The implantation of stents to keep the ductus arteriosus patent in cyanotic congenital heart disease is an alternative to the modified Blalock-Taussig surgery (mBT) in high-risk patients. This study describes the immediate and medium-term outcomes of stent implantation in neonates and infants with duct-dependent pulmonary circulation.
MethodsThis was a descriptive and prospective study including different cyanotic congenital heart diseases treated between 2014 and 2015.
ResultsFourteen patients with a mean age of 46 days, and mean weight of 4.5kg were assessed, and pulmonary artresia with interventricular communication was the most treated condition. The femoral artery approach was used in 70% of procedures; carotid approach was used in the remaining cases. Stents of 3.5 x 12mm were used in most cases, and implant success was achieved in 78% of interventions (11/14). The failed cases were referred to surgery – one of them was an emergency, which resulted in death. Ductal spasm occurred in < 48hours in three patients who required mBT, with favorable outcome. Complications after discharge and within the first 30 days included stent thrombosis (2/11), one of which was controlled with redilation, another progressed to death, and one sudden death (1/11). The overall mortality was 21.4% (3/14). A patent ductus arteriosus in the first 6 months was present in five cases, which underwent palliative surgery.
ConclusionsThe initial experience of ductal stenting showed favorable immediate outcomes, but in the medium term, little more than a third of the cases maintained a patent ductus arteriosus within 6 months.
O implante de stents para manter o ducto arterial patente na cardiopatia congênita cianótica é uma alternativa à cirurgia de Blalock-Taussig modificada (BTm) em pacientes de alto risco. Descrevemos os resultados imediatos e de médio prazo do implante de stent em neonatos e lactentes com circulação pulmonar ducto-dependente.
MétodosTrata-se de estudo descritivo e prospectivo, que incluiu diferentes cardiopatias congênitas cianóticas tratadas entre 2014 e 2015.
ResultadosAvaliamos 14 pacientes, com média de idade de 46 dias e pesando 4,5kg, sendo a atresia pulmonar associada à comunicação interventricular a cardiopatia mais tratada. A abordagem pela artéria femoral ocorreu em 70% dos procedimentos e, nos demais, por via carotídea. Stents de 3,5 12mm foram usados na maioria dos casos, e o sucesso do implante foi obtido em 78% das intervenções (11/14). Os casos de insucesso foram encaminhados para cirurgia uma delas em situação de urgência, que resultou em óbito. Ocorreu espasmo ductal < 48 horas em três pacientes que necessitaram de BTm, com evolução favorável. Complicações após a alta e nos primeiros 30 dias incluíram trombose de stent (2/11), uma delas controlada com redilatação e outra que evoluiu para óbito, e uma morte súbita (1/11). A mortalidade total foi de 21,4% (3/14). A patência do ducto arterial nos primeiros 6 meses foi obtida em 5 casos que foram submetidos à cirurgia paliativa.
ConclusõesA experiência inicial de implante de stent ductal mostrou resultados imediatos favoráveis, e, em médio prazo, mais de um terço dos pacientes com circulação pulmonar ducto-dependente manteve seus canais patentes.
Congenital heart disease patients with duct-dependent pulmonary circulation require urgent treatment in the first days of life to ensure pulmonary blood flow before the duct closes. Prostaglandin E1 infusion is generally effective in maintaining the patency of the ductus arteriosus.1 Pulmonary atresia with intact ventricular septum, extreme tetralogy of Fallot, tricuspid atresia with pulmonary atresia, and critical pulmonary stenosis, among others, are conditions dependent on continuing patency of the ductus arteriosus.
Traditionally, the first treatment option is the modified Blalock-Taussig (mBT) surgery; however, it can be limited by the lack of experience of the service or due to patient-related factors such as very severe disease or very low birth weight.2 The mBT surgery consists of a systemic pulmonary shunt, and is mostly performed as a palliative surgery to improve pulmonary blood flow prior to the definitive surgery. Although this surgery has been used for over 50 years, there is still high morbidity and mortality, particularly among newborns and in early infancy.3,4
In 1992, Gibbs et al. first described maintenance of ductal patency with stenting during cardiac catheterization.5 Since then, stenting has achieved wide acceptance as a reliable alternative, considered to be as safe and effective as mBT surgery in high-risk patients. Moreover, it has other potential advantages such as reducing the number of palliative surgeries and optimize the time for definitive surgical repair.6,7
This study presents an early experience with this technique in newborns and infants with cyanotic heart disease and duct-dependent pulmonary circulation undergoing ductal stenting as an alternative to conventional surgery. The immediate and medium-term outcomes and complications are presented.
MethodsNewborns and infants with duct-dependent congenital heart disease underwent ductal stenting from January 2014 to March 2015, at the Instituto Nacional de Salud del Niño de Lima and at the National Hospital Dos de Mayo, in Lima, Peru, with prior informed consent.
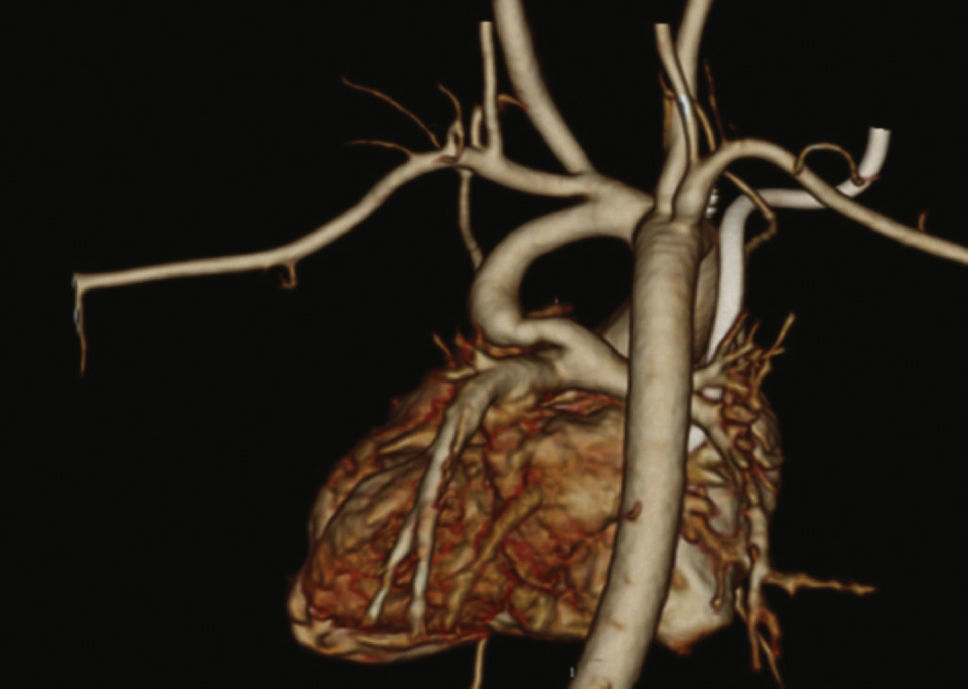
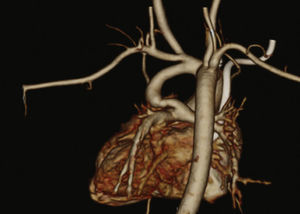
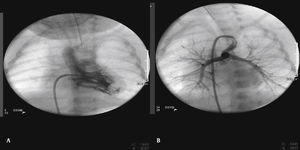
Patients were selected by a medical team, and presented with echocardiography and, in most cases, CT angiography (Fig. 1). These patients were considered to be at high surgical risk, so they were referred to percutaneous intervention.
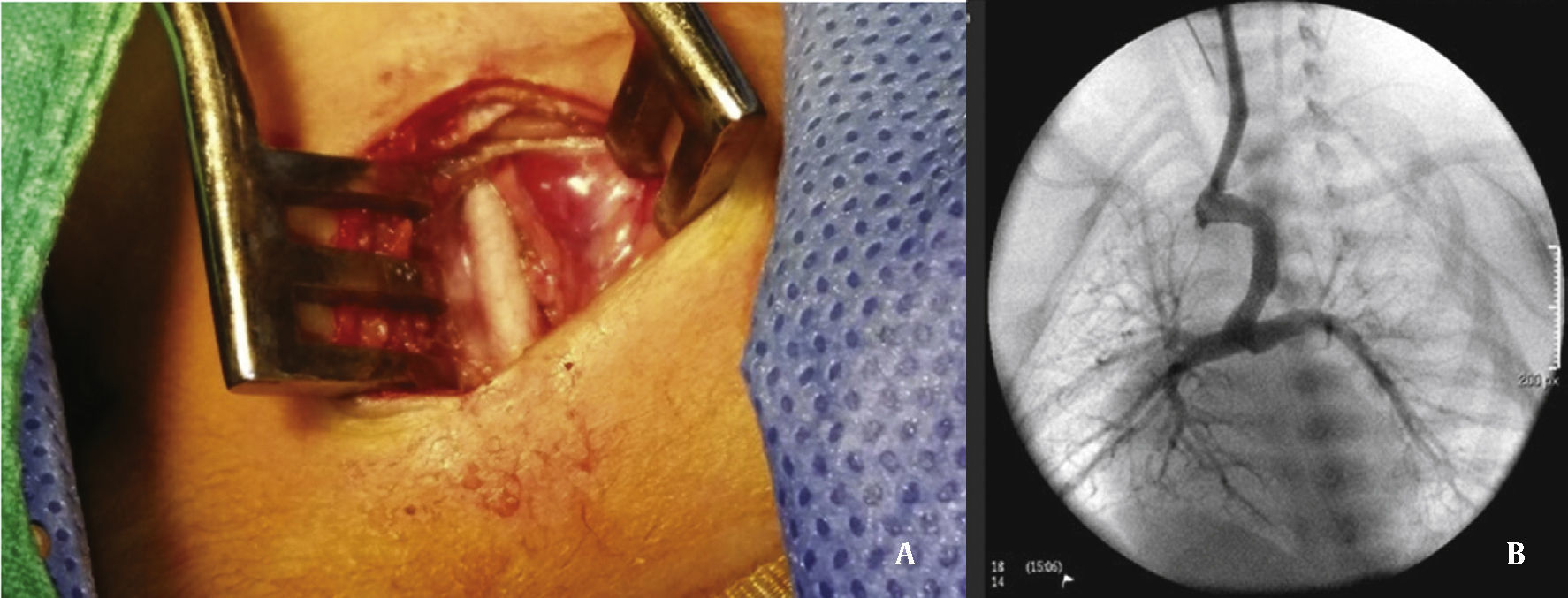
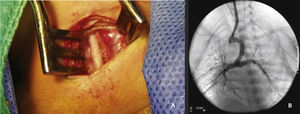
Under general anesthesia and endotracheal intubation, the approach was made considering the most favorable access to the duct in accordance with its origin in the aortic arch or descending aorta8 (previously defined in CT angiography, or if not available, by echocardiogram). An 5 F sheath was placed using Seldinger technique in the femoral access (whether venous or arterial), or through direct dissection and puncture when in the carotid artery.
All patients were started on prostaglandin E1 infusion, and the dose was gradually reduced or suspended when the duct was approached, and the guide passed through the narrowest area; this allowed for a clear definition of the constriction, minimizing the risk of hypoxic crisis.
Angiography was performed with pigtail catheter or, on other occasions, through a guide catheter near the duct.
A catheter guide was used to address the duct and then to cross the narrowest area with a 0.014” angioplasty guide. Stents 3.0/12mm, 3.5/16mm, or 4.0/12mm were used in this study.
At the beginning of the procedure, 100 U/kg intravenous heparin was given; at the end, antiplatelet therapy was started with acetylsalicylic acid 5mg/kg and clopidogrel 2mg/kg.
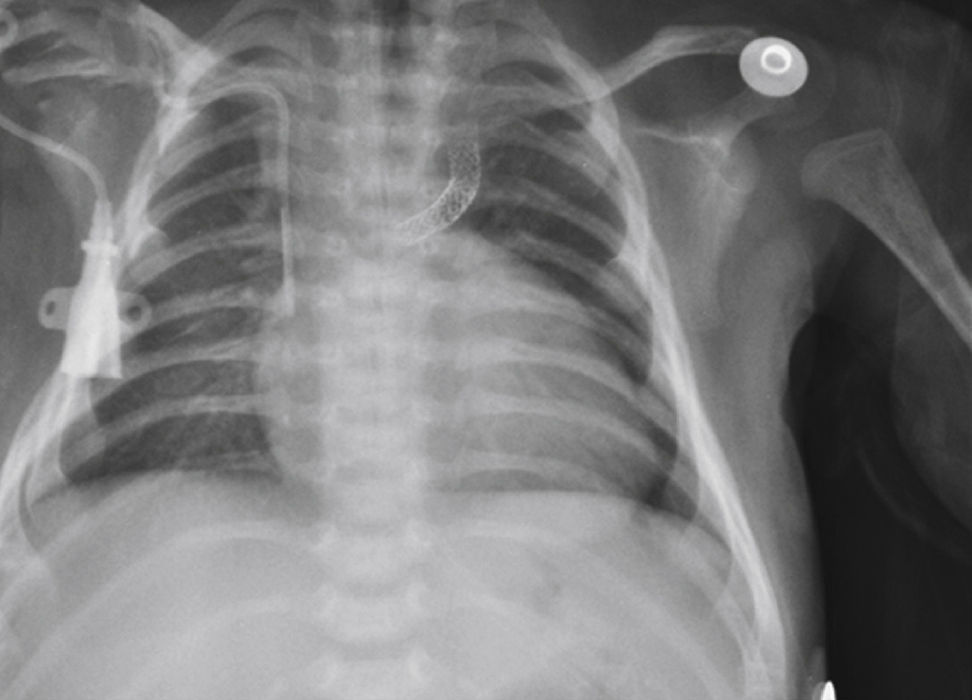
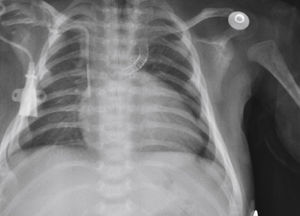
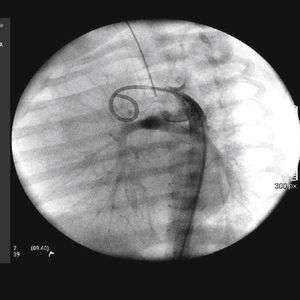
The implantation was considered successful when oxygen saturation above 75% was achieved, along with complete patency of the duct with adequate flow to the pulmonary branches. The follow-up was clinical: a 24-hour control echocardiogram was performed after the procedure, and radiological controls were performed as needed (Fig. 2).
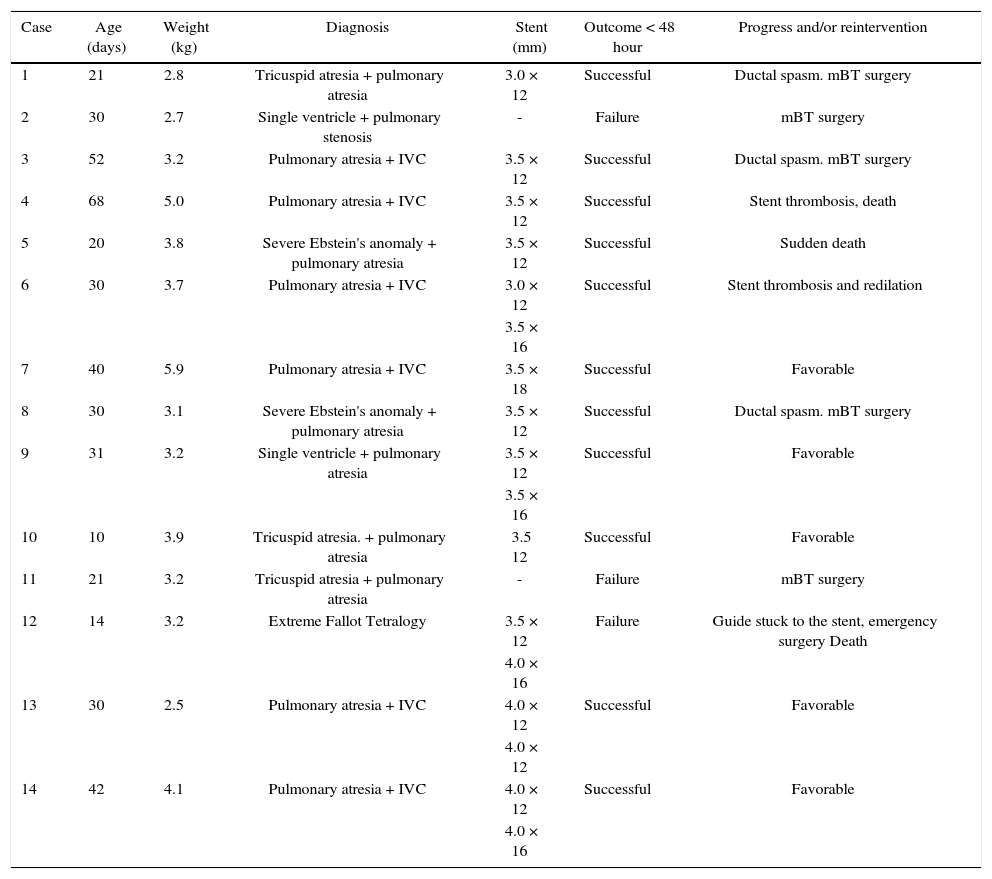
ResultsFourteen patients with several cyanotic congenital heart diseases and a mean age of 46 days (10 to 68 days) and mean weight of 4.5kg (2.5 to 5.9kg) were studied. The heart diseases were type I-A tricuspid atresia (3/14), pulmonary atresia and interventricular communication (7/14), Ebstein's anomaly and pulmonary atresia (2/14), and single ventricle and pulmonary atresia (2/14) (Table 1).
Clinical data and complications.
| Case | Age (days) | Weight (kg) | Diagnosis | Stent (mm) | Outcome < 48 hour | Progress and/or reintervention |
|---|---|---|---|---|---|---|
| 1 | 21 | 2.8 | Tricuspid atresia + pulmonary atresia | 3.0 × 12 | Successful | Ductal spasm. mBT surgery |
| 2 | 30 | 2.7 | Single ventricle + pulmonary stenosis | - | Failure | mBT surgery |
| 3 | 52 | 3.2 | Pulmonary atresia + IVC | 3.5 × 12 | Successful | Ductal spasm. mBT surgery |
| 4 | 68 | 5.0 | Pulmonary atresia + IVC | 3.5 × 12 | Successful | Stent thrombosis, death |
| 5 | 20 | 3.8 | Severe Ebstein's anomaly + pulmonary atresia | 3.5 × 12 | Successful | Sudden death |
| 6 | 30 | 3.7 | Pulmonary atresia + IVC | 3.0 × 12 | Successful | Stent thrombosis and redilation |
| 3.5 × 16 | ||||||
| 7 | 40 | 5.9 | Pulmonary atresia + IVC | 3.5 × 18 | Successful | Favorable |
| 8 | 30 | 3.1 | Severe Ebstein's anomaly + pulmonary atresia | 3.5 × 12 | Successful | Ductal spasm. mBT surgery |
| 9 | 31 | 3.2 | Single ventricle + pulmonary atresia | 3.5 × 12 | Successful | Favorable |
| 3.5 × 16 | ||||||
| 10 | 10 | 3.9 | Tricuspid atresia. + pulmonary atresia | 3.5 12 | Successful | Favorable |
| 11 | 21 | 3.2 | Tricuspid atresia + pulmonary atresia | - | Failure | mBT surgery |
| 12 | 14 | 3.2 | Extreme Fallot Tetralogy | 3.5 × 12 | Failure | Guide stuck to the stent, emergency surgery Death |
| 4.0 × 16 | ||||||
| 13 | 30 | 2.5 | Pulmonary atresia + IVC | 4.0 × 12 | Successful | Favorable |
| 4.0 × 12 | ||||||
| 14 | 42 | 4.1 | Pulmonary atresia + IVC | 4.0 × 12 | Successful | Favorable |
| 4.0 × 16 |
mBT: modified Blalock-Taussig surgery; IVC: interventricular communication.
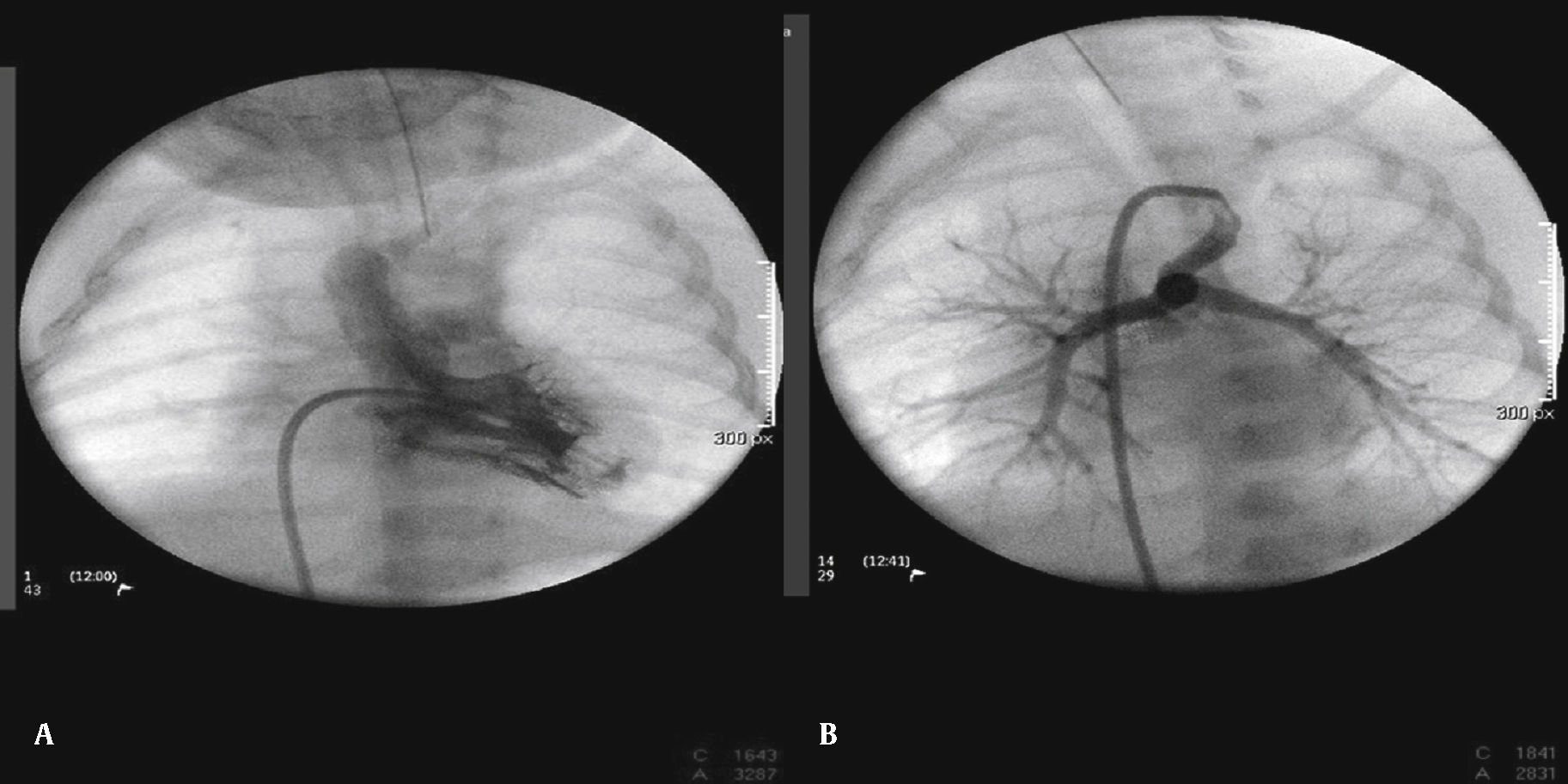
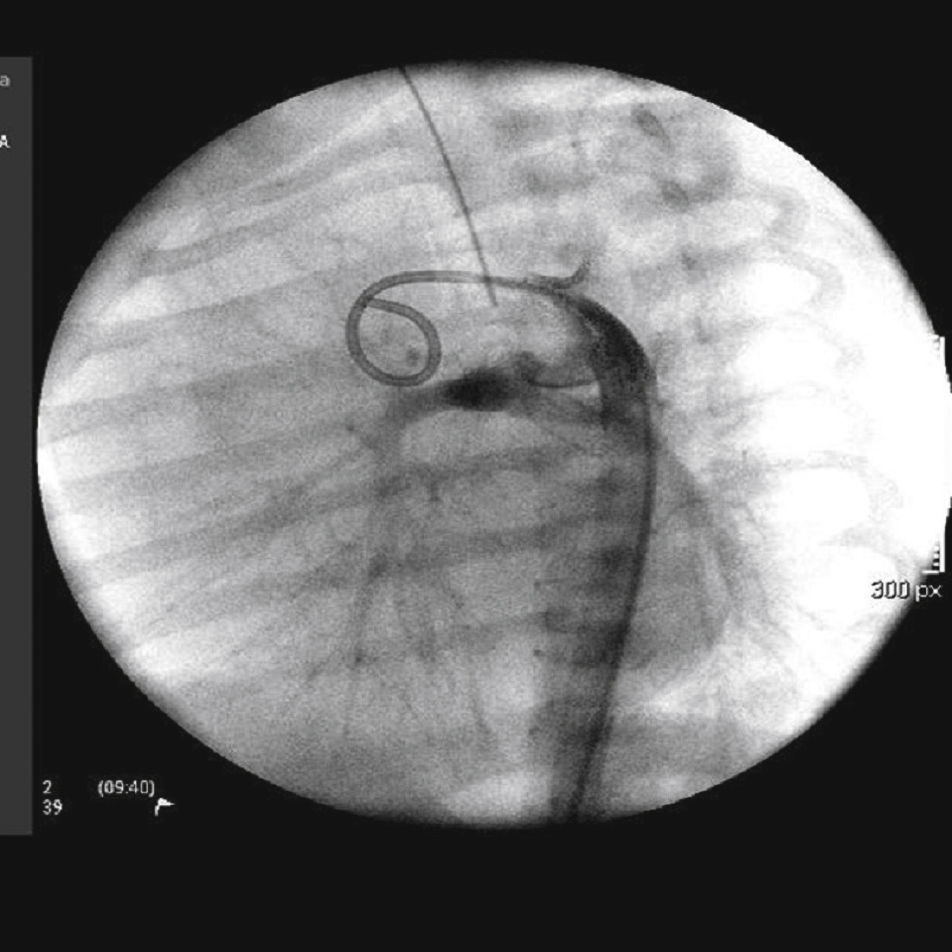
The arterial or venous femoral artery approach was used in 71% of cases (Figs. 3 and 4); and through the carotid artery in the remaining cases, with the assistance of a cardiovascular surgeon (Fig. 5).
Anterograde access through the femoral vein (5/14), retrograde access through the femoral artery (5/14), and through the carotid artery (4/14) were used. Regarding the type of duct, we found the elongated type, originating in the descending aorta; the curved type, originating in the distal aortic arch; or the tortuous type, originating in the vertical aortic arch.
The mean procedure time and mean fluoroscopy time were 95 ± 18minutes and 36 ± 12minutes, respectively. Stents 3.5/12mm were used in 75% of cases and 3.0/16mm or 4.0/12mm in the remaining cases; depending on the length of the duct, up to two stents were placed, as needed.
The implant success rate was 79% (11/14). In two cases, stenting was not possible, and mBT surgery was required. In the third case, there was a major complication during the procedure, which required emergency surgery and led to a fatal outcome. Regarding other complications associated with the procedure, three patients who required mBT surgery, presented ductal spasm before 48hours, with favorable outcome.
Extra-hospital complications in the first 30 days included stent thrombosis, with one improving after stent redilation, and the other evolving to death; there was also one sudden death. The overall mortality was 21.4% (3/14).
The medium-term evolution was favorable with presence of patent duct for over 4 to 6 months in five patients, who underwent palliative surgery.
DiscussionIn this institution, mBT palliative surgery has been routinely performed for over 25 years; however, as in other centers, it still has considerable morbidity and mortality rates due to possible complications such as pulmonary artery distortion and stenosis, pleural effusion, chylothorax, phrenic and vagus nerve paralysis, and differential branch pulmonary artery growth, as well as the patients clinical profile (severe hypoxemia, low birth weight, prematurity, among others).9
Conversely, there are several limitations of infrastructure, and insufficient human resources to meet the growing demand for cardiac surgeries in increasingly complex patients. Under these circumstances, the development of percutaneous ductal stent techniques enables treating patients with duct-dependent cyanotic heart disease, establishing itself as an alternative palliative approach.
Ductal stenting and surgical shunts are the most common procedures in patients who have no other effective pulmonary arterial source other than the ductus arteriosus. The patency of the duct through the stent is as effective as mBT surgery in promoting the overall growth of the pulmonary artery in duct-dependent congenital heart diseases.10,11
In the present study, the immediate outcomes were favorable in most cases, although duct anatomy was not optimal (70% were large and tortuous ducts). When comparing this experience with other series,12,13 similarities in the clinical characteristics of patients regarding diagnosis, age, and weight could be observed; the implantation success rate was 79%, very close to the reported average rates (80 to 100%).
Regarding complications related to the procedure, they are uncommon, including major complications such as stent migration (in the present study, one case, 9%) and acute stent thrombosis (two cases, 18%); similar to other series.14 In addition, congestive heart failure was observed in four patients, related to excessive pulmonary blood flow, which improved with diuretics and water restriction; and one of the patients also had moderate pericardial effusion. These complications are not usually observed. In the present study, immediate mortality was 7.1%, as a result of emergency surgery due to guide wire entrapment behind one of the stents, with subsequent migration of the device into the left pulmonary branch.
However, the patency of the stent in our group in the medium term was different than that reported in other studies.15 Just over a third of cases showed patent stents, condition associated with the use of 3.0 and 3.5mm stents, and the incomplete coverage of the duct. As the procedure was consolidated, larger caliber (4mm) stents were implanted, multiple stents were used, covering thus all the duct extension, increasing the chances of maintaining stent patency. Moreover, the last cases were approached by direct dissection and carotid artery puncture, allowing the use of larger stents and facilitating the access to the duct.
The literature indicates that stenosis usually develops over a period of 6 months to 1 year of stent implantation.16 Therefore, surgical interventions, such as Glenn surgery for univentricular heart or total correction in biventricular hearts, should be performed as soon as possible. However, it is possible to successfully dilate the stent in some of these patients to gain more time before surgery. In this study, one patient required stent redilation and placement of a larger-caliber stent to increase the flow to the pulmonary branches and improve oxygen saturation.
In recent years, stent implantation in the ductus arteriosus gained wide acceptance as a reliable alternative to the systemic-pulmonary surgery in patients with duct-dependent congenital cyanotic heart disease. This option is considered safer and more effective than palliative surgery in high-risk patients, as it is possible to adapt the stent to the patient size and pulmonary anatomy.17 However, the ability of ductus stenting to promote a significant growth of the pulmonary artery branches has not been specifically evaluated. Theoretically, if the stent is fitted to the size and angulation of the pulmonary artery, an even distribution of pulmonary blood flow could take place, thereby promoting uniform vascular development.18 This hypothesis is currently being evaluated, but with limited data so far.
ConclusionsDuctal stenting had favorable immediate outcomes; however, in the medium term, ductal patency for over 6 months was maintained in less than 50% of cases.
The optimal outcome requires the duct to not be too big or too tortuous, a condition that was present in most of the present patients. The authors believe that in the future, with better-selected cases (more favorable ductal anatomy) and the use of larger-caliber stents (4 to 4.5mm), the immediate success rate will improve, and a medium term patency will be achieved, optimizing the results for the benefit of patients.
Funding sourceNone.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.