Transesophageal echocardiography (TEE) is the most widely used method to guide the percutaneous treatment of atrial septal defect (ASD) and patent foramen ovale (PFO), but the necessity of another professional to perform it and the need for general anesthesia are potential disadvantages. Intracardiac echocardiography (ICE) is seen as an alternative to TEE, as it can be performed by the interventionist and requires only local anesthesia with mild or no sedation. The aim of this study was to report our experience with ASD/PFO occlusion guided by ICE.
MethodsThe ICE uses an ultrasound catheter, which is intravenously inserted in the right heart chambers and acquires images for the intervention through variable positioning of the transducer. Success and complication rates of the procedure were evaluated.
ResultsFrom 2011 to 2015, 201 procedures guided by ICE were performed, comprising 139 in patients with ASD and 62 in those with PFO. Most patients were female (64.2%), ages ranged from 7 to 78 years (36.6 ± 19.3 years), and weight ranged from 28 to 92kg (62.5 ± 13.0kg). Occlutech Figulla® prostheses were used and all interventions were successful, with fluoroscopy time of 5.7 ± 2.4minutes and procedure time of 21.5 ± 6.4minutes. Two patients (2.0%) had transient supraventricular tachycardia and two others had arteriovenous fistula at the access site, with spontaneous resolution in the first month of follow-up.
ConclusionsICE provided accurate anatomical information to guide the closure of the ASD/PFO and successfully eliminated the main drawbacks of TEE.
O ecocardiograma transesofágico (ECO-TE) é o método mais utilizado para guiar o tratamento percutâneo da comunicação interatrial (CIA) e do forame oval (FOP), mas a necessidade de um outro profissional para realizá-lo e de anestesia geral constituem inconvenientes para seu emprego. O ecocardiograma intracardíaco (ECO-IC) apresenta-se como alternativa ao ECO-TE, pois pode ser realizado pelo próprio operador e demanda apenas anestesia local, com leve ou nenhuma sedação. Nosso objetivo foi relatar a experiência do serviço com a oclusão de CIA/FOP guiada por ECO-IC.
MétodosO ECO-IC utiliza cateter de ultrassom, que é introduzido por via venosa em câmaras cardíacas direitas e, por meio de posicionamento variável do transdutor, obtém as imagens adequadas para a intervenção. Foram avaliadas as taxas de sucesso do procedimento e as complicações.
ResultadosDe 2011 a 2015, foram realizados 201 procedimentos guiados pelo ECO-IC, sendo 139 em pacientes com CIA e 62 com FOP. A maioria dos pacientes era do sexo feminino (64,2%), as idades variaram de 7 a 78 anos (36,6 ± 19,3 anos) e o peso variou de 28 a 92kg (62,5 ± 13,0kg). Foram utilizadas próteses Occlutech Figulla®, e todas as intervenções tiveram sucesso, com tempos de fluoroscopia de 5,7 ± 2,4 minutos e tempo de procedimento de 21,5 ± 6,4 minutos. Dois pacientes (2,0%) apresentaram taquicardia supraventricular transitória e outros dois pacientes evoluíram com fístula arteriovenosa na via de acesso, com resolução espontânea no primeiro mês.
ConclusõesO ECO-IC forneceu informações anatômicas precisas para guiar o fechamento da CIA/FOP com sucesso e eliminou as principais desvantagens do ECO-TE.
Percutaneous occlusion of intracardiac septal defects has been successfully carried out with low morbidity and mortality, constituting the procedure of choice for the treatment of atrial septal defects (ASD) and patent foramen ovale (PFO). Obtaining good images is fundamental and indispensable to the safety and success of the procedures. Classically, transesophageal echocardiography (TEE) is the most widely used method, as it allows, for instance, the analysis of the size and morphology of the ASD, the septal edges, the position of the prosthesis, and its relation with adjacent structures. However, it has some disadvantages, such as the need for a specialized professional to perform it, sedation or general anesthesia with endotracheal intubation, and the fact that it is poorly tolerated in lengthy procedures. In turn, intracardiac echocardiography (ICE) provides the same safety and accuracy as the TEE, but it can be performed with only local anesthesia or mild sedation, and by the same professional in charge of the intervention, which allows greater freedom in the medical schedule. This article aims to report the experience of this service in the treatment of interatrial defects in adults guided by ICE.
MethodsA retrospective study of patients submitted to percutaneous closure of ASD/PFO guided by ICE between April 2011 and January 2015, carried out by one operator, in two hospitals in Belo Horizonte, (MG), Brazil. All participants gave informed consent.
Patients were evaluated through pre-intervention TEE to confirm the diagnosis; to define the size, location and number of atrial septal defects; and to rule out other associated abnormalities. Micro-bubble transcranial Doppler, associated with Valsalva maneuver, was performed in patients referred for PFO closure.
The procedures were performed under mild sedation or local anesthesia only. Cephalothin was employed at a single dose of 30mg/kg pre-procedure. The right femoral vein was punctured below the inguinal ligament at two distinct points, using a conventional vascular puncture needle, with a distance between the punctures of approximately 1 to 2cm. The punctures could be performed, if needed, in left femoral vein or in both femoral veins. One of the accesses was used for cardiac catheterization and prosthesis implantation, and the other for introduction of the ICE catheter. After the puncture, heparin was administered at a dose of 100 units/kg.
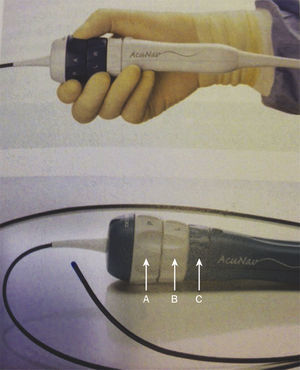
The AcuNav® system (Siemens-Acuson, Inc., Mountain View, United States) was used (Fig. 1). The procedures were performed with 11 F or 9 F sheaths, when ICE probes 10 F or 8 F were respectively employed. The second venous access was used for cardiac catheterization and prostheses implantation. The ICE probe was introduced using a diagnostic catheter inserted in the second orifice as a guide. In the presence of any resistance, a manual contrast injection was carried out and, whenever necessary, a small rotation of the ultrasound catheter was performed. The catheter was advanced smoothly, in a neutral position, up to the middle portion of the right atrium. Catheters and sheaths were washed with saline solution/heparin at a concentration of 2 units/mL.
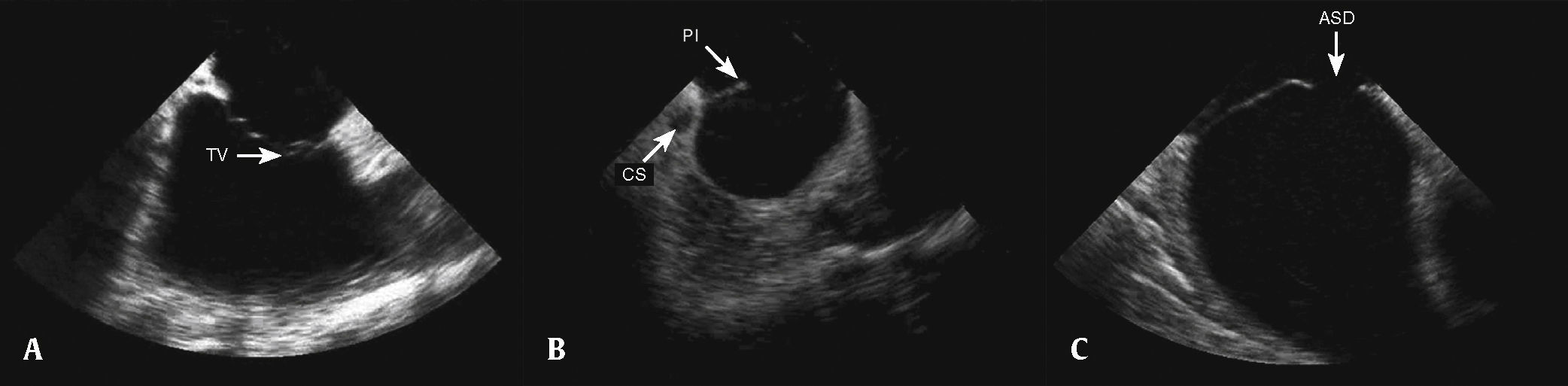
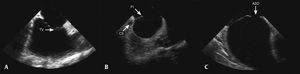
After the catheter was positioned in the right atrium, small adjustments were performed, with cranial or caudal movements, until images of the tricuspid valve, the right ventricular outflow tract, and the longitudinal aorta were obtained, the so-called home view or neutral view (Fig. 2A). Obtaining this image is the reference point for other echocardiographic views and, if it cannot be clearly obtained, new catheter adjustments are performed, with slight clockwise/counterclockwise, caudal/cranial rotation and, whenever necessary, posterior movements. When it was difficult to obtain other views, or when unwanted intermediate views were obtained, the operator returned to the initial view and the maneuvers were reinitiated. After viewing the right ventricular inflow and outflow tracts, a clockwise rotation of the probe was performed, until a clear image of the coronary sinus, interatrial septum, and especially the posteroinferior border was obtained (Fig. 2B). The next step was the clockwise rotation of the catheter to obtain the short axis, with the visualization of the aorta in a cross-sectional view and the anterior portion of the interatrial septum. From this point on, the clockwise movement of the catheter was accentuated and, whenever necessary, the medial/lateral movement was performed, in order to visualize the superior vena cava, the interatrial septum in the long axis, and the pulmonary veins (Fig. 2C).
Positioning the probe to obtain images of the cardiac structures. (A) Neutral view, with the probe located in the middle portion of the right atrium, showing the tricuspid valve (TV) and the right ventricular inflow tract. (B) Clockwise rotation of the probe to obtain images of the coronary sinus (CS), the posteroinferior (PI) septal border, and the atrial septal defect (ASD). (C) Longitudinal view showing the ASD.
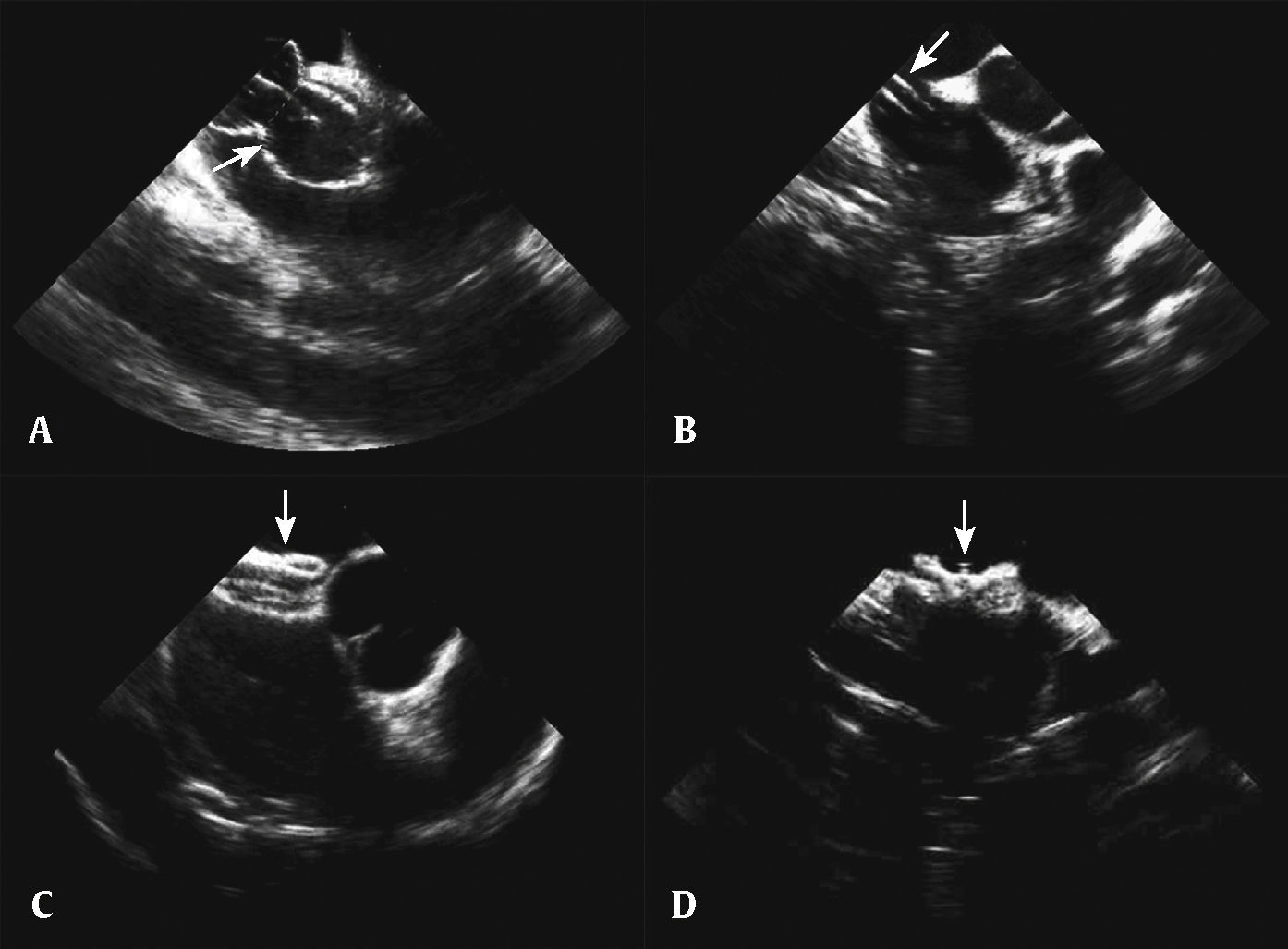
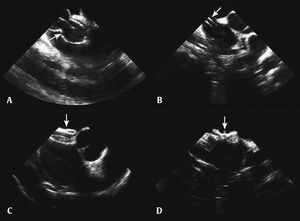
After the analysis of the morphology and size of the interatrial septum and its association with surrounding structures, of the cardiac function, and after ruling out an intracardiac thrombus, the stages of percutaneous occlusion were performed: diameter measurement with balloon, introduction of the long sheath, prosthesis preparation and insertion, and release of the left disc, the neck, and the right disc. After verifying its good positioning, without interference from adjacent structures, the prosthesis was released (Fig. 3). Occlutech Figulla® (Occlutech GmbH, Jena, Germany) prostheses were used. After the procedure, the sheaths were removed and manual compression was performed, until hemostasis was achieved.
Percutaneous occlusion of atrial septal defect. (A) Balloon visualization to measure the distended diameter of the atrial septal defect. (B) Long sheath visualized inside the left atrium. (C) Prosthesis visualization in the short axis. (D) Visualization of the prosthesis released at the vena cava view.
Patients remained under observation in the hospital until the day after the procedure, and ambulation was allowed 6hours after procedure completion. Examination of the puncture site was performed before discharge and 1 week after the procedure. Doppler of the femoral vessels was requested in the presence of murmurs. Electrocardiogram and transthoracic echocardiography were scheduled for 1, 6, and 12 months after percutaneous treatment. Acetylsalicylic acid 200mg/day and endocarditis prophylaxis were prescribed for 6 months.
The quantitative and qualitative variables were described, respectively, as mean and standard deviation, and as absolute frequency and percentage.
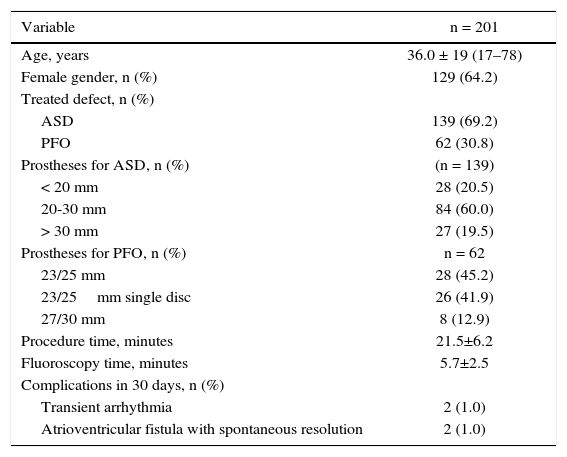
ResultsA total of 201 cases were treated, 139 with ASD and 62 with PFO. Right femoral vein puncture was performed in 196 patients (97.5%), in both femoral veins in 4 (2.0%) patients, and in 1 (0.5%) patient the left femoral vein was used. In all cases, punctures were performed in two different points of the same vessel, except those in which both femoral veins were punctured and in 2 cases treated through the right femoral vein, with 2 sheaths being inserted in a same orifice (Table 1).
Patient data.
| Variable | n = 201 |
|---|---|
| Age, years | 36.0 ± 19 (17–78) |
| Female gender, n (%) | 129 (64.2) |
| Treated defect, n (%) | |
| ASD | 139 (69.2) |
| PFO | 62 (30.8) |
| Prostheses for ASD, n (%) | (n = 139) |
| < 20 mm | 28 (20.5) |
| 20-30 mm | 84 (60.0) |
| > 30 mm | 27 (19.5) |
| Prostheses for PFO, n (%) | n = 62 |
| 23/25 mm | 28 (45.2) |
| 23/25mm single disc | 26 (41.9) |
| 27/30 mm | 8 (12.9) |
| Procedure time, minutes | 21.5±6.2 |
| Fluoroscopy time, minutes | 5.7±2.5 |
| Complications in 30 days, n (%) | |
| Transient arrhythmia | 2 (1.0) |
| Atrioventricular fistula with spontaneous resolution | 2 (1.0) |
ASD: atrial septal defect; PFO: patent foramen ovale.
Of the 139 cases of ASD closure, 28 (20.5%) prostheses smaller than 20mm were implanted; 84 (60.0%) were between 20 and 30mm; and 27 (19.5%) were larger than 30mm. In the 62 cases of PFO occlusion, 23/25mm prostheses were implanted in 28 patients (45.2%), 23/25mm with single disc in 26 (41.9%), and 27/30mm in 8 (12.9%). The success of the procedure was confirmed in all cases.
Two female patients had transient supraventricular tachycardia with spontaneous resolution and, in one of them, the arrhythmia was attributed to the position of the ICE probe. Two other patients had continuous murmur at the access site, with arteriovenous fistulas diagnosed by Doppler, which spontaneously occluded within the first month after the procedure.
DiscussionPercutaneous occlusion of interatrial septal defects, under the echocardiogram vision, has been safely and accurately performed. It corresponds to the method of choice for guiding the procedure in most services. Although it is the most widely used method, due to its image quality and the possibility of using the three-dimensional mode, the TEE has as disadvantages the need for sedation or general anesthesia to tolerate the endoesophageal probe, and the need for another professional to perform it. In turn, the ICE can be performed by the interventionist, under local anesthesia or mild sedation only, facilitating the procedure schedule.1,2 It is simple to perform and has a short learning curve. The technique consists of conducting a probe through a venous access, with the femoral vein being the most often used. The use of a diagnostic catheter as a guide or for contrast injection in the lateral part of the sheath allows the probe to be easily conducted. Once in the right atrium, the images can be obtained clearly and safely through the probe movements, such as clockwise/counterclockwise rotation, anterior/posterior and lateral/medial movements, as well as cranial and caudal movements whenever necessary.3,4
In literature, studies show the efficacy and safety of the images obtained by the ICE compared to those obtained by TEE.1,5 Based on the authors’ experience, it has been observed that a second venipuncture carries little risk and has a manual compression time similar to that employed when performing only one puncture. Although some authors recommend the “Figure-of-Eight” suture6 to shorten the puncture site compression time, these results were not reproduced in the present cases.
Some publications report the use of ICE for interventions in children,7,8 which can be safely performed, especially in those weighing more than 15kg, by puncturing the two femoral veins using a 8 F sheath. However, in the present series, ICE was not used in children, as the need for general anesthesia eliminated one of its advantages, and thus, it was preferred in these cases to use TEE with a pediatric probe.
When the procedures guided by ICE started, the authors already had experience with more than 600 cases performed with the TEE, a fact that allows us to make a critical analysis of the two methods. It was found that the good quality of the images obtained by ICE provides the same safety as the TEE. According to the results of this study and other publications, the ICE is safe, easy to perform, and effective for guiding percutaneous interventions in interatrial septal defects.9,10 However, multicenter randomized studies comparing the two imaging modalities have not yet been performed; these studies are necessary to compare the accuracy, reproducibility, and clinical outcomes.
LimitationsA current limitation of ICE is the considerable size of the sheaths 11 F and 9 F. Potential risks include the formation of arteriovenous fistulae or perivascular bleeding, thrombosis, and embolism, which were not observed in this study. Another limitation was related to the high cost of probes, which should be considered against simpler procedures, without the need for general anesthesia. The re-sterilization and reuse of probes are allowed in Germany and other countries of Eastern Europe, which helps to reduce costs. Finally, it is necessary to supplement the training of interventionists, considering that the learning curve is faster for those with prior training in echocardiography.
ConclusionsIntracardiac echocardiography provided precise anatomical information to guide the successful occlusion of interatrial septal defects, eliminating the major disadvantages of transesophageal echocardiography and giving the interventionist procedural control, without the need for additional echocardiographic support.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.