Chikungunya virus infection has become a public health problem, due to its immediate effect on the health and quality of life of patients and their families, as well as complications in the medium and long term. Its necessary to determine immunological characteristics of this afection as an important step for the future development of strategies to reduce its incidence and aggressiveness.
ObjectiveTo characterize immunologically a population from Colombian Caribbean with serologic and clinic diagnosis of chikungunya virus infection.
Material and methodsA descriptive, longitudinal, and prospective study was conducted on in 109 patients with a clinical diagnosis and serological confirmation of chikungunya virus infection and attended in the emergency department of the Fundación Hospital Universitario Metropolitano and the Orthopaedic and Rheumatology Centre. Immunoglobulin G or M type antibodies against Chikungunya virus were determined in a peripheral blood sample using immuno-enzymatic serological and immunological test in order to diagnose rheumatic diseases.
ResultsTests were positive for immunoglobulin G type antibodies against chikungunya virus in all of the 109 patients. The results for anti-cyclic citrullinated peptide antibodies, rheumatoid factor, antinuclear, and anti-DNA antibodies were negative in almost all of the 109 patients.
ConclusionPatients were not in a viral replication process that characterizes the acute phase of the disease. There were no positive results in laboratory test related to rheumatic diseases. High concentrations of certain pro-inflammatory interleukins were found in patients, and the clinical manifestations in these, suggest an inflammatory joint process with severe arthralgia that can mimic the symptoms of a rheumatic disease.
La infección por el virus chikungunya se ha convertido en un problema de salud pública, tanto por su afección inmediata sobre la salud y calidad de vida de los pacientes y sus familias, como por las complicaciones a medio y largo plazos. Es necesario llevar a cabo la caracterización inmunológica de esta afección, como paso importante para el futuro desarrollo de estrategias tendentes a disminuir su incidencia y agresividad.
ObjetivoCaracterizar inmunológicamente una población del Caribe colombiano con diagnóstico clínico y serológico de infección por el virus chikungunya.
Material y métodosEstudio descriptivo, longitudinal, prospectivo, en 109 pacientes con diagnóstico clínico y confirmación serológica de infección por el virus chikungunya, atendidos en el Servicio de Emergencias de la Fundación Hospital Universitario Metropolitano y en la consulta externa del Centro de Reumatología y Ortopedia. A partir de la toma de muestra de sangre periférica, se determinaron anticuerpos tipo inmunoglobulina G o M contra el virus chikungunya, por ensayo inmunoenzimático y pruebas de serología inmunológicas, orientadas al diagnóstico de enfermedades reumatológicas.
ResultadosSe obtuvieron resultados positivos para anticuerpos tipo inmunoglobulina G contra el virus chikungunya en 109 pacientes. Los resultados para anticuerpos antipéptido cíclico citrulinado, factor reumatoide, anticuerpos antinucleares, anticuerpo anti-ADN, fueron negativos en la mayoría de los sujetos.
ConclusiónLos pacientes no se encontraban en un proceso de replicación viral, característico de la fase aguda de la enfermedad. No hubo resultados positivos en las pruebas de laboratorio relacionadas con enfermedades reumatológicas. Las concentraciones altas halladas son sugerentes de un proceso inflamatorio articular con artralgias severas, que puede mimetizar las condiciones de una enfermedad reumatológica.
The chikungunya virus infection (CHIKV) is acquired by the bite of the female mosquito Aedes aegypti or Aedes albopictus.1–3 The virus enters the subcutaneous capillaries, initiating its replication in the macrophages of the dermis, fibroblasts and endothelial cells, from where it is transported to the lymph nodes adjacent to the inoculum, and it spreads by the circulation to tissues and organs such as the liver, muscles and joints.4,5
Most infected patients have acute, subacute and chronic manifestations, although between 3 and 12% are asymptomatic.6,7 During the silent incubation period (2–4 days), gets involved the innate immune system,8–11 and later the specific immune response12 which, in the majority of cases, accomplishes an initial sweep of the virus.13,14
During the initial inflammatory response, the interferon 1α, when it binds to its cell surface receptor, produces the activation of tyrosine kinases, which lead to the production of several enzymes that inhibit the replication of the virus in the infected cells, which have escaped the action of the natural killer cells (NK).15–17
The viral nucleic acid induces the secretion of interferon 1β which, when it joins its cell surface receptor, releases enzymes that slow down the protein synthesis, inhibiting the transduction of the viral RNA and thus degrading the viral messenger RNA. The production of interferon γ is mediated by T lymphocytes activated by antigens that are presented to it; this interferon acts on NK cells, macrophages, and T and B lymphocytes, modifying the production of antibodies. Subsequently, the specific immune response develops, with release of cytokines that attract leukocytes to the site of viral replication.18–20
Meanwhile, the cellular immunity is mediated by cytotoxic T lymphocytes (CTL CD8+), responsible for neutralizing and destroying the infected cells, through the release of enzymes, previous binding of the CD8+T membrane receptor, with the major histocompatibility complex I (HLA I) on the surface of the infected cells.21
The CD4+T (ThL) lymphocyte response, is based on the release of specific interleukines (IL). The Th1 cells produce: IL2, interferon gamma (INFγ), tumor necrosis factor (TNFα), with proinflammatory characteristics. The Th2 cells, secrete IL4, IL5, IL6 and IL10 and promote the production of antibodies mediated by B lymphocytes.22,23
During the acute phase of the CHIKV infection, there is an elevation of interferon alpha (INFα), mediated by IL-1, IL-2 and TNFα. IFNα is produced by T lymphocytes, B lymphocytes, macrophages, fibroblasts, endothelial cells, NK cells and osteoblasts, among others; its antiviral effects include the inhibition of viral replication, and the activation of macrophages and NK cells.
The effects of INFα are potentiated by INFγ, once it is secreted by the activated Th1 cells. The symptoms such as muscle pain and fever are related to the production of interferons.24–26
Considering that the immune response regulates the control of the disease, this will depend on the underlying immune situation in each patient, such as the presence of a premorbid rheumatic state (rheumatoid arthritis).27–29
In the regional environment, so far, there have not been found works showing the immunological characterization of the patients infected with CHIKV, and therefore arose the need to carry out this work, with the purpose of analyzing their immune response, relating the cases of more severe arthralgia as the result of a defect in the defense mechanisms.22,21,30,31
Materials and methodsA descriptive, longitudinal and prospective study conducted from October 2014 to August 2015 in 109 patients with clinical and serological diagnosis of CHIKV infection, aged between 22 and 82 years, treated in the Fundación Hospital Universitario Metropolitano (FHUM) and in the Orthopedic and Rheumatologic Center of the city of Barranquilla. The peripheral blood samples were obtained from the subjects after the onset of symptoms, in an average time of 49 days, for the determination of immunologic laboratory tests and measurement of the concentrations of INF and IL, according to the Affymetrix Bioscience protocol.
The ELISA (quantitative enzyme-linked immunosorbent assay, second generation) test was carried out in all samples in order to determine IgG and IgM antibodies against CHIKV, immunophenotypification of lymphocyte populations by flow cytometry, with monoclonal antibodies (anti-CD3, anti-CD4, anti-CD8), concentration of IL-1β, IL-2, IL-6, IL8, IL-10, IL-12, IL-17, IFNγ, IFNα, TNFα, rheumatoid factor, citrullinated peptide, C-reactive protein, anti-DNA and antinuclear antibodies.
The ELISA test used belonged to the commercial firm eBioscience, Inc – An affymetrix Company, and was performed in the city of Barranquilla.
The patients included in the study, who met the inclusion criteria to participate therein, were selected consecutively during their attendance in the emergency service of the FHUM and in the outpatient service of the Orthopedic and Rheumatologic Center of Barranquilla.
The established inclusion criteria were: suspected case (fever higher than 38.5°C, severe arthralgia or arthritis of acute onset, with no other medical condition that explains it and rash). Patients with positive anti-CHIKV type IgM or IgG, by ELISA. Dully completed and signed informed consent.
The patients with previous or presumptive diagnosis of rheumatologic disease, or with positive results for: citrullinated peptide, rheumatoid factor, ASO, uric acid, antinuclear antibodies with titer higher than 1/160 or those who did not sign the consent were excluded.
During the gathering of the information, it was applied a form that allowed to analyze: the time of onset and duration of the symptoms, pain or inflammation, affections of the joints, arthralgia, myalgia, headache, back pain, fever, rash, nausea and incapacity for work and, in some cases the diagnosis of rheumatoid arthritis (RA), according to the criteria established by ACR/EULAR.36 Periodically, the patients were clinically evaluated at 9 months.
The statistical, descriptive and inferential analyses of the clinical manifestations allowed us to observe the behavior and the immuno-rheumatologic affectation.
EthicsThe study was approved by the Ethics Committee of the Metropolitan University and of the FHUM. The participating patients were properly informed, and they completed and signed the consent. The Guidelines for Good Clinical Practice in Research were applied during the study.
Statistical analysisAll data were entered in the program Microsoft Excel version 2010, and for the statistical analysis was used the program Statgraphics XVI, where the mean and standard deviation were obtained for the quantitative variables, and the frequency and percentages for the qualitative variables.
The Student's t-test was used for the quantitative variables in order to establish the existence or not of significant differences among the mean values. The measure of association Odds ratio was calculated to measure the level of relationship between the symptoms and the determined laboratory markers, considering the values of the healthy control group for each test.
ResultsA total of 109 patients, from whom a peripheral blood sample was taken, were studied. The age of the population ranged between 22 and 82 years, with a higher percentage (56%) between the ages of 41 and 60 years. Regarding the activity and persistence of the symptoms, the age was not decisive. As for the gender, it was found a female predominance, which corresponds to 89% of the analyzed population, being 40% of them housewives.
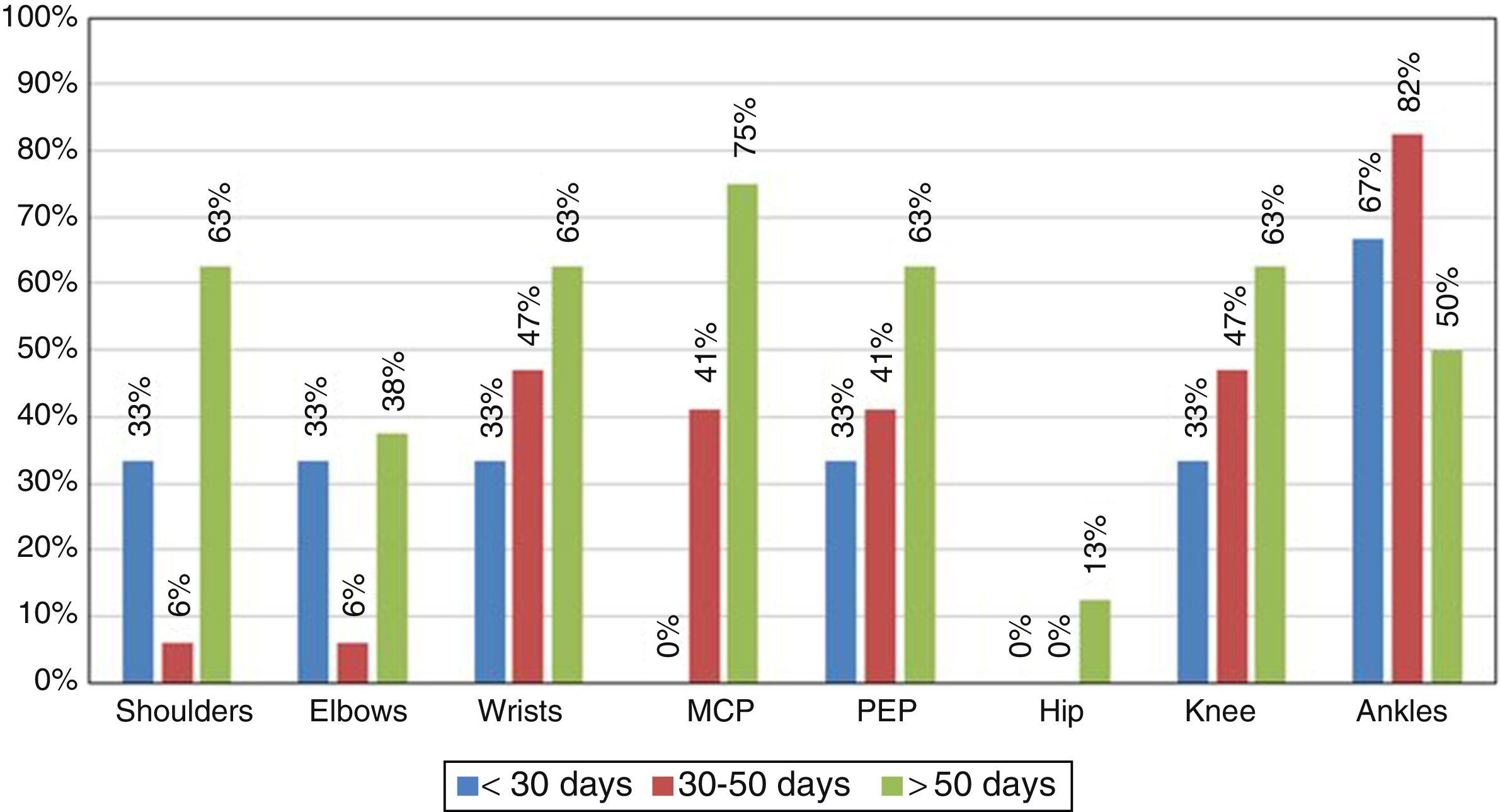
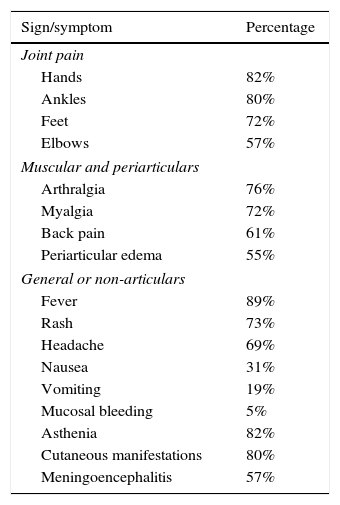
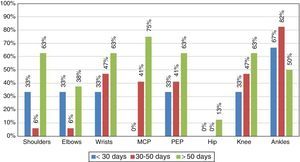
Although the main objective of the study was to characterize the immune response of the patients infected with CHIKV, it is important to highlight the clinical manifestations found during the medical attention (Table 1). In this context, the most characteristic symptoms were: symmetrical polyarthritis in small joints and ankles with a persistence of the symptoms for more than 6 weeks, with which the patients met the criteria for RA (Fig. 1).
Signs and symptoms present in the participants.
| Sign/symptom | Percentage |
|---|---|
| Joint pain | |
| Hands | 82% |
| Ankles | 80% |
| Feet | 72% |
| Elbows | 57% |
| Muscular and periarticulars | |
| Arthralgia | 76% |
| Myalgia | 72% |
| Back pain | 61% |
| Periarticular edema | 55% |
| General or non-articulars | |
| Fever | 89% |
| Rash | 73% |
| Headache | 69% |
| Nausea | 31% |
| Vomiting | 19% |
| Mucosal bleeding | 5% |
| Asthenia | 82% |
| Cutaneous manifestations | 80% |
| Meningoencephalitis | 57% |
Source: FHUM tracking template of laboratory results.
At the time of the initial collection of the information, all patients had at least one persistent arthralgia (Table 1), and 10% of them had symptoms of polyarthritis, so that according to the time of evolution the disease was classified as in sub-acute stage (persistence of the symptoms for a time longer than 8 days and shorter than 3 months). No patients in the study were in the acute phase.
After 9 months it was conducted a new clinical evaluation of the patients, 72% of them showed persistence of the arthralgia and periarticular edema predominantly in the ankles, which indicated chronicity of the disease.
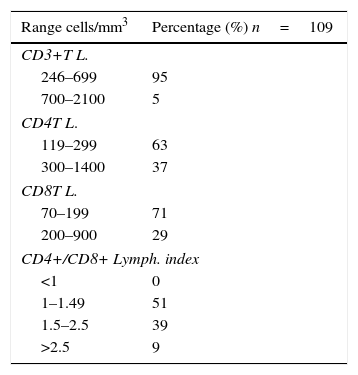
On the other hand, all patients included in the study had positive results for anti-CHIKV IgG type antibodies. The populations and subpopulations of T lymphocytes were found decreased: 95% of the population expressed less than 700 CD3T lymphocytes+/mm3, those of CD4T lymphocytes+ were also decreased in 63% of the participants, as well as 71% of them regarding the CD8T lymphocytes. As for the CD4/CD8 ratio, 51% of the participants exhibited values between 1 and 2.5, denoting a balance of the subpopulation of CD4 lymphocytes with respect to the CD8 lymphocytes (Table 1).
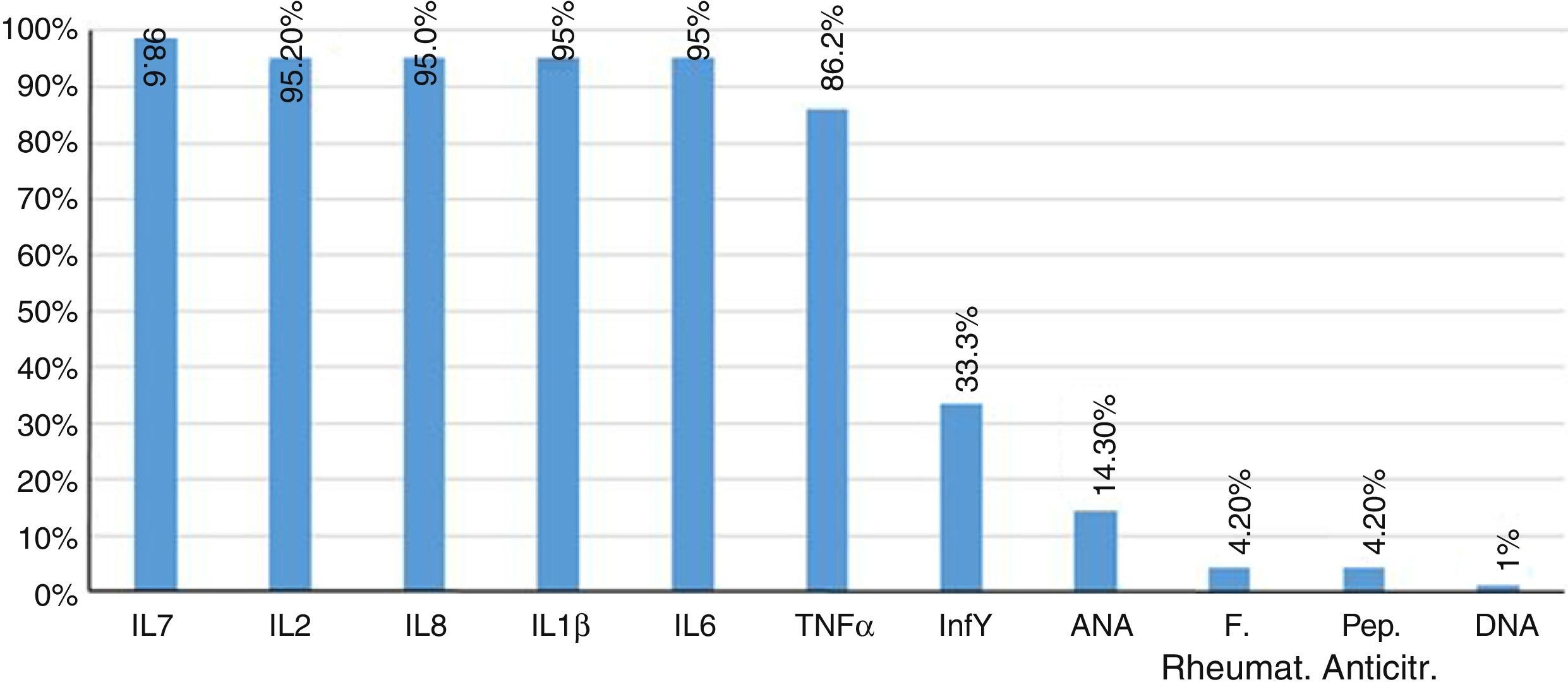
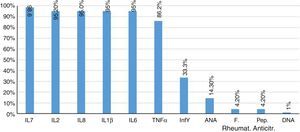
Furthermore, 85.7% of patients had negative results of antinuclear antibodies. Two patients showed titers higher than 1/80 dilution, and only one patient had a positive DNA. The rheumatoid factor and the anti-citrullinated peptide were negative in 95.8% of patients. Finally, the concentrations of proinflammatory interleukins, IL1β, IL2, IL6, IL8, IL17, IFNγ, TNFα, were found increased in 95% of patients (Fig. 2).
DiscussionAlthough 75% of the patients included in the study had more than 10 joints affected, fulfilling the criteria for articular affectation established by the ACR/EULAR, the clinical presentation of manifestations such as rash, fever and coexistence of the epidemic outbreak in the city, marked a difference with the diagnosis of RA and leaned the clinical diagnosis toward a viral infection.
It was found a decrease in the immune response, mediated by the populations and subpopulations of T lymphocytes, which would indicate a diagnosis of lymphocytopenia secondary to the viral infection. But unlike the lymphocyte decrease found in the infection with HIV, the balance between CD4/CD8, in the patients infected with CHIKV, is maintained in favor of the TH cells (CD4+).
The CD4/CD8 ratio was greater than 1, highlighting the functional capacity for production of a specific immune response in these patients, as well as the partial balance between the lymphocyte subpopulations. Unlike what is found in RA, where there is also a positive balance between CD4/CD8, in this entity we found increased the subpopulations of THL.37
The lymphocytopenia at the expense of the CD4+ lymphocytes, which is seen in the patients with HIV, differs from the decrease of the cellular immune response found in the patients with CHIKV, since in the latter the CD4/CD8 ratio is positive, so that 63% of the population that presented less than 300 CD4 lymphocytes/mm3, had likewise less than 200 CD8 lymphocytes/mm3, which would show a transient immunodepletion, possibly as a result of an immunomodulatory effect. In contrast, the lymphocytopenia in HIV is accompanied by a lymphocyte imbalance with predominance of CD8 cells, with immunosuppressive effect and associated opportunistic infections.38
TNFα, as well as interleukins IL17 (Fig. 1), IL1β (Fig. 2) and IL8 are substantially increased, which coincides with the values regularly found in active inflammatory processes of viral nature, widely referenced.39,40
The concentrations of INFα, close to the values of the healthy control group, would be explained by the fact that this protein increases during the acute phase of the disease and there were no patients in that phase.
Due to the clinical mimicry that CHIK fever exhibits with rheumatologic diseases such as RA, it is important to compare both entities within the framework of the immune response. In the serum of the infected patients it was found an increase of the analyzed proinflammatory interleukins, mainly IL2 and IL8, which exceeded about 2 times the value of the healthy control group.
This response would arise from the stimulus that the decrease of CD4 lymphocytes, found in the patients infected with CHIKV, exerts on the production of IL2. These data are consistent with those published by other authors, after analyzing the cytokines profile in adults and young people with RA, but which unlike our population were positive for anti-citrullinated peptides antibodies.16,17,31
The different levels of expression of the cytokines account for the pleiotropism, in the course of the same disease of among different rheumatological diseases; in our study the levels of IL6 and TNFα, were above those of the healthy control group, with a behavior similar to that found in the RA group, within a comparative study conducted between RA and juvenile idiopathic arthritis, in which, although these cytokines were increased in both entities, they were higher in the RA group than in the juvenile idiopathic arthritis.18,32,33
Regarding the expression of the anti-inflammatory IL10, there was no elevation and therefore its immunoregulatory and suppressor effects on IL1β and IFNγ were not evidenced.19,34,35
The negative results of the rheumatoid factor, antinuclear antibodies, anti-DNA and anticitrulline, indicate that in the CHIKV infection there is not an autoimmune response, for this reason, is considered that it is a joint swelling with inflammatory mediators very similar to what we might find in rheumatologic diseases, especially in RA, although it was not possible to determine that it would exist an autoimmune response developed by the viral infection, found in rheumatologic processes. However, it would be necessary to wait in a next assessment of the patients with persistent joint symptoms, the immunologic laboratory tests (ANA, DNA, RF, anti-citrulline) and observe autoimmune reactions.
It is considered to conduct studies in the synovial fluid, in order to establish if the decrease of CD4T and CD8T cells in peripheral blood is due, as in animal models, to the fixation and activation of these lymphocytes in the tissues.
ConclusionIt was found a viral immune response of the participants in the study, although no specific antibodies were produced.
Most of the participants exhibited a big component of persistent polyarticular pain, so we consider important to make a follow up of the clinical evolution and the immune profile, and to continue research during the acute, hyperacute and chronic phases of the CHIKV fever.
Due to the great similarity in the clinical symptomatology between CHIKV fever and RA, rheumatologists should be alert with the patients who show involvement of small joints and who somehow meet the ACR/EULAR criteria for the diagnosis of RA, and they must also take into account the importance of a differential diagnosis in the places which are endemic for CHIKV (Table 2).
Values of T lymphocytes: CD3, CD4, CD8 and CD4+/CD8+ lymphocyte index.
| Range cells/mm3 | Percentage (%) n=109 |
|---|---|
| CD3+T L. | |
| 246–699 | 95 |
| 700–2100 | 5 |
| CD4T L. | |
| 119–299 | 63 |
| 300–1400 | 37 |
| CD8T L. | |
| 70–199 | 71 |
| 200–900 | 29 |
| CD4+/CD8+ Lymph. index | |
| <1 | 0 |
| 1–1.49 | 51 |
| 1.5–2.5 | 39 |
| >2.5 | 9 |
Source: FHUM tracking template of laboratory results.
The authors declare that no experiments were performed on human beings or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent from patients and/or subjects referred in the article. This document is held in the possession of the corresponding author.
FundingMetropolitan University, Fundación Hospital Universitario Metropolitano and Orthopedic and Rheumatologic Center.
Conflict of interestWe declare that we have no commercial or associative interest that represents a conflict of interest with the presented work.
Please cite this article as: Jaller Raad J, Segura Rosero A, Vidal Martínez J, Parody A, Jaller Raad R, Caballero Tovar D, et al. Respuesta inmunitaria de una población del Caribe colombiano infectada con el virus chikungunya. Rev Colomb Reumatol. 2016;23:85–91.