To analyze the body distribution of Erdheim-Chester disease (ECD) and determine the utility of 2-[18 F]FDG PET/CT compared to other imaging techniques. Additionally, to assess the aggressiveness and extent of the disease based on the presence/absence of the BRAFV600E mutation.
Materials and methodsThe 2-[18F]FDG-PET/CT scans of all patients diagnosed with ECD between 2008 and 2021 were reviewed, including 19 patients. The affected territories were classified as detectable by PET/CT or detectable only by other imaging techniques (bone scintigraphy, contrast-enhanced CT, or MRI). Descriptive analysis and correlation of the BRAF mutation with the affected organs and maximum SUV were performed using the Student's t-test.
ResultsOut of the 19 patients (14 males; mean age 60.3 years), 11 had the BRAFV600E mutation. A total of 127 territories (64 organ-systems) affected were identified using different imaging modalities, of which 112 were detected by PET/CT, and an additional 15 territories were solely identified by cerebral and cardiac MRI. The presence of BRAFV600E mutation was associated with greater organ involvement (p < 0.05) without differences in SUVmax (p > 0.05).
Conclusion2-[18F]FDG PET/CT is a highly effective diagnostic tool in patients with ECD, detecting the majority of affected territories. MRI was the only imaging modality with additional findings in territories showing high physiological uptake of 2-[18F]FDG (cerebral and cardiac). The presence of the BRAFV600E mutation correlated with a higher extent of the disease.
Analizar la distribución corporal de la enfermedad Erdheim-Chester (ECD) y determinar la utilidad de la 2-[18F]FDG-PET/TC frente a otras técnicas de imagen. Asimismo, evaluar la agresividad y extensión de la enfermedad según la presencia/ausencia de mutación BRAFV600E.
Material y métodosSe revisaron las 2-[18F]FDG-PET/TC de todos los pacientes diagnosticados con ECD entre 2008 y 2021, incluyendo 19 pacientes. Los territorios afectados se clasificaron como detectables por PET/TC o detectable solamente por otras técnicas de imagen (gammagrafía ósea, TC con contraste yodado o RM). Se realizó análisis descriptivo y correlación de la mutación BRAF con los órganos afectados y SUVmáx mediante la prueba t de Student.
ResultadosDe los 19 pacientes (14 hombres; edad media 60,3 años), 11 presentaban la mutación BRAFV600E. Se detectaron un total de 127 territorios (64 órgano-sistemas) afectados utilizando las diferentes modalidades de imagen, de los cuales 112 fueron detectados por la PET/TC y 15 territorios adicionales fueron identificados únicamente por la RM cerebral y cardiaca. La presencia de mutación BRAFV600E se asoció con mayor afectación orgánica (p < 0,05) sin diferencias en el SUVmáx (p > 0,05).
ConclusiónLa 2-[18F]FDG-PET/TC es una prueba de alto rendimiento diagnóstico en pacientes con ECD detectando la mayoría de los territorios afectados. La RM fue la única prueba de imagen con hallazgos adicionales en territorios con alta captación fisiológica de 2-[18F]FDG (cerebral y cardíaca). La presencia de mutación del BRAFV600E se correlacionó con mayor extensión de la enfermedad.
Erdheim-Chester Disease (ECD) is a rare systemic histiocytosis of non-Langerhans cells that is characterized by the infiltration of histiocytic cells in the stroma of several organs, producing inflammation, thickening, and an increase in density and fibrosis in these organs. Despite having first been described in the literature in 1930 by the pathologists William Chester andJ akob Erdheim, ECD continues to be a diagnostic challenge due to the diversity of clinical manifestations and the scarcity of cases published worldwide, with fewer than 1000 cases having been reported to date.1,2
Prior to the advances in understanding the disease, there was uncertainty regarding whether ECD was a benign or inflammatory disorder or if it had malignant characteristics. This is due, in part, to the difficulties in determining the cellular clonality and identifying the causative mutations. However, in 2012, there was an important finding with the discovery of recurrent activating kinase mutations and fusions that affect the mitogen-activated protein kinase (MAPK) (RAS-RAF-MEK-ERK) and lipid kinase phoshoinositide-3-kinase (PI3K-AKT) signaling pathways in a large proportion of patients with ECD.3 These discoveries provided conclusive evidence that ECD is a neoplastic clonal disorder caused by constitutive MAPK signaling in most cases and also provided one of the first objectives for molecular therapy in histiocytosis.4 As a result of this advance, the World Health Organization (WHO) included ECD in the classification of neoplasias of hematopoietic and lymphoid tissue (Table 1).2
The World Health Organization classification of neoplasia of hematopoietic and lymphoid tissue. ECD is included within the neoplasias of histiocytes and dendritic cells and related diseases.
| I. | B cell neoplasias |
| II. | T cell neoplasias |
| III. | Hodgkin lymphoma (Hodgkin disease) |
| IV. | Lymphoproliferative diseases associated with immunodeficiency |
| V. | Neoplasias of histiocytes and dendritic cells and related diseases |
| VI. | Acute leukemias and myelodysplastic syndromes |
| VII. | Chronic myeloproliferative diseases |
It is believed that histiocytic disorders originate from mononuclear phagocytic cells, such as macrophages and dendritic cells, also known as histiocytes. This category of disorders is subdivided into Langerhans cell (LCH) and non-Langerhans cell histiocytosis (NLCH). The name LCH was given due to the supposed derivation of the Langerhans cells, which are specialized dendritic cells found in the skin and the mucosa. On the other hand, it is thought that the NLCH originate from the lineage of monocytes and macrophages.5,6
Most patients with ECD present somatic mutations (that is, acquired and non-hereditary) in BRAF or other components of the signaling pathway dependent on MAPK. Recent studies have shown that approximately half of the cases of ECD have the BRAFV600 mutation, although it is likely that the incidence is greater when more sensitive techniques are used.7–9 The presence of the activated BRAF mutation leads to an increase in cell proliferation and survival by activating the RAS/RAF/MEK/MAPK signaling pathway.10 The histiocytes in ECD also present a profile of expression of proinflammatory cytokines and chemokines that contribute to the recruitment and activation of additional histiocytes.11
The clinical presentation of ECD may vary according to the extension and distribution of the organs affected. Most patients present bone compromise at diagnosis and the vast majority also show at least one organ of extraosseous involvement. It is crucial to determine the grade of involvement of ECD in each person for different reasons. Firstly, the choice of treatment is based on the phenotype and the severity of the disease, and these are influenced by the pattern of organ system involvement, especially the nervous and cardiovascular systems.12,13 Secondly, patients with ECD may present a wide range of not only generalized but also focalized symptoms.12,14 However, they often have concomitant diagnoses that may cause independent symptoms, such as autoimmune diseases15 and other neoplasias.16 Therefore, the identification of the territories affected by ECD is essential to distinguish the symptoms of infiltrative disease from those that are not attributable to ECD. Lastly, determination of the extension of ECD may influence eligibility for clinical trials and provide evaluable target lesions to assess response to treatment.
At present, there is scarce evidence of the optimal diagnostic images for characterizing the extension of ECD without treatment. However, studies in small cohorts suggest that [¹⁸F]Fluorodeoxyglucose (2-[18F]FDG) positron emission tomography/computerized tomography (PET/CT) with is an effective option for evaluating organ involvement in ECD.17–21 In fact, the last consensus of the American Society of Hematology (ASH) established that although bone scintigraphy (BS) is the most sensitive for detecting bone lesions, 2-[18F]FDG PET/CT is the preferred diagnostic test due to its capacity to evaluate extraosseous involvement.3 In addition, studies such as that by Arnaud et al. have shown that 2-[18F]FDG PET/CT is very useful in the follow-up of patients with ECD who are receiving treatment.22
Therefore, the objective of our study was double. First, we aimed to describe the distribution of corporal and multisystemic involvement in ECD and compare the images obtained by 2-[18F]FDG PET/CT with other imaging tests such as BS, CT and magnetic resonance (MR) to determine which study offers the best diagnostic performance. Second, we correlated the presence or absence of the BRAF mutation (BRAFV600E or BRAFwt) with the involvement of different organs and the intensity of uptake in the PET.
Material and methodsThis was a retrospective observational study carried out in the Department of Nuclear Medicine -PET (IDI) of the Hospital Universitario de Bellvitge, Spain. We included all the patients with a final histopathological diagnosis of ECD referred from the Department of Internal Medicine between 2008 and 2021. All had undergone a 2-[18F]FDG PET/CT study. Patients with suspicion or a previous diagnosis of ECD referred for disease staging were included as well as patients without suspicion of ECD at the time of referral to our department who were diagnosed with histiocytosis following the PET/CT study. A total of 19 patients were recruited, always evaluating the images prior to any type of treatment whenever possible.
Demographic and clinical data were collected including sex, age at the time of ECD diagnosis, the presentation of symptoms at diagnosis, diagnostic delay (from the first symptom to histopathological verification) and the presence or absence of a BRAF mutation (BRAFV600E or BRAFwt).
The images of the BS, CT with iodine contrast and MR corresponding to these patients were reviewed. The territories and organ systems affected by the disease were classified into two categories: detectable by PET/CT and detectable only by other imaging techniques (BS/CT/MR).
A descriptive analysis was made and the Student’s t test was used to compare the number of organs affected and the maximum standard uptake value (SUVmax) between the groups of patients according to the mutation in the BRAF gene.
The PET/CT images were acquired using Discovery ST and Discovery IQ (General Electric Milwaukee, USA) equipment. The patients had fasted for 4−6 hours and with glycemia levels less than 10 mMol/L, a dose of 2-[18F]FDG of 3-4 MBq/kg was administered, depending on the acquisition equipment. Following a period of 60 min, a whole body study was made from the vertex to the feet with the arms above the head whenever possible. The CT acquisition parameters were 110 keV, modulation of the amperage with a maximum of 80 mA, and a slice thickness of 3.75 mm for the Discovery ST equipment and 2.5 mm for the Discovery IQ scanner. The PET acquisition parameters were 3D, 2.5 min per field on the trunk and 1.5 min per field in the extremities with a matrix of 128 × 128 for the Discovery ST equipment and 256 × 256 for the Discovery IQ. Iterative reconstruction was used in the ST equipment and the Q Clear technology in the IQ equipment. Both scanners had EARL accreditation (EANM-Research-Ltd).
A GE Healthcare AW Server work station was used for image processing and the SUVmax value was quantified. The SUVmax was measured by semi-automatically drawing a 3D region of interest (VOI) on the lesion in the PET images corrected by attenuation.
The studies of other imaging techniques used in the diagnostic process of ECD were reviewed. In total we reviewed 13 whole body BS, 37 CT with iodine contrast (8 of the cranium, 18 thorax-abdomen, 3 of the knees, 3 of the orbits, 2 of the lower extremities, among others) and 28 MR (13 of the brain, 7 cardiac, 3 of the orbits, 2 retroperitoneal, among others).
The involvement of several organ systems was evaluated by different imaging techniques. Within each organ system, we analyzed the presence of involvement of different specific territories. The organ systems evaluated included: the musculo-skeletal system (made up of the appendicular skeleton territory, facial bones and others), cardiovascular system (cardiac and aortic involvement), nervous system (brain/cerebellum, hypophysis, spinal cord and orbits/periorbital space), respiratory system (paranasal sinus, lung/pleura), urinary system (kidneys/perinephric and retroperitoneal space), digestive system (mesentery and hepatopancreatic), lymphatic system (lymph nodes) and the integumentary system.
In addition, the correlation between the BRAF mutation, the number of organs affected and the SUVmax of the lesion with greatest uptake of the radiotracer was analyzed with the Student’s t test.
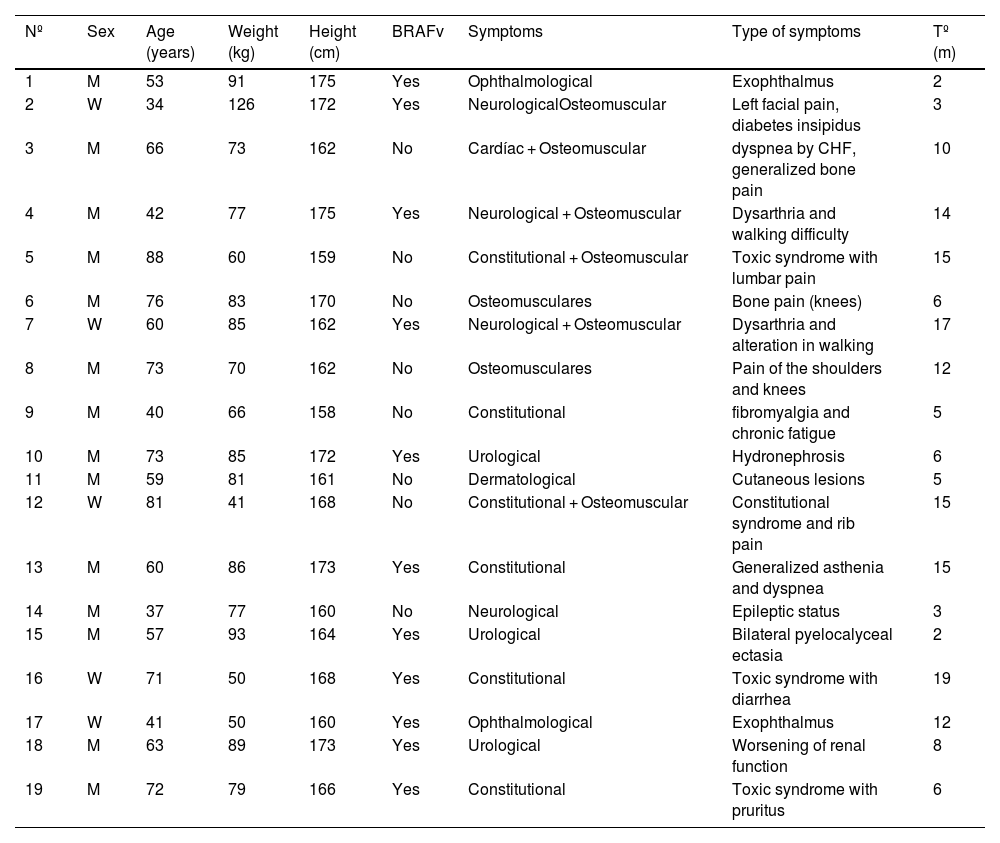
ResultsOf the 19 patients included, 14 were men (mean age: 61 years; range 37–88) and 5 were women (mean age: 57 years; range 34–81). The BRAFV600E gene was mutated in 11 patients (Table 2).
Summary of the demographic characteristics, presence of a BRAF mutation, the clinical presentation and the time to diagnosis.
| Nº | Sex | Age (years) | Weight (kg) | Height (cm) | BRAFv | Symptoms | Type of symptoms | Tº (m) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 53 | 91 | 175 | Yes | Ophthalmological | Exophthalmus | 2 |
| 2 | W | 34 | 126 | 172 | Yes | NeurologicalOsteomuscular | Left facial pain, diabetes insipidus | 3 |
| 3 | M | 66 | 73 | 162 | No | Cardíac + Osteomuscular | dyspnea by CHF, generalized bone pain | 10 |
| 4 | M | 42 | 77 | 175 | Yes | Neurological + Osteomuscular | Dysarthria and walking difficulty | 14 |
| 5 | M | 88 | 60 | 159 | No | Constitutional + Osteomuscular | Toxic syndrome with lumbar pain | 15 |
| 6 | M | 76 | 83 | 170 | No | Osteomusculares | Bone pain (knees) | 6 |
| 7 | W | 60 | 85 | 162 | Yes | Neurological + Osteomuscular | Dysarthria and alteration in walking | 17 |
| 8 | M | 73 | 70 | 162 | No | Osteomusculares | Pain of the shoulders and knees | 12 |
| 9 | M | 40 | 66 | 158 | No | Constitutional | fibromyalgia and chronic fatigue | 5 |
| 10 | M | 73 | 85 | 172 | Yes | Urological | Hydronephrosis | 6 |
| 11 | M | 59 | 81 | 161 | No | Dermatological | Cutaneous lesions | 5 |
| 12 | W | 81 | 41 | 168 | No | Constitutional + Osteomuscular | Constitutional syndrome and rib pain | 15 |
| 13 | M | 60 | 86 | 173 | Yes | Constitutional | Generalized asthenia and dyspnea | 15 |
| 14 | M | 37 | 77 | 160 | No | Neurological | Epileptic status | 3 |
| 15 | M | 57 | 93 | 164 | Yes | Urological | Bilateral pyelocalyceal ectasia | 2 |
| 16 | W | 71 | 50 | 168 | Yes | Constitutional | Toxic syndrome with diarrhea | 19 |
| 17 | W | 41 | 50 | 160 | Yes | Ophthalmological | Exophthalmus | 12 |
| 18 | M | 63 | 89 | 173 | Yes | Urological | Worsening of renal function | 8 |
| 19 | M | 72 | 79 | 166 | Yes | Constitutional | Toxic syndrome with pruritus | 6 |
Nº = patient number (the order of the patients is according to the data of histopathological diagnosis); W = women; M = man, BRAFv = presence of BRAFV600F mutation; TD = time until diagnosis (from the appearance of the first symptom until the histopathological diagnosis in months).
Among the most frequent clinical presentations, osteo-muscular pain was of note and was present in 8 patients (42%), with generalized pain and knee pain being the most common. The constitutional syndrome characterized by weight loss, anorexia, asthenia, among others, was observed in 6 patients (32%). Neurological symptoms, such as diabetes insipidus, were present in 4 patients (21%), while 3 patients (16%) presented urological symptoms. Ophthalmological symptoms were observed in 2 patients (11%) while cardiac and dermatological symptoms were presented in 1 patient each (5% in both cases).
The mean time from the appearance of the first symptom to diagnosis was 9.2 months (range: 2–19 months). The patients with the greatest diagnostic delay were those presenting unspecific symptoms, such as the constitutional syndrome, with a mean time to diagnosis of approximately one year.
Image acquisition was done using the Discovery IQ PET/CT in 13 patients and the Discovery ST in 6 patients. For the patients with initial clinical suspicion of ECD (total of 8 patients), we performed whole body 2-[18F]FDG PET/CT including the cranium and the lower extremities. In the remaining patients without initial clinical suspicion of ECD, different types of acquisitions were made: 7 patients underwent a study of the trunk (from the base of the skull to the thighs) followed by selective images of the lower extremities; in 3 patients images of only the trunk were made, and one patient underwent a study of the trunk and selective images of the cranium. In 16 cases, the PET/CT was performed before receiving any treatment for ECD, while in 3 cases it was carried out after the initiation of chemotherapy/immunotherapy but without receiving kinase inhibitors.
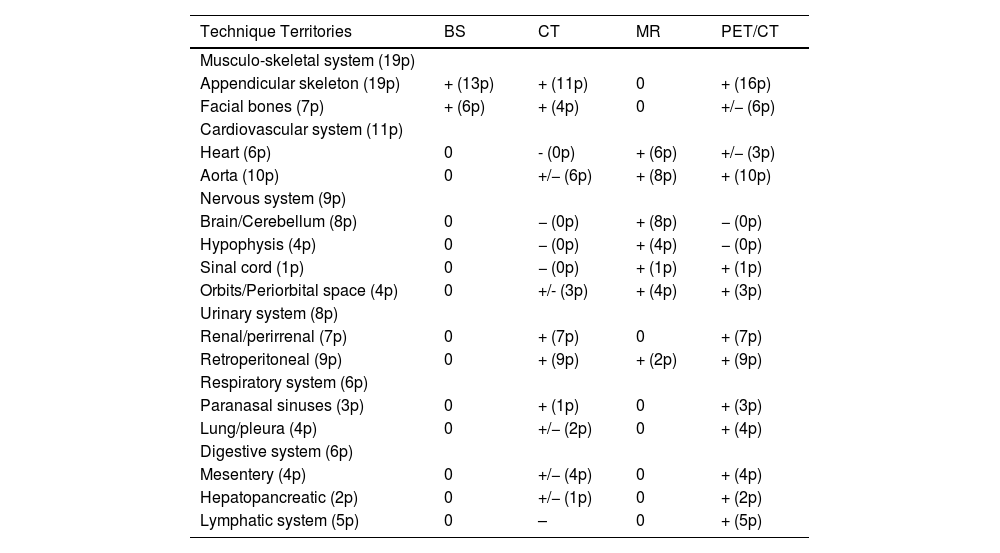
The use of 2-[18F]FDG-PET/CT detected the involvement of 112 territories (59 organ systems, corresponding to 88% of the total of 127 territories (64 organ systems) affected in all the metabolic and anatomical imaging studies. Table 3 shows the imaging techniques used during the diagnostic process and their capacity to detect some of the territories affected. The anatomical studies that provided additional information to the PET were the brain and cardiac MRs which detected 15 additional territories (5 organ systems). It is important to note that the CT with iodine contrast did not provide additional information. However, in the physical study, this study detected cutaneous involvement that was not visible in other imaging studies in 5 patients.
Capacity of the imaging techniques to detect the involvement of the different organ systems. In parenthesis we indicate the number of patients (p) with infiltration detected by the different imaging techniques.
| Technique Territories | BS | CT | MR | PET/CT |
|---|---|---|---|---|
| Musculo-skeletal system (19p) | ||||
| Appendicular skeleton (19p) | + (13p) | + (11p) | 0 | + (16p) |
| Facial bones (7p) | + (6p) | + (4p) | 0 | +/− (6p) |
| Cardiovascular system (11p) | ||||
| Heart (6p) | 0 | - (0p) | + (6p) | +/− (3p) |
| Aorta (10p) | 0 | +/− (6p) | + (8p) | + (10p) |
| Nervous system (9p) | ||||
| Brain/Cerebellum (8p) | 0 | − (0p) | + (8p) | − (0p) |
| Hypophysis (4p) | 0 | − (0p) | + (4p) | − (0p) |
| Sinal cord (1p) | 0 | − (0p) | + (1p) | + (1p) |
| Orbits/Periorbital space (4p) | 0 | +/- (3p) | + (4p) | + (3p) |
| Urinary system (8p) | ||||
| Renal/perirrenal (7p) | 0 | + (7p) | 0 | + (7p) |
| Retroperitoneal (9p) | 0 | + (9p) | + (2p) | + (9p) |
| Respiratory system (6p) | ||||
| Paranasal sinuses (3p) | 0 | + (1p) | 0 | + (3p) |
| Lung/pleura (4p) | 0 | +/− (2p) | 0 | + (4p) |
| Digestive system (6p) | ||||
| Mesentery (4p) | 0 | +/− (4p) | 0 | + (4p) |
| Hepatopancreatic (2p) | 0 | +/− (1p) | 0 | + (2p) |
| Lymphatic system (5p) | 0 | – | 0 | + (5p) |
. "+": The study was able to detect lesions in this territory. "−": The study could not detect involvement in this territory. "+/−": The study could not detect involvement in some patients. "0": This imaging study was not performed for evaluation of this territory.
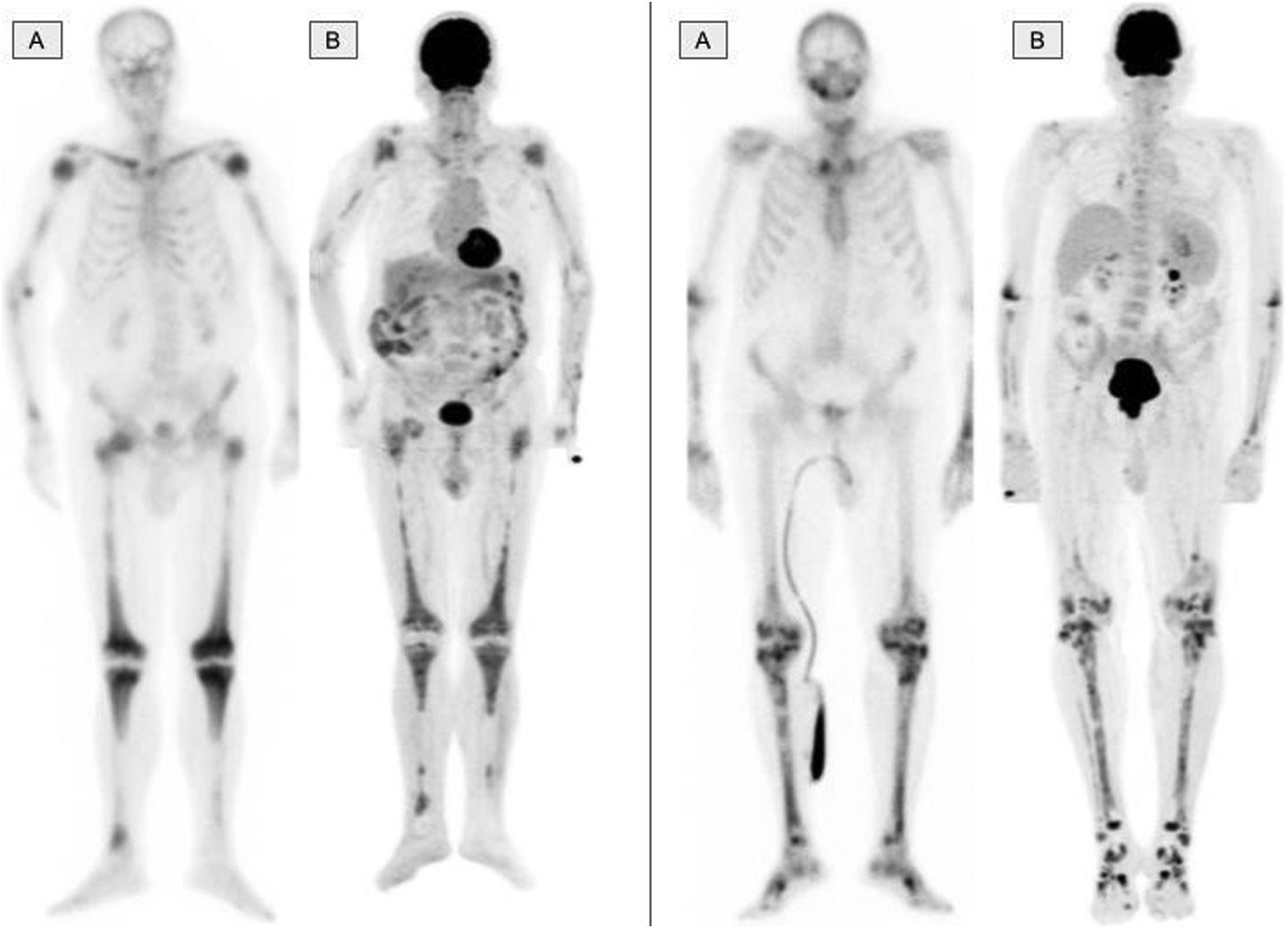
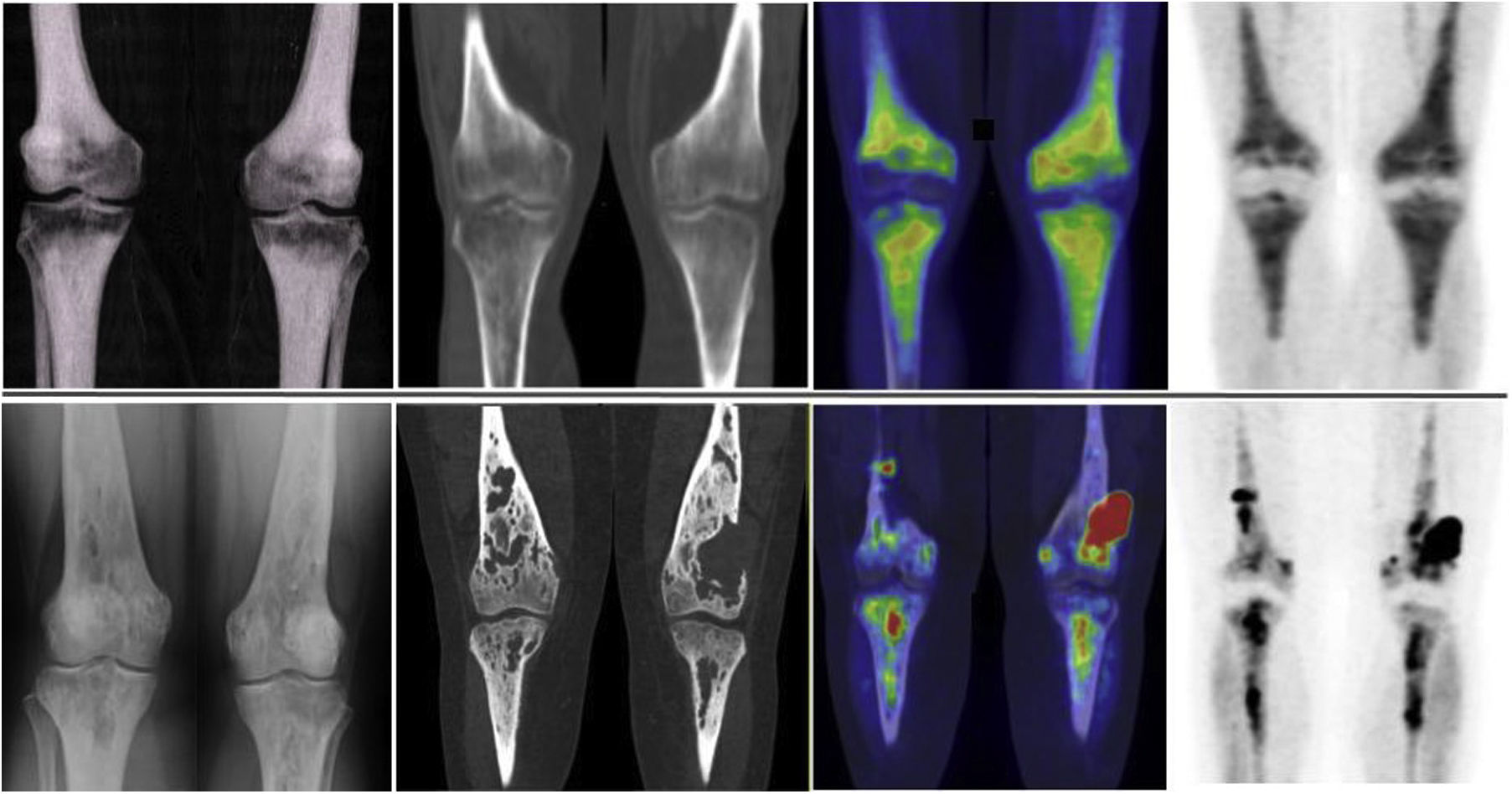
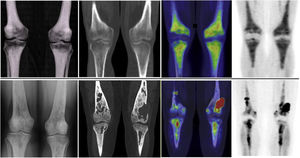
The musculo-skeletal system was affected in all the patients. A constant finding in all the patients was bilateral and symmetric involvement of the long bones of the lower extremities, while those of the upper extremities only presented involvement in 10 patients. These findings were consistent in both the BS and the 2-[18F]FDG-PET/CT, as shown in Fig. 1. The most typical and practically pathognomonic manifestation was the presence of symmetric diaphyseal and metaphyseal osteosclerosis in the femurs (distal metaphysis) and the tibias (proximal metaphysis) associated with a greater uptake of bisphosphonates in the BS and intense glycidic metabolism in the 2-[18F]FDG-PET. In advanced stages, the sclerotic lesions with homogeneous uptake evolved and aggregated lytic components resulting in a more heterogeneous uptake as shown in Fig. 2. Other bones that were frequently affected were the facial bones (Fig. 3) observed in 7 patients, specifically in the bones of the orbit, and the upper and lower jaw. In addition, lesions were detected in the cranial calotte in 2 patients, especially in the sphenoid and temporal bones, in the pelvis (iliac bones) in 4 patients and in the clavicle in 1 patient.
The bone scintigraphy findings (A) and in the maximum intensity projection (MIP) image of the 2-[18F]FDG-PET (B) in two different patients showing a similar distribution of the radiopharmaceutical at the appendicular skeletal level. The involvement of the diaphyseal-metaphyseal regions in the proximity of the knees, as shown in these two cases, is a practically pathognomonic image in patients with ECD.
The most frequent finding is bilateral hypermetabolic sclerotic involvement, which involves the diaphyseal-metaphyseal regions close to the knee. These sclerotic lesions with homogeneous uptake of the radiopharmaceutical are associated with lytic lesions and more heterogeneous uptake in advanced stages as can be seen in the image. The first line of images shows the typical involvement in initial stages and the second line shows images in more advanced stages. The images are in the following order (from left to right): simple X-ray of knees; a coronal slice at the level of the knees in the CT, fusion PET/CT and PET, respectively.
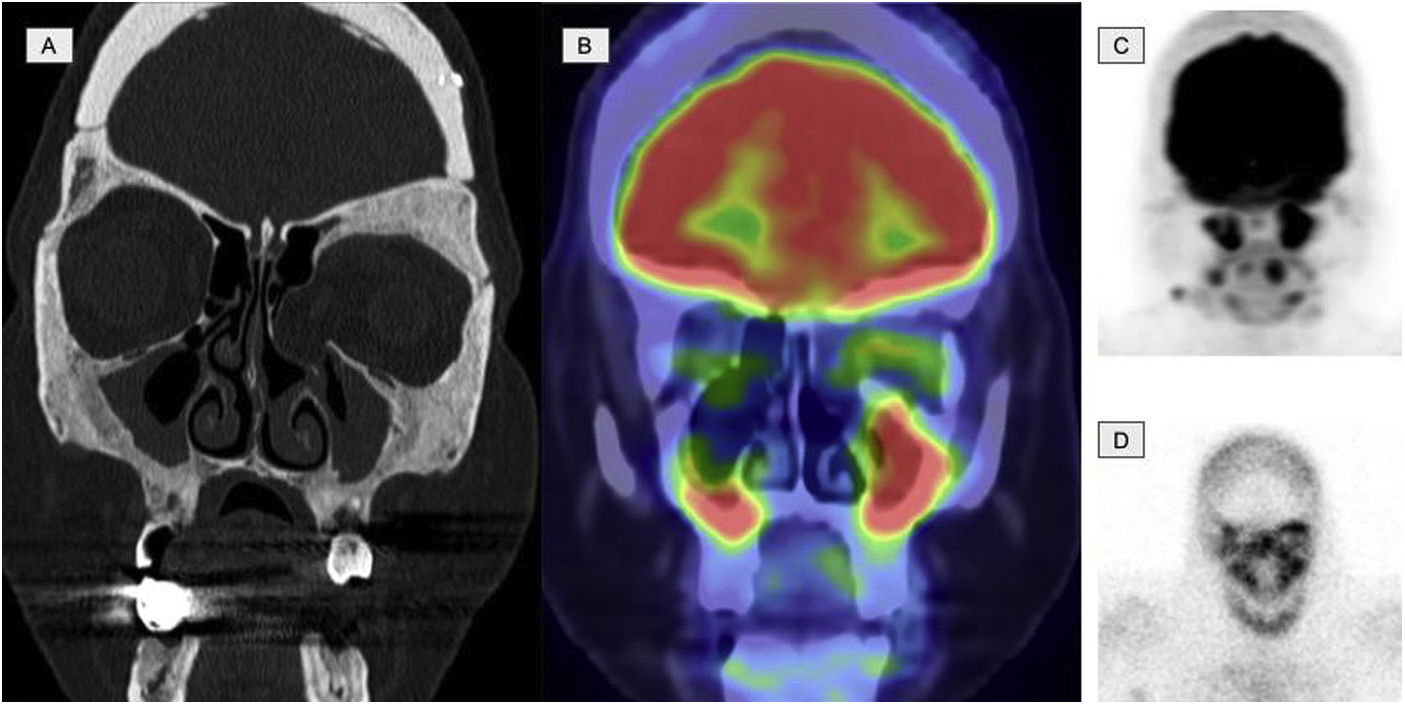
Images of a 45-year-old patient diagnosed with ECD. A. Coronal slice of the CT at the level of the facial mass showing osteosclerosis with thickening of the walls of the frontal, sphenoid and maxillary sinuses. B. Coronal slice of the 2-[18F]FDG-PET/CT showing hypermetabolism at the level of the paranasal sinuses with thickening of the mucosa. C. Maximum intensity projection (MIP) image of the cranial 2-[18F]FDG-PET showing metabolic activity at the level of the paranasal sinuses. D. Planar image of a bone scintigraphy at the cranial level showing osteogenic activity at the level of the paranasal sinuses comparable with image C.
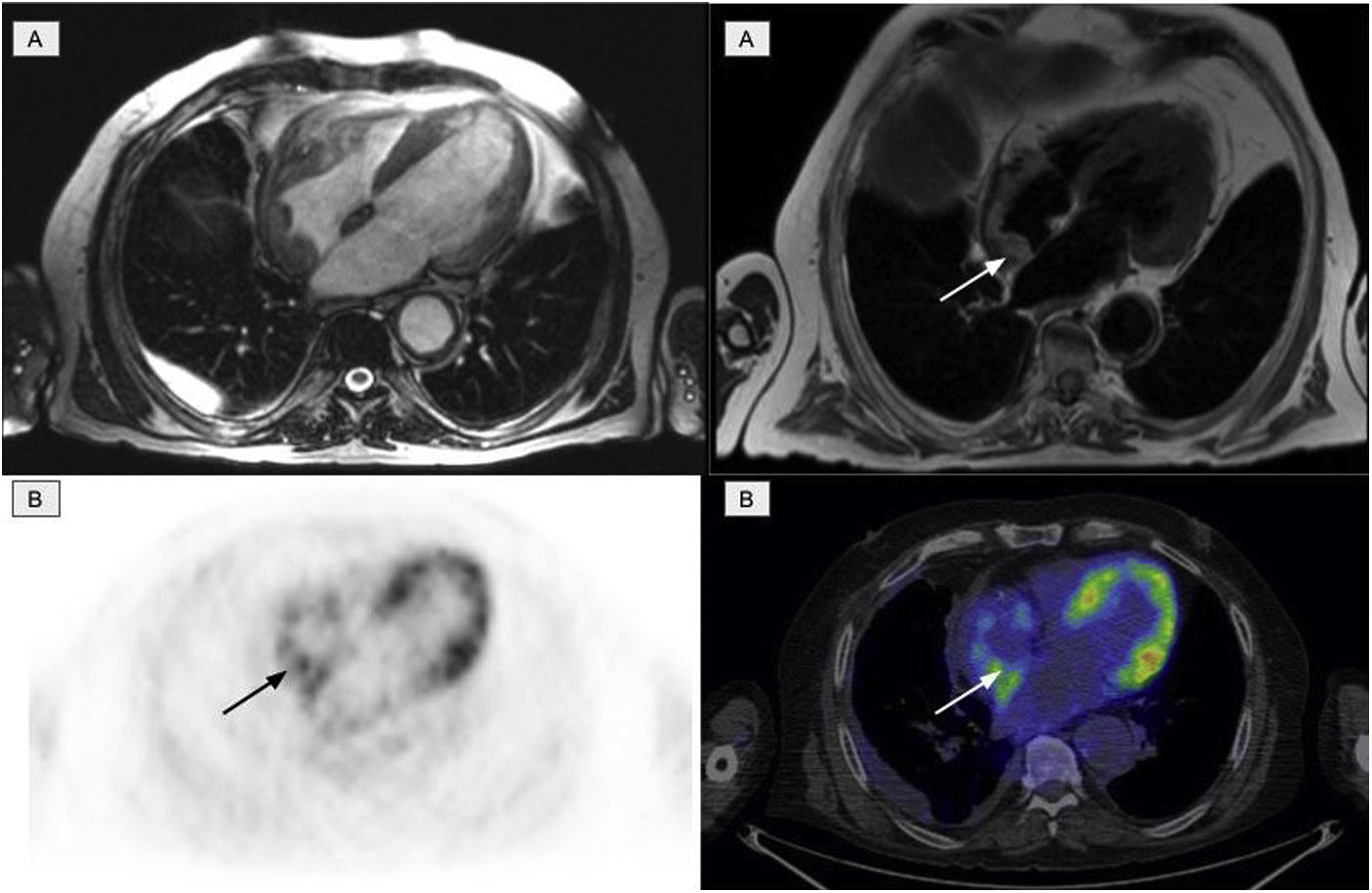
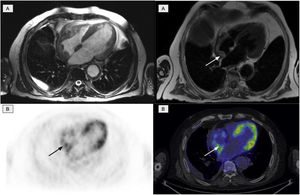
The second most affected system was the cardiovascular system, which was affected in 11 patients. Of these, 10 presented involvement of the aorta (7 patients with the thoracic aorta, 1 with abdominal aorta and 2 patients presented the involvement of both), manifested as an increase in periaortic density in the morphological images, with intense uptake of 2-[18F]FDG. Six patients with cardiac involvement were detected, visualized by PET in 3 (Fig. 4). The most frequent findings included the presence of myocardial masses/pseudotumors, mainly in the right atria/ventricles, valve diseases and infiltration of the pericardium with the presence of pericardial effusion.
Cardiac involvement in the form of a myocardial mass in the right atrium. A: cardiac MR images identifying involvement of the right atrium. B: PET/CT images showing physiological activity in the left ventricle and an increase in glycidic metabolism in the right atrium in accordance with the existing cardiac involvement.
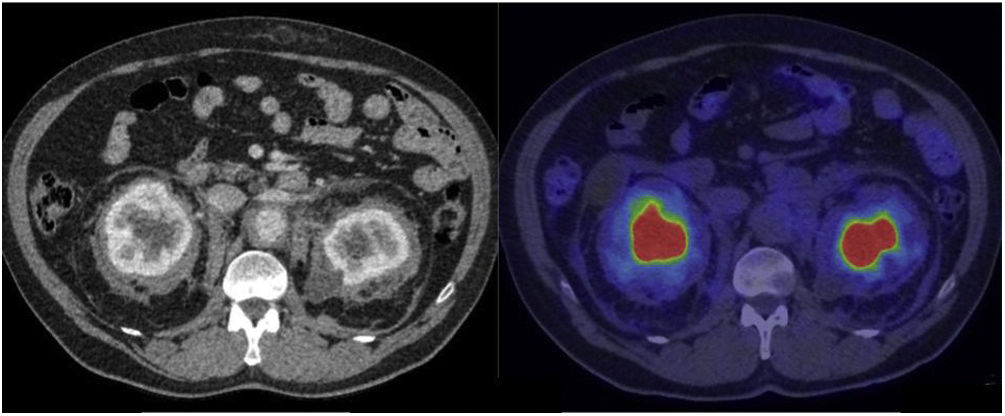
The third and fourth most affected organ systems were the nervous system in 9 patients and the urinary system in 8 patients. In relation to the nervous system and specifically the central nervous system (CNS), MR demonstrated to be superior in terms of diagnosis compared to PET. MR detected involvement of the brain hemispheres in 6 patients and of the cerebellum in 2 patients, while involvement of the hypophysis was detected in 4 patients. Involvement of the spinal cord was detected by both PET and MR in 1 patient. Involvement of the orbit was observed in 4 patients presenting retrobulbar masses detected by PET in 3 patients and edema of the optic nerve only detected by MR in 1 patient. In regard to the urinary system, involvement of the retroperitoneum (without including the abdominal aorta) was observed in the form of fibrosis that included the proximal ureters, causing obstruction and pyelocalyceal dilation in several patients, requiring derivation of the urinary pathway in most of the cases. Renal/perinephric involvement was observed in 7 patients, 4 of whom presented an image characteristic of a hairy kidney, manifested as symmetric, bilateral and irregular tissue infiltration of the fat of the perinephric space. In the CT, these lesions are shown as bands of spiked, hypodense and homogeneous edges, with variable 2-[18F]FDG uptake (Fig. 5).
CT images with contrast and 2-[18F]FDG-PET/CT showing renal/pararenal involvement, with visualization of the hairy kidney sign. This sign refers to the ring of soft tissue of perinephric infiltration observed in the axial imaging studies in ECD and is considered pathognomonic of this disease. The description of “hairy” refers to the associated thickening of the perinephric septa (Kunin septa), which are bands of fibrous tissue that extend between the renal capsule and the perinephric fascia. The metabolic activity in these alterations is variable and even scarce, such as in the case shown here.
The fifth, sixth and seventh positions include the respiratory, digestive and integumentary systems, with 6 patients each. Within the respiratory system, the paranasal sinuses are included, being involved in 3 patients, presenting thickening of the mucosa with elevated metabolic activity. Involvement of the pleura and the lungs was observed in 4 patients, presenting as thickening of the pleura and fissures (generally bilateral and symmetric), pleural effusion, thickening of the interlobar septa, the presence of small centrilobular nodules, parenchymatous consolidations and ground glass opacities. The uptake of 2-[18F]FDG varied in these lesions, with pleural uptake in all the cases, while the micronodular patterns and the thickening of the septa occasionally did not show metabolic activity. In relation to the digestive system, the involvement was mainly due to the infiltration of the mesenteric fat, presenting an image similar to that of mesenteric panniculitis. This manifestation was characterized by an increase of density or infiltration by soft tissue in the adipose tissue of the mesentery with elevated metabolic activity. On the other hand, cutaneous involvement was detected on physical exploration, with xanthelasma, mainly of the eyelids as well as multifocal papulonodular lesions, which were not visible in the PET.
Evaluation of the lymphatic system was more complicated by PET, due to the need to perform a differential diagnosis between reactive and infiltrative adenopathies. However, involvement was identified in 5 patients.
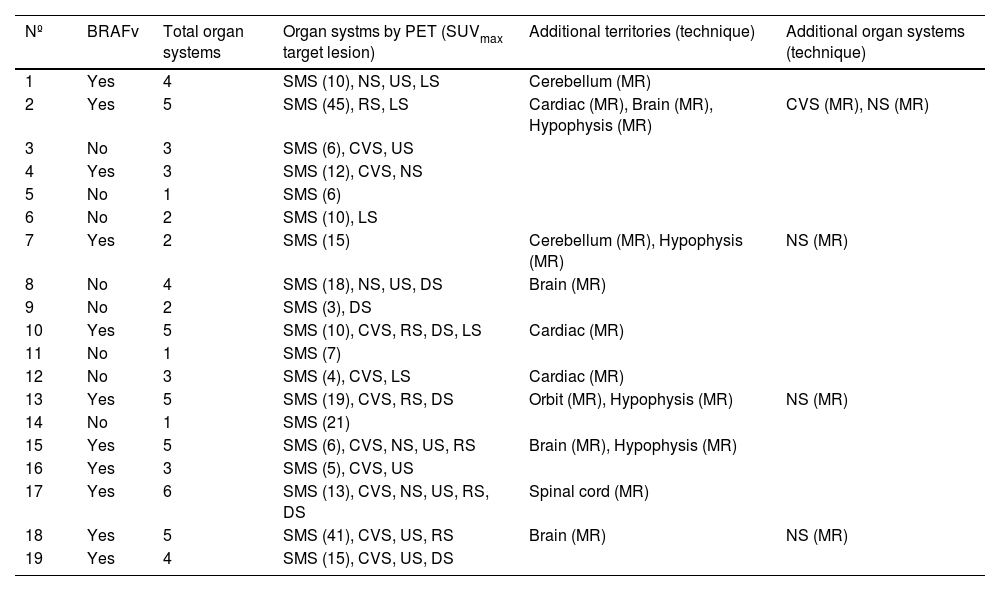
Table 4 shows the correlation between the BRAF mutation and the different organ systems affected together with the SUVmax value of the radiopharmaceutical in the lesion with greatest affinity for the radiopharmaceutical. In the 11 patients with the BRAFV600E mutation, a mean of 4.27 organ systems were affected (standard deviation [SD] +/− 1.19) with a mean SUVmax of 17.5 (SD +/− 13.5). In addition, 81% of these patients presented involvement of the cardiovascular system and 64% of the nervous system. On the other hand, the 8 patients with a BRAFwt mutation had a mean of 2.13 organ systems affected (SD +/− 1.13) and a mean SUVmax of 9.4 (SD +/− 6.6). In this group, 25% presented involvement of the cardiovascular system and only 13% presented nervous system involvement. The Student’s t test was used to compare the number of organs affected between these two groups and showed statistically significant differences with a p value of 0.001 (95% confidence interval [CI]: 1.01–3.29). However, the Student’s t test to compare the SUVmax values between the two groups showed no statistical differences, with a p value of 0.1388 (95% CI: −2.90−19.05).
Correlation of the BRAFV600E mutation with the organ systems affected (detected by PET or other techniques) and the SUVmax of the organ system with greatest avidity for the radiopharmaceutical, in parenthesis.
| Nº | BRAFv | Total organ systems | Organ systms by PET (SUVmax target lesion) | Additional territories (technique) | Additional organ systems (technique) |
|---|---|---|---|---|---|
| 1 | Yes | 4 | SMS (10), NS, US, LS | Cerebellum (MR) | |
| 2 | Yes | 5 | SMS (45), RS, LS | Cardiac (MR), Brain (MR), Hypophysis (MR) | CVS (MR), NS (MR) |
| 3 | No | 3 | SMS (6), CVS, US | ||
| 4 | Yes | 3 | SMS (12), CVS, NS | ||
| 5 | No | 1 | SMS (6) | ||
| 6 | No | 2 | SMS (10), LS | ||
| 7 | Yes | 2 | SMS (15) | Cerebellum (MR), Hypophysis (MR) | NS (MR) |
| 8 | No | 4 | SMS (18), NS, US, DS | Brain (MR) | |
| 9 | No | 2 | SMS (3), DS | ||
| 10 | Yes | 5 | SMS (10), CVS, RS, DS, LS | Cardiac (MR) | |
| 11 | No | 1 | SMS (7) | ||
| 12 | No | 3 | SMS (4), CVS, LS | Cardiac (MR) | |
| 13 | Yes | 5 | SMS (19), CVS, RS, DS | Orbit (MR), Hypophysis (MR) | NS (MR) |
| 14 | No | 1 | SMS (21) | ||
| 15 | Yes | 5 | SMS (6), CVS, NS, US, RS | Brain (MR), Hypophysis (MR) | |
| 16 | Yes | 3 | SMS (5), CVS, US | ||
| 17 | Yes | 6 | SMS (13), CVS, NS, US, RS, DS | Spinal cord (MR) | |
| 18 | Yes | 5 | SMS (41), CVS, US, RS | Brain (MR) | NS (MR) |
| 19 | Yes | 4 | SMS (15), CVS, US, DS |
BRAFv = presence of BRAFV600E mutation. SMS = Musculo-skeletal system, CVS = cardiovascular system, NS = nervous system, RS = respiratory system, US = urinary system, DS = digestive system, LS = lymphatic system; MR: magnetic resonance.
Erdheim-Chester disease is a rare disease that may present a wide variety of clinical manifestations depending on the extension and distribution of the organs affected. According to the last consensus of ECD by the ASH in 2021,3 a whole body study with 2-[18F]FDG-PET/CT is recommended in all cases suspected of ECD. This imaging technique has a high sensitivity for detecting the extension of bone and extraosseous disease in the same study.
According to the literature available, bone involvement is observed in more than 95% of the cases of patients with ECD,3 although pain of the lower extremities is present in close to 50% of the cases.23 These statistics coincide with the findings of the present series, which confirmed bone involvement in all the patients with 42% presenting pain in these areas. Bilateral and symmetric, poorly defined osteoblastic lesions of the metaphyseal-diaphyseal bones around the knees are practically pathognomonic of ECD and were consistently identified in all of our patients in whom evaluation of the lower extremities was included in both the BS and 2-[18F]FDG-PET/CT. Therefore, in cases with initial clinical suspicion of ECD, it is necessary to perform a whole body PET/CT study including the lower extremities because of its great capacity to detect subtle alterations and this study can direct biopsies with greater accuracy.24 Nonetheless, in the case of diagnosis of the disease after performing 2-[18F]FDG-PET/CT with a protocol of the trunk (from the base of the skull to the upper third of the lower extremities) which did not include acquisition of the lower extremities, a whole body study with BS can be considered to determine the extension of bone disease, given the concordance observed in our series.
Involvement of the axial skeleton is detected In approximately half of the patients,8 overall as infiltration of the facial mass in concordance with our results (47%). All of the bone structures should, therefore, be evaluated with caution, especially in the facial and cranial region, which can sometimes be difficult due to physiological cerebral and inflammatory sinus activity.
The identification of infiltration of the cardiovascular and CNS is of crucial importance since it is associated with a greater risk of morbidity and mortality.20 The physiological activity of these systems with elevated glucose consumption hinders evaluation by 2-[18F]FDG-PET/CT. Thus, the study with greatest diagnostic performance in both territories is usually MR, as suggested by our results in which the only test that surpassed PET was MR at the cerebral and cardiac level.
The most frequent cardiovascular involvement is aortic, which appeared in between 46% and 62% of the patients,14 and may be present in any branch as well as the main branches of the aorta. This involvement is normally asymptomatic and does not involve severe complications.23 On the other hand, in a non-negligible percentage of patients cardiac involvement may appear in any of the layers,9 the most frequent being infiltration of the pericardium with pericardial effusion. These alterations in the PET/CT image are visible even without myocardial suppression, while visualization of involvement of the remaining cardiac layers requires adequate preparation of the patient with prolonged fasting, a diet rich in fatty acids and low in carbohydrates and even the administration of sodium heparin prior to administering 2-[18F]FDG. Another frequent and characteristic finding is the presence of a pseudotumor at the level of the right atrium (as in the patient shown in Fig. 4), followed by infiltration of the atrioventricular sulcus. Cohen-Aubart et al. described a significant association between BRAFV600E and the presence of atrial pseudotumor in patients with ECD,14 as well as an increased risk of cardiac and aortic infiltration, and our results corroborate this. Thus, in patients with a BRAFV600E mutation, it is a priority to perform cardiac evaluation with MR and myocardial suppression for the 2-[18F]FDG-PET study.
Brain metabolism only depends on an energetic substrate: glucose, and thus, cerebral uptake of 2-[18F]FDG is intense and it is not possible to suppress the uptake. ECD has a preference for the brain parenchyma of the posterior fossa and the spinal cord,25 with the two generating symptoms such as cognitive deterioration, dementia cerebellar ataxia, loss of hearing, headache and peripheral neuropathy.23 However, it can also be associated with involvement of the hypophysis, the dura mater, the facial sinuses and the orbit. In the latter case, the most common symptomatology is exophthalmos, which is often bilateral due to infiltration of the soft tissues of the retroorbital space.14,23 PET/CT can detect involvement of the facial sinuses, the orbits and retroorbital spaces, although it is recommended to complete the study with MR or PET/MR, if there is clinical suspicion of CNS involvement. As occurred with cardiac involvement, our series showed an association between the presence of a BRAFV600E mutation and involvement of the CNS. This fact reinforces the greater risk of aggressiveness in patients with ECD that present this mutation.14,15
It is interesting to note that in addition to observing involvement of organs of great risk - that is, the brain and heart or both - in our series the patients with BRAFV600E showed greater disease extension, with these findings being statistically significant compared to patients with BRAFwt. Therefore, the findings of the 2-[18F]FDG PET/CT allowed the identification of patients in whom evaluation of the BRAF gene (mutated or not mutated) is obligatory since they may benefit from targeted molecular therapies.
Despite 2-[18F]FDG PET/CT providing the best diagnostic method for delimiting the extension of the disease, it is fundamental to evaluate the morphologic component of the CT in the hybrid study, especially at the pulmonary and renal level. Although the sign of hairy kidneys is one of the most frequent and characteristic in patients with ECD,21,26 it does not always show an increase in metabolism as occurred in some of our patients. On the other hand, in the case of patients with retroperitoneal or urinary system involvement, it is essential to identify the patients who might evolve to hydronephrosis16,23,27 since they may require the more or less urgent placement of a urinary catheter (double J).
Among the limitations of our study it is important to mention that it was a retrospective observational study. The lack of clinical suspicion of ECD in some patients may have led to variability in image acquisition, such as the non-inclusion of the extremities or the lack of myocardial suppression. These factors may induce bias in the evaluation of disease extension at not only the level of the extremities but also at the cardiac level. Another important bias is due to the acquisition of the studies in two different types of equipment with different algorithms of reconstruction, which condition the variability of the SUVmax values evaluated. In addition, it should be taken into account that in 3 patients the 2-[18F]FDG-PET/CT was performed after having initiated treatment and the treatment may have altered the distribution, especially the intensity of 2-[18F]FDG uptake in the affected tissues. Prospective controlled studies are needed to confirm and amplify our findings.
As future perspectives, in addition to increasing the availability of PET/CT, PET/MR equipment are being introduced, which allow simultaneous acquisition of anatomical images of high spatial and temporal resolution, together with metabolic images. We believe that this technique will, in a single study, improve the evaluation of the extension of the disease, especially in relation to cardiac and CNS involvement.
On the other hand, knowledge of the BRAFV600E mutation together with adequate determination of disease extension by PET, allows the selection of patients with greater risk, and thus, the use of personalized therapies with a positive impact on the prognosis and the response to treatment of patients with ECD.
In conclusion, the imaging technique with the greatest diagnostic performance in patients with ECD is 2-[18F]FDG PET/CT. Acquisition of whole body images (from the vertex to the feet) is crucial to adequately evaluate the extension of the disease at both the bone level as well as extraosseous areas. It is important to assess not only the metabolic component (PET) but also the morphological component (CT) of the images since the findings may sometimes have limited glucose translation or be close to organs of high glycidic metabolism, such as in orbital and facial areas. Taking into account that high cerebral and cardiac metabolic activity may hinder detection of the disease in these localizations, adequate preparation with myocardial suppression is fundamental and careful evaluation of both territories is of vital importance due to their implication in the prognosis of the patients. If there is suspicion of involvement in these areas that is not clearly defined with PET/CT, it is recommended to perform cardiac and/or cerebral MR. In relation to the BRAF phenotypes in ECD, it can be concluded that the presence of this mutation is associated with a greater extension of the disease identified by FDG-PET/CT as well as greater involvement of organs of risk such as the brain and heart. This suggests that patients with this mutation have a more aggressive disease.
Conflict of interestThe authors declare that they have no conflict of interest.
FinancingThe authors received no financial support for the research, authorship, and/or publication of this article.






![The bone scintigraphy findings (A) and in the maximum intensity projection (MIP) image of the 2-[18F]FDG-PET (B) in two different patients showing a similar distribution of the radiopharmaceutical at the appendicular skeletal level. The involvement of the diaphyseal-metaphyseal regions in the proximity of the knees, as shown in these two cases, is a practically pathognomonic image in patients with ECD. The bone scintigraphy findings (A) and in the maximum intensity projection (MIP) image of the 2-[18F]FDG-PET (B) in two different patients showing a similar distribution of the radiopharmaceutical at the appendicular skeletal level. The involvement of the diaphyseal-metaphyseal regions in the proximity of the knees, as shown in these two cases, is a practically pathognomonic image in patients with ECD.](https://static.elsevier.es/multimedia/22538089/0000004300000001/v1_202401120524/S2253808923000800/v1_202401120524/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Images of a 45-year-old patient diagnosed with ECD. A. Coronal slice of the CT at the level of the facial mass showing osteosclerosis with thickening of the walls of the frontal, sphenoid and maxillary sinuses. B. Coronal slice of the 2-[18F]FDG-PET/CT showing hypermetabolism at the level of the paranasal sinuses with thickening of the mucosa. C. Maximum intensity projection (MIP) image of the cranial 2-[18F]FDG-PET showing metabolic activity at the level of the paranasal sinuses. D. Planar image of a bone scintigraphy at the cranial level showing osteogenic activity at the level of the paranasal sinuses comparable with image C. Images of a 45-year-old patient diagnosed with ECD. A. Coronal slice of the CT at the level of the facial mass showing osteosclerosis with thickening of the walls of the frontal, sphenoid and maxillary sinuses. B. Coronal slice of the 2-[18F]FDG-PET/CT showing hypermetabolism at the level of the paranasal sinuses with thickening of the mucosa. C. Maximum intensity projection (MIP) image of the cranial 2-[18F]FDG-PET showing metabolic activity at the level of the paranasal sinuses. D. Planar image of a bone scintigraphy at the cranial level showing osteogenic activity at the level of the paranasal sinuses comparable with image C.](https://static.elsevier.es/multimedia/22538089/0000004300000001/v1_202401120524/S2253808923000800/v1_202401120524/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![CT images with contrast and 2-[18F]FDG-PET/CT showing renal/pararenal involvement, with visualization of the hairy kidney sign. This sign refers to the ring of soft tissue of perinephric infiltration observed in the axial imaging studies in ECD and is considered pathognomonic of this disease. The description of “hairy” refers to the associated thickening of the perinephric septa (Kunin septa), which are bands of fibrous tissue that extend between the renal capsule and the perinephric fascia. The metabolic activity in these alterations is variable and even scarce, such as in the case shown here. CT images with contrast and 2-[18F]FDG-PET/CT showing renal/pararenal involvement, with visualization of the hairy kidney sign. This sign refers to the ring of soft tissue of perinephric infiltration observed in the axial imaging studies in ECD and is considered pathognomonic of this disease. The description of “hairy” refers to the associated thickening of the perinephric septa (Kunin septa), which are bands of fibrous tissue that extend between the renal capsule and the perinephric fascia. The metabolic activity in these alterations is variable and even scarce, such as in the case shown here.](https://static.elsevier.es/multimedia/22538089/0000004300000001/v1_202401120524/S2253808923000800/v1_202401120524/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

