Evaluate the therapy impact of initial staging in patients diagnosed with prostate cancer by 18F-Choline PET/MRI hybrid technique.
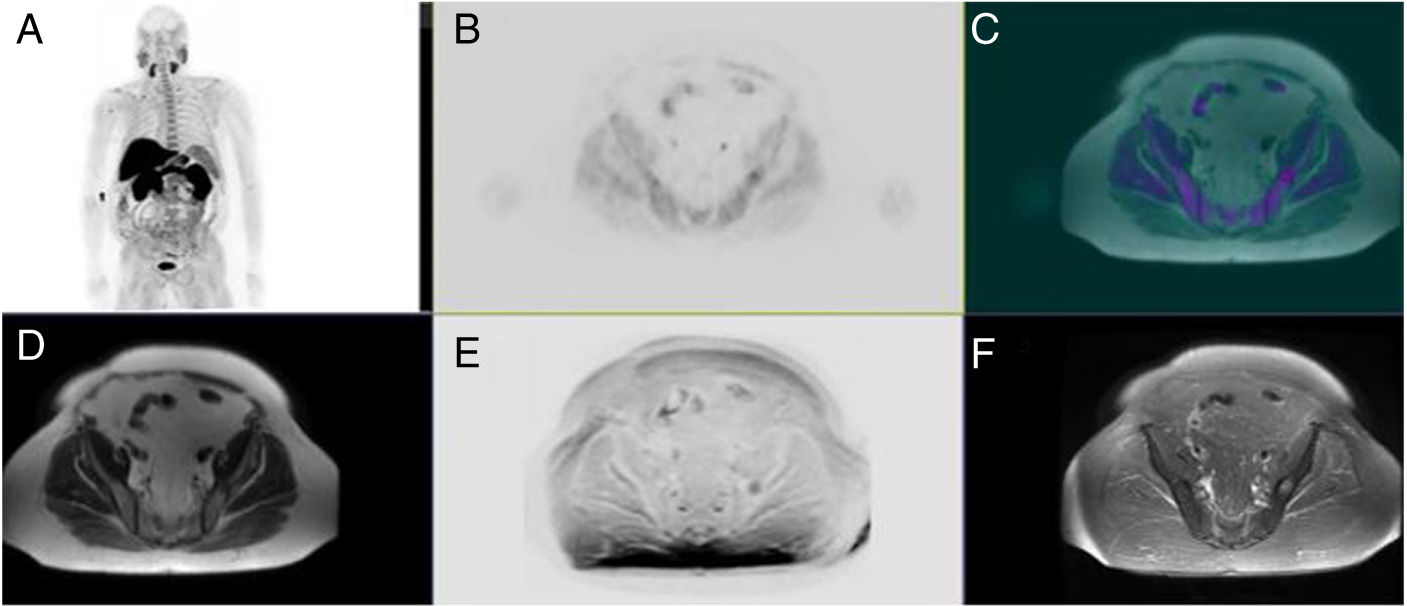
MaterialA prospective study which included 31 patients diagnosed with prostate cancer; Gleason >7; mean PSA 13.6 ng/mL (range 6.3–20.6) PET/MRI studies were acquired simultaneously with hybrid equipment (SIGNA.3T, GE) following intravenous injection of 185 ± 18.5 MBq of 18F-Choline:
- Early/prostate imaging: PET emission + Multiparametric MR: DIXON-T1-T2-diffusion-gadolinium.
- Late/whole-body imaging: PET emission + MR: DIXON-T1-T2-diffusion-STIR sequences. Images were visually evaluated. SUV & ADC & Textures were also calculated. Treatment selection was based upon Oncology Committee consensus decision.
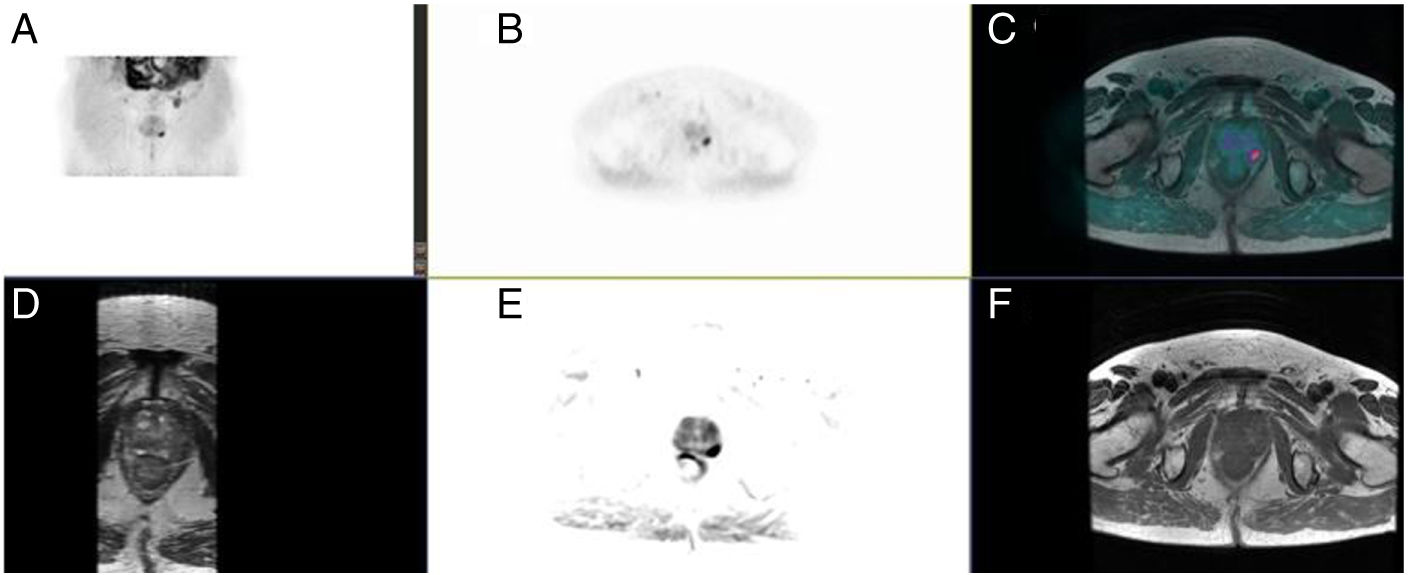
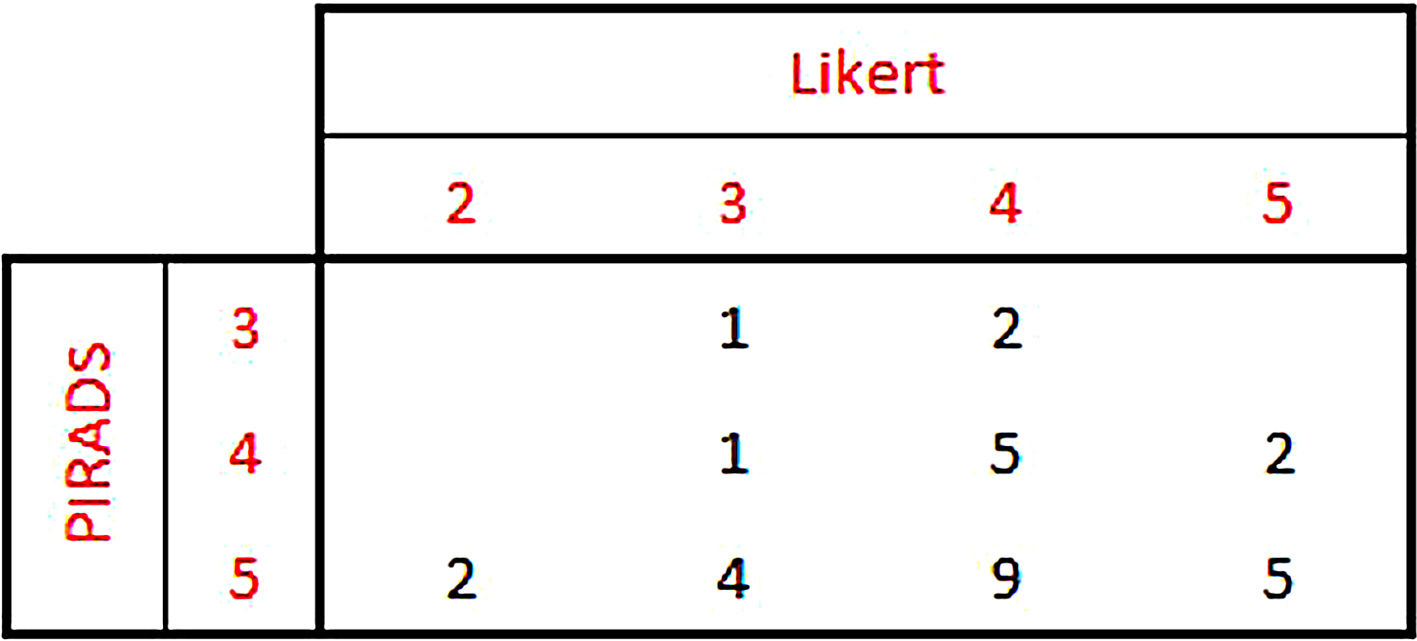
ResultsProcedure was well tolerated in all patients, and no artefacts were reported. MRI was superior in T staging in 8 patients (25.8%) (Likert: 2–3), whereas PET increased MRI sensitivity in 3 patients (9.7%) (PIRADS: 3).
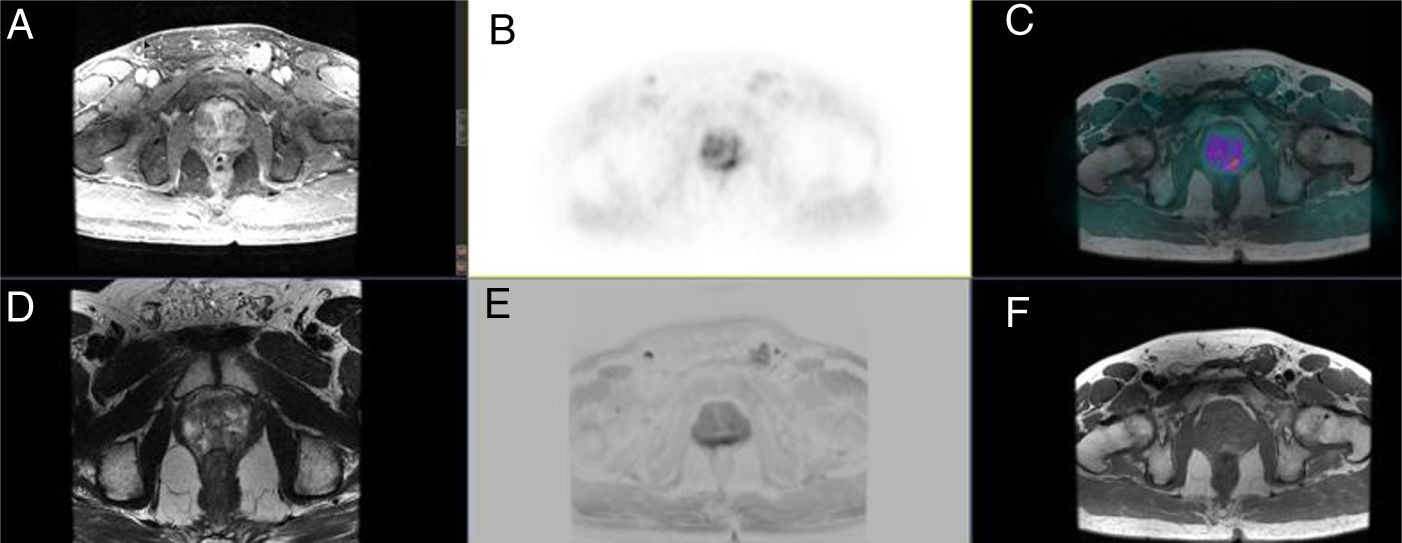
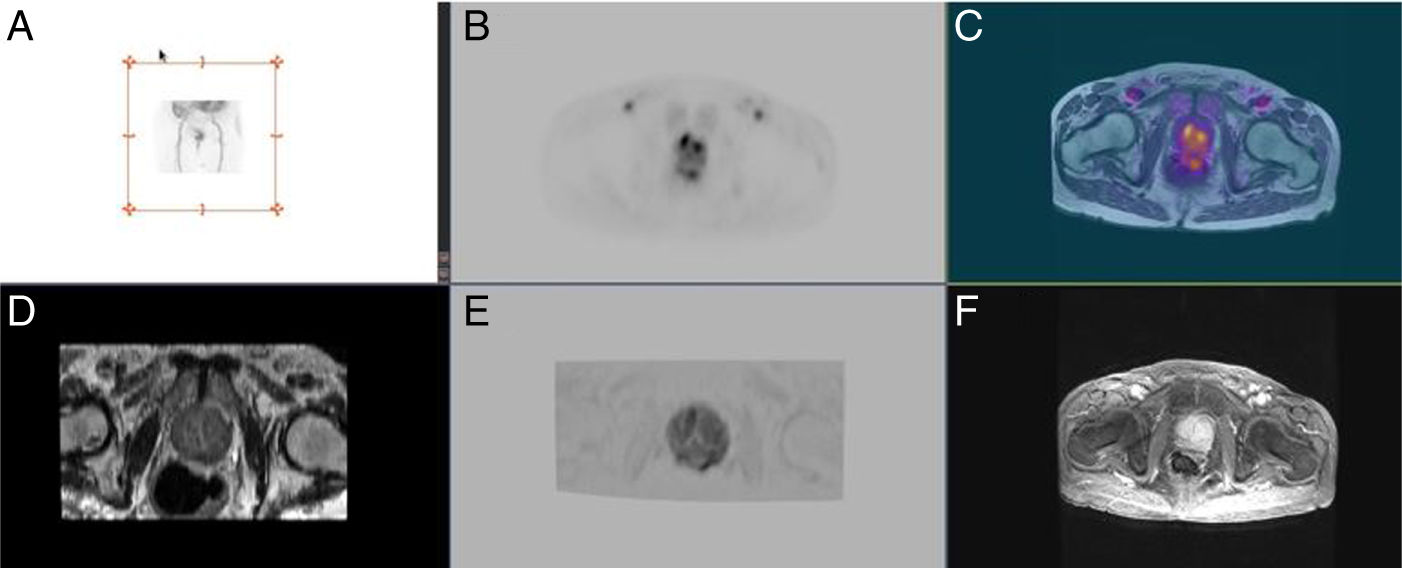
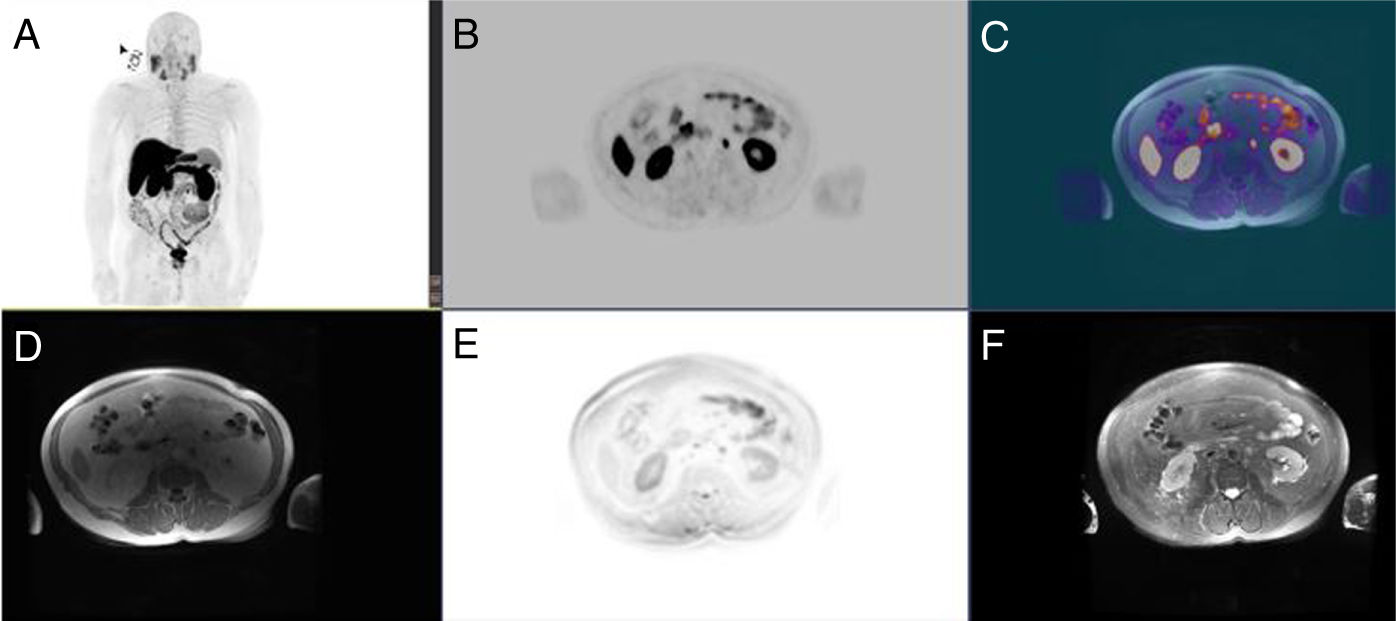
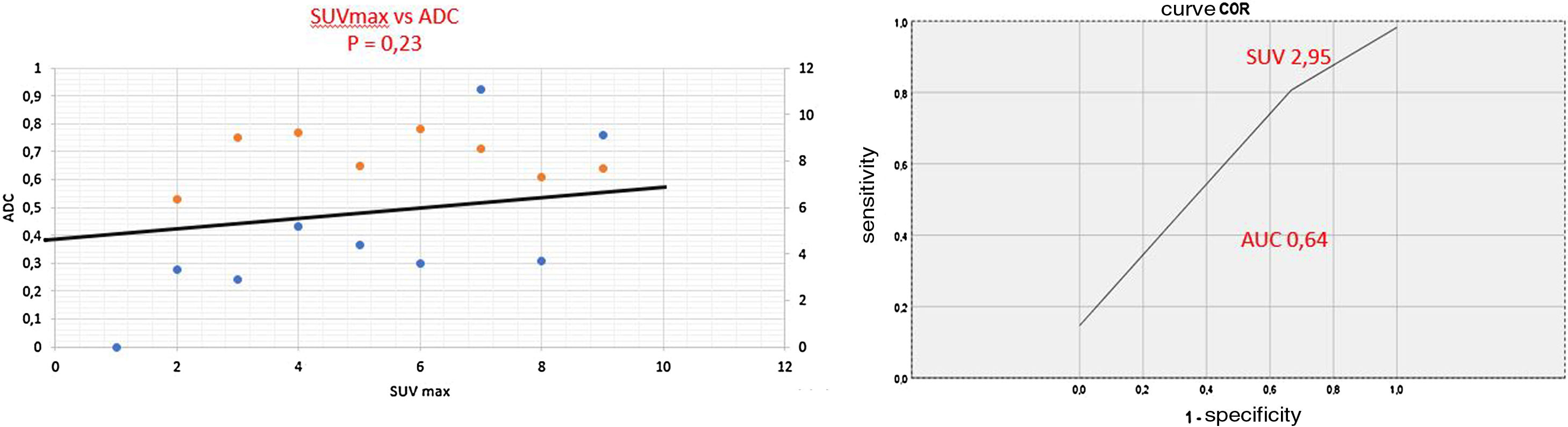
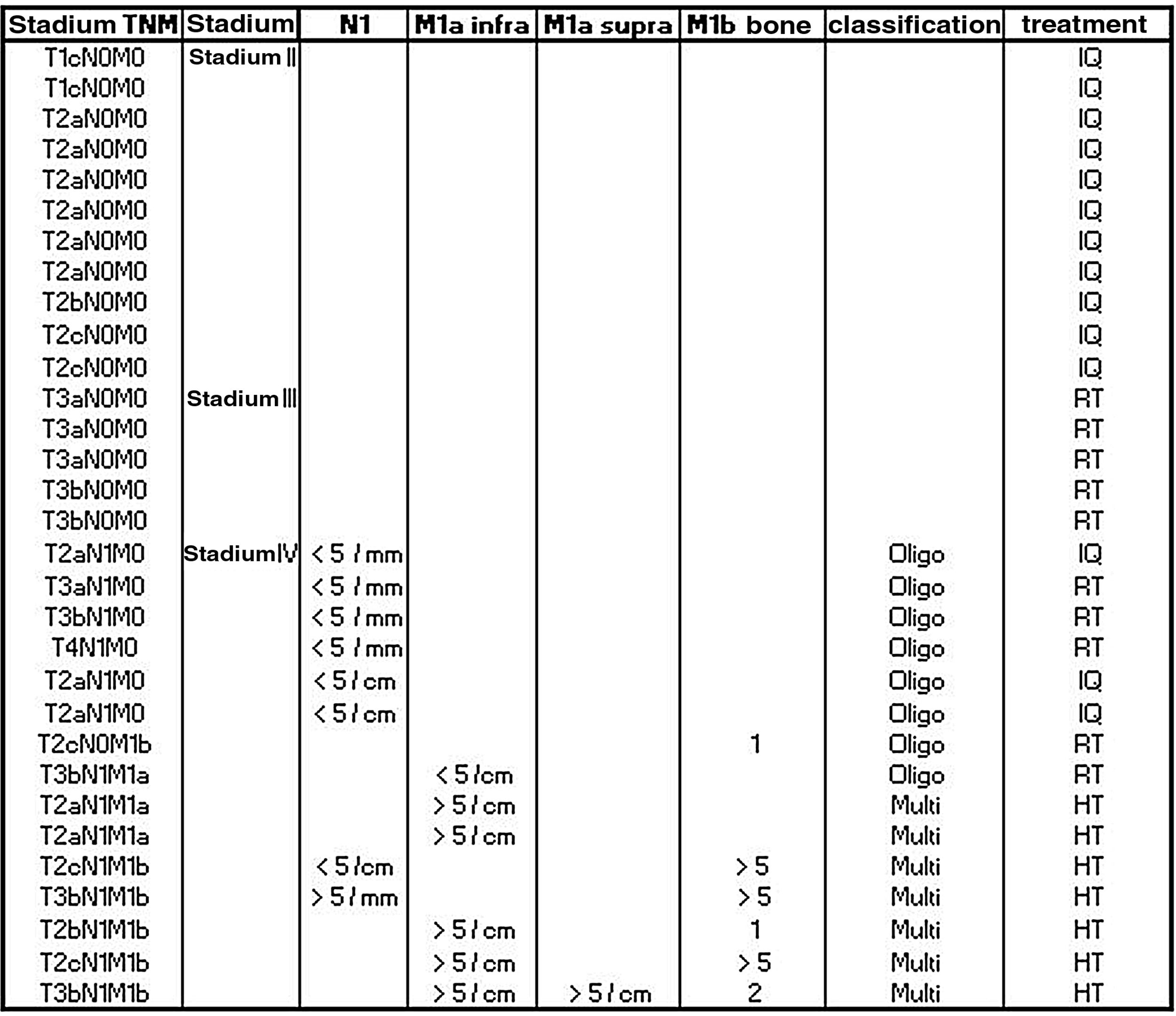
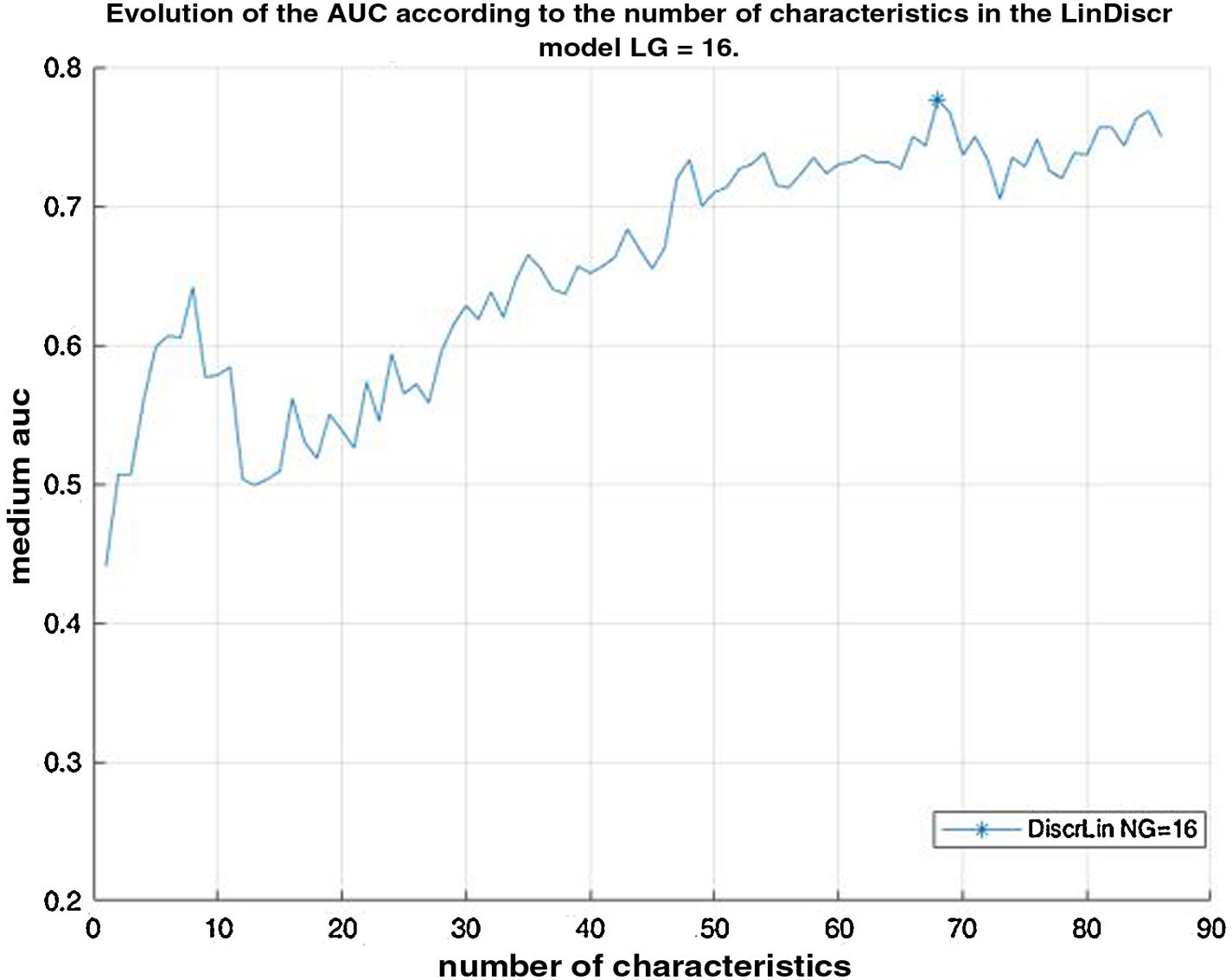
Prostate lesion locationPeripheral 91.4%, transitional 8.6%. SUVmax threshold: 2.95: sensitivity 92.9%, specificity 66.7%. No correlation SUV vs ADC. Radiomics: Better distinctiion between stage T2 vs T3 using the DiscrLin model with NG = 16 (AUC 0,7767 ± 0,3386). 68 variables, more frequently PET than T2 (0.588 vs 0.412) Seventeen patients (54.8%) were staged ≥ T3, with surgical treatment being contraindicated. Fifteen patients (48.4%) presented with extra-prostatic disease: 8/31 oligometastatic and 7/31 multiple metastasis. Therapy approach following PET/MRI was: radical treatment in 24/31 patients (77.4%): 14 radical prostatectomy and 10 MRI-guided radiotherapy; systemic treatment in 7/31 patients (22.6%).
Conclusion18F-Choline PET/MRI had a complementary role for the T staging, with a high detection rate for NM infiltration. PET/MRI findings allowed patients to be directed either to prostatectomy or MRI-guided radiotherapy, and thus avoiding radical treatment in 22.6% of patients.
Evaluar el impacto terapéutico de la estadificación inicial de pacientes con cáncer de próstata mediante la exploración integrada 18F-Colina PET/RM.
MaterialEstudio prospectivo de 31 pacientes diagnosticados de cáncer de próstata: Gleason >7; PSA: 13,6 ng/mL (6,3–20,6). Adquisición simultánea en cámara PET/RM tras la administración de 185 ± 18,5 MBq de 18F-Colina:
- Fase precoz/prostática (1-bed): emisión PET + RM multiparamétrica: DIXON-T1-T2- difusión-Gadolinio.
- Fase tardía/cuerpo completo: emisión PET + RM: DIXON-T1-T2-difusión-STIR. Interpretación visual y cálculo de SUV & ADC & Texturas. Selección del tratamiento en comité oncológico.
ResultadosTodos los pacientes toleraron la exploración, sin artefactos. En 8 pacientes (25,8%) la RM fue superior en la estadificación T (Likert:2–3), mientras que la PET aumento la sensibilidad de la RM en 3 pacientes (9,7%) (PIRADS:3).
Localización TZona periférica 91,4%, transicional 8,6%. Umbral SUVmax 2,95: sensibilidad 92,9%, especificidad 66,7%. Sin correlación SUV vs ADC. Mejor distinción estadio T2 vs T3 mediante el modelo DiscrLin con NG = 16 (AUC 0,7767 ± 0,3386). 68 variables, con mayor frecuencia PET que T2 (0.588 vs 0.412) En 17 pacientes (54,8%) la estadificación T fue ≥ T3, contraindicando la opción quirúrgica. En 15 pacientes (48,4%) presentaron enfermedad extra-prostática: oligometástasis (8/31), multimetástasis (7/31). La decisión terapéutica tras PET/RM fue: 77,4% tratamiento radical (24/31), prostatectomía en 14 y radioterapia radioguiada en 10; 22,6% tratamiento sistémico (7/31).
ConclusiónLa 18F-Colina PET/RM muestra un carácter complementario en la estadificación T, con elevada tasa de detección de la infiltración NM. Los hallazgos de la exploración permiten la selección de los candidatos a prostatectomía o radioterapia radioguiada, evitando una terapia radical en un 22,6% de los pacientes.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)