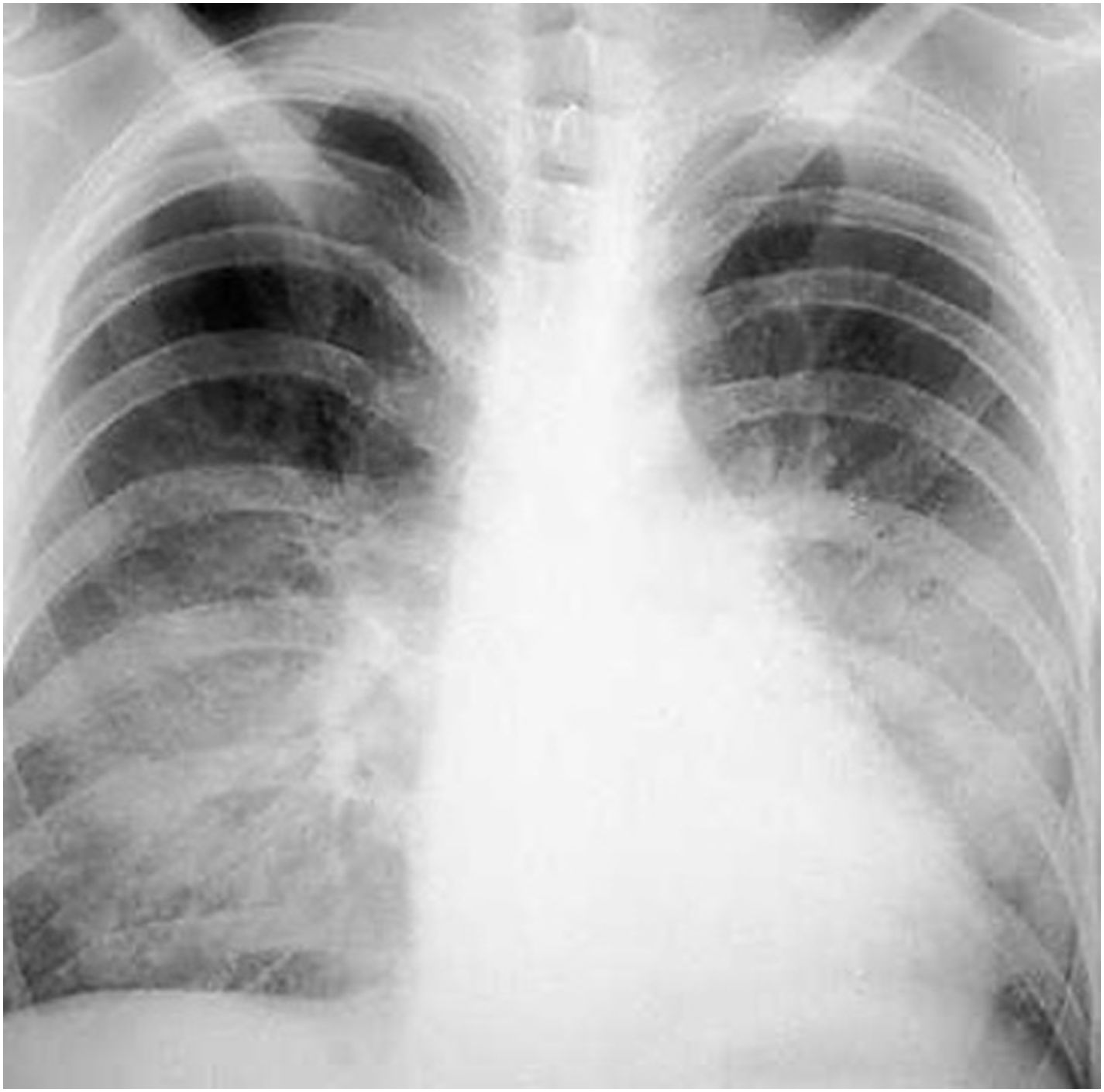
Serial blood cultures were performed at different times, and 48h after incubation positive results were reported for the three aerobic bottles. Yeasts were observed by light microscopy, and a treatment with liposomal amphotericin B was, thus, started. During this period the patient clearly worsen, and due to the clinical criteria and hemodynamic instability, she was admitted to ICU; two days later she lost consciousness and presented with gaze deviation, skin, and mucous paleness. A new intervention for prosthesis replacement was necessary, and both the catheter and the valve were sent to the laboratory for microbiological culture. Some days later the patient suffered a pulmonary infiltration with purulent secretion (Fig. 2). C. albicans was observed in a culture of a bronchial washing. The patient died in the ICU after weeks in a critical condition.
DiscussionThe aortic valve prosthesis replacement, the rheumatoid arthritis, and the corticosteroid treatment, were predisposing factors for acquiring the mycosis, as previously documented in the literature. Other conditions (e.g., uncontrolled diabetes) allows the infection by opportunistic microorganisms, such as Candida.3,7,8,11,15 However, the guidelines for differentiating between bacterial and fungal endocarditis in the clinical practice are still ambiguous. Despite the efforts for properly treating fungal endocarditis, international literature lacks consensus on the diagnosis and treatment of infectious endocarditis.
In general, the valve prosthesis replacement, together with an antifungal therapy, is the preferred practice, with the higher success for these conditions.6,9 The omission of such measures often leads to a relapse in the first year of treatment. In our case, although the valve replacement was the correct procedure,12 sustained prophylaxis with fluconazole for three years when an antifungal susceptibility test in vitro was not performed is questionable. A previous retrospective cohort study found a higher survival rate, independent of surgical intervention, when using induction monotherapy with liposomal amphotericin B in the first 30 days after the onset of the fungal endocarditis. In contrast, the study showed that long-term therapy with fluconazole did not enhance 6 month-survival rates.14 Meena et al.10 observed that monotherapy and combined antifungal treatment schemes have no significant difference in the survival of patients with fungal endocarditis. In the absence of standard therapy, it would be wise to establish closer monitoring of pathogens and supervision of the prescribed antifungal treatment.
Among the etiologies of fungal endocarditis, C. albicans is the most common. However, other Candida species (i.e., Candida glabrata) have become more common depending on the geographical region, underlying conditions, and administered antimycotics.4,5 Nevertheless, fungal resistance occurs; therefore, it is important to highlight that the surveillance and antifungal susceptibility tests are essential for detecting resistant strains and giving an effective therapy. Unfortunately, in developing countries like Mexico, just a few laboratories offer this test.13
In conclusion, antifungal resistance could become an emergent health problem worldwide. The absence of a surveillance program for resistant fungal strains in hospitals, and the poor therapeutic control in immunosuppressed patients, contribute to this outcome. Therefore, healthcare services and users could benefit from standardizing clinical practice guidelines in fungal endocarditis and carrying out antifungal susceptibility tests.
FundingThe authors declare that they did not receive any grant from public, private, or non-profit funding agencies for this research.
Conflicts of interestThe authors have no conflicts of interest to declare.