To describe persistent pulmonary abnormalities detected on HRCT after 18 months of SARS-CoV-2 pneumonia, and to determine their extension and correlation with pulmonary function.
Patients and methodsA prospective cross-sectional study with an initial cohort of 90 patients in follow-up due to persisting lung abnormalities on imaging, functional respiratory impairment and/or respiratory symptoms. Of these, 31 (34%) were selected for analysis due to the persistence of their lung abnormalities on HRCT at 18 months after infection. A double reading was performed for each HRCT (62 observations).
ResultsOf the 31 patients included: 20 (65%) were men; mean age was 67 years; 17 (55%) were smokers/ex-smokers. The mean hospitalisation time was 38 days. Eighteen (58%) patients were admitted to intensive care units. Five patients (16%) suffered an acute pulmonary thromboembolism and three (9.7%) had a pneumothorax. The mean time between the onset of pneumonia and the follow-up HRCT was 20.34 months. Nineteen percent of patients suffered from total lung function abnormalities; and ground-glass opacities and reticulation were present in 12% and 4.5% respectively. The findings of the 62 readings were: ground-glass opacities (100%), reticulation (83%), subpleural curvilinear lines (62%), parenchymal bands (34%), traction bronchiectasis (69%), displacement of vessels/fissures (46%) and honeycombing (4.9%).
Pulmonary function 18 months after the acute episode revealed a mean FVC of 92% of predicted value, with an FVC < 80% of predicted value in 11 patients (35.4%). Mean DLCO was 71% of predicted value, with a DLCO < 80% in 22 patients (70%).
We observed a statistically significant relationship between total lung function abnormalities on HRCT and FVC (P < 0.05), and a trend towards statistical significance with DLCO (P = 0.051); there was a statistically significant relationship between the presence of ground-glass opacities and FEV1/FVC (P < 0.01). The relationships between reticulation and FVC, FVC%, FEV1, FEV1% and DLCO% were also considered statistically significant (P < 0.05).
ConclusionPersistent interstitial lung abnormalities are seen on HRCT for a subset of patients infected with SARS-CoV-2 pneumonia. Seventy percent of these patients suffered a slight decrease in DLCO.
Describir después de 18 meses, de una neumonía por SARS-CoV-2, las alteraciones pulmonares persistentes en la TCAR, valorar la extensión de las lesiones y su relación con la función respiratoria.
Pacientes y métodoEstudio transversal prospectivo de 90 pacientes en seguimiento por persistencia de alteraciones radiológicas, funcionales y/o síntomas respiratorios. Se seleccionaron 31 pacientes (34%) que mantenían alteraciones pulmonares en la TCAR a los 18 meses. Se realizó una doble lectura de las tomografías (62 observaciones).
ResultadosDe los 31 pacientes: 20 eran hombres (65%), edad media 67 años; 17 pacientes (55%) eran exfumadores o fumadores; el tiempo medio de hospitalización fue de 38 días, necesidad de cuidados respiratorios intermedios en 18 pacientes (58%). Cinco pacientes (16%) tuvieron tromboembolismo pulmonar agudo, y tres neumotórax (9,7%). El tiempo medio desde la neumonía hasta la TCAR fue de 20,3 meses. La extensión de las alteraciones pulmonares totales, de las opacidades de vidrio deslustrado y de la reticulación fue del 19%, 12% y 4,5%, respectivamente. Los hallazgos de las 62 lecturas fueron vidrio deslustrado (100%), reticulación (83%), opacidades curvilíneas subpleurales (62%), bandas parenquimatosas (34%), bronquiectasias por tracción (69%), desplazamiento de vasos o cisuras (46%) y panalización (4,9%).
La situación funcional respiratoria a los 18 meses mostró una FVC media de 92% del valor predicho, con FVC menor del 80% del valor predicho en 11 pacientes (35,4%), y una DLCO media del 71% del valor predicho, con una DLCO menor del 80% en 22 pacientes (70% ).
Se observó relación significativa entre la extensión total de las alteraciones en la TCAR con la FVC (P < 0,05), con la DLCO hubo una tendencia a la relación significativa (P = 0,051); existió una relación significativa entre la extensión de las opacidades de vidrio deslustrado y FEV1/FVC (P<0,01). La extensión de la reticulación presentó asociación significativa (P < 0,05) con la FVC, la FVC%, la FEV1, la FEV1% y la DLCO%.
ConclusiónUn porcentaje de pacientes que han padecido neumonía por SARS-CoV-2 persisten con alteraciones intersticiales en la TCAR. El 70% mostró una disminución leve de la DLCO.
More than three years into the pandemic caused by SARS-CoV-2, there is still no clear evidence on the proportion of survivors who, after suffering severe pneumonia, will have long-term pulmonary sequelae or develop the progressive fibrosing interstitial lung disease (ILD).1–9
Several publications10,11 have presented imaging findings of SARS-CoV-2-induced pneumonia from the initial diagnosis to later stages. Acute manifestations include ground-glass opacities, crazy-paving pattern (ground glass with superimposed septal thickening) and alveolar consolidations, the extent of which vary. These lesions are consistent with patterns of organising pneumonia, acute fibrinous and organising pneumonia or diffuse alveolar damage.4 Some of the most frequent complications described in acute infection caused by SARS-CoV-2 are pulmonary embolism, pneumothorax, pneumomediastinum and infections.10,11
The most frequent persistent radiological abnormalities on high-resolution computed tomography (HRCT) are ground-glass opacities, reticulation, subpleural curvilinear lines, parenchymal bands and fibrotic interstitial abnormalities (in at least 25% of cases).4–9
The clinical course of the disease can vary from asymptomatic patients with no pulmonary function impairment to those with pulmonary sequelae and pulmonary function impairment and/or impact on quality of life. Numerous questions remain as regards the persistence of symptoms, functional and/or radiological abnormalities, as well as the possibility that these will develop into progressive pulmonary fibrosis secondary to SARS-CoV-2-induced pneumonia,1,3,12 all of which must be evaluated by specialised units. There are few publications on the most appropriate protocols for the follow-up of these patients.12–16
The proportion of imaging-detected persistent lung lesions varies widely in different publications depending on factors such as the initial severity of the condition, the duration of follow-up or the type of consultation. Some articles have shown sequelae on HRCT in up to 30–38% of patients with severe pneumonia in the acute stage.6,17–19 Certain radiological patterns that persist after several months may indicate progression to fibrotic forms.2,9,17,20
This study aims to describe the pulmonary imaging findings from HRCT of the chest starting at 18 months after the acute episode of SARS-CoV-2-induced pneumonia.
Patients and methodsWe conducted a prospective cross-sectional study, approved by the hospital's drug research ethics committee. We followed good clinical practice standards and current legislation on personal data protection at all times. Patients signed the informed consent form to participate in the study.
The inclusion criteria required patients to be over 18 years of age, diagnosed with SARS-CoV-2-induced pneumonia which required hospitalisation or respiratory intensive care and who were attending a specialist ILD clinic from May 2020 to July 2022. We had clinical data for all patients included in the study from the acute episode as well as the results from pulmonary function tests (PFTs) starting at 18 months after the acute episode.
Patients with suspected ILD prior to infection caused by SARS-CoV-2 were excluded. This diagnosis was established on the basis of detailed respiratory medicine records and the assessment of imaging studies from prior to the acute onset of pneumonia. We also excluded patients whose abnormalities on HRCT had nearly entirely resolved, or patients whose pulmonary lesions were not attributable to SARS-CoV-2-induced pneumonia sequelae (extensive pulmonary emphysema, scarring, post-inflammatory bronchiectasis).
Chest HRCT scans were performed on a 64-row multidetector scanner (General Electric). The volumetric acquisition mode was used with thin slices of 1.25 mm or smaller, using a high spatial frequency reconstruction algorithm. Images were acquired at maximum inspiration from lung apices to bases. We also obtained multiplanar reconstructions. No intravenous contrast was administered.
We followed the visual system described by Goh et al.21 to determine the total extent of lung abnormalities, ground-glass opacities and reticulation in percentages on HRCT images of the chest. For each lobe we recorded the presence of the following findings: traction bronchiectasis, subpleural curvilinear lines, parenchymal bands, architectural distortion (abnormal displacement of bronchi, vessels, fissures or septa due to lung disease) and honeycombing (according to the Fleischner Society nomenclature).22 The presence of any of the following findings was considered indicative of fibrotic subtype: coarse reticulation, traction bronchiectasis, architectural distortion and/or honeycombing.15,20
We noted the following clinical variables: age, sex, tobacco use, length of hospitalisation for acute pneumonia and need for admission to the respiratory intensive care unit, pulmonary embolism or pneumothorax during the acute episode. Pulmonary function was tested throughout follow-up. For this study, we used PFTs taken within 30 days of the HRCT of the chest taken 18 months after the acute episode of SARS-CoV-2-induced pneumonia. The variables collected were FVC (litres), predicted FVC%, FEV1 (litres), predicted FEV1%, FEV1/FVC ratio and predicted DLCO%.
All images were analysed by two radiologists with 25 and 30 years of experience in thoracic radiology, in a blinded and independent manner. A total of 62 observations were inputted into the model for the CT analysis.
Statistical analysisFor the statistical analysis, a univariate model was used in which the repeated measures (readings from the two observers) are nested for each patient. In this way the effect of the reader was corrected for. Since there were 31 patients, each with two readings, 62 observations were evaluated.
Because the dependent variables derived from the radiological study (percentage of disease extent according to Goh et al. criteria21) follow a gamma distribution, we used a generalised linear model in R to adapt the regression study to our sample, which does not follow a normal distribution. Subsequently, we grouped the data by patient (31 groups) and repeated the univariate analysis, with the aim of reducing both intra- and inter-patient variability. We established p < 0.05 as the threshold for statistical significance.
We used the kappa concordance index to evaluate the interobserver variability of the qualitative variables (traction bronchiectasis, subpleural curvilinear lines, parenchymal bands and architectural distortion [abnormal displacement of bronchi, vessels, fissures, or septa]). We considered the following concordance ranges: 0–0.2; 0.21–0.40; 0.41–0.60; 0.61–0.80; 0.81–1 as poor, slight, moderate, high and very high concordance, respectively. We calculated concordance of the quantitative variables (overall/ground-glass/reticulation extent in percentage) using Pearson's rho correlation coefficient.
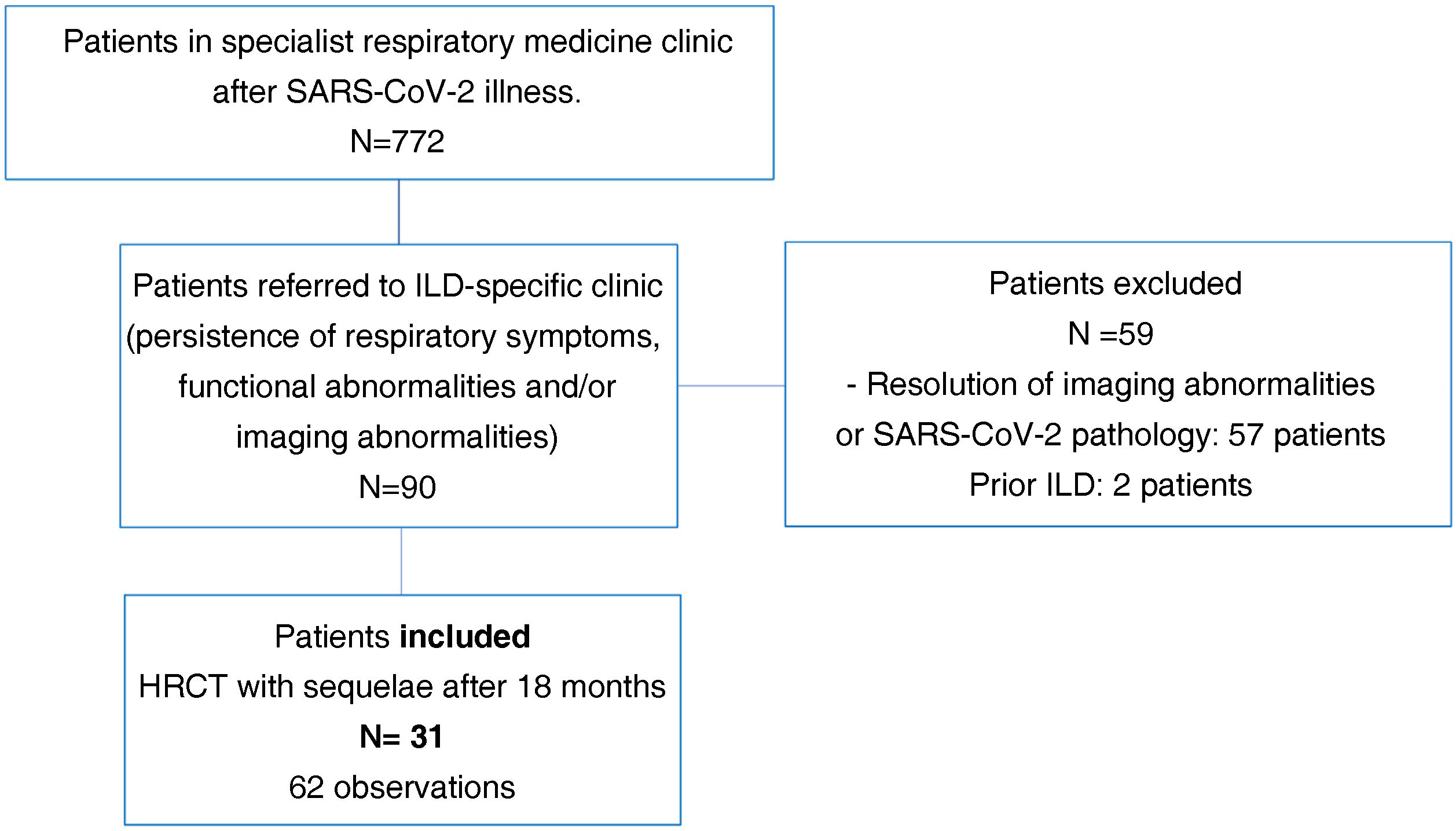
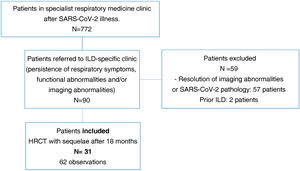
ResultsA total of 772 patients were assessed after discharge from hospital in post-COVID-19-specific respiratory medicine clinics. Of these, 90 patients were referred to the specialist ILD clinic at the same centre due to persistent radiological and functional abnormalities and/or persistent respiratory symptoms.
We excluded two patients suspected of a prior ILD and 57 patients with near-total resolution of abnormalities on HRCT or findings not attributable to SARS-CoV-2-induced pneumonia sequelae.
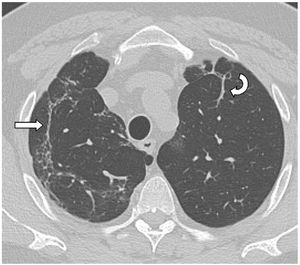
Finally, we selected 31 patients (34%) for analysis. All had a follow-up volumetric HRCT that continued to show imaging abnormalities from 18 months after the acute episode of SARS-CoV-2-induced pneumonia during the inclusion period (Fig. 1).
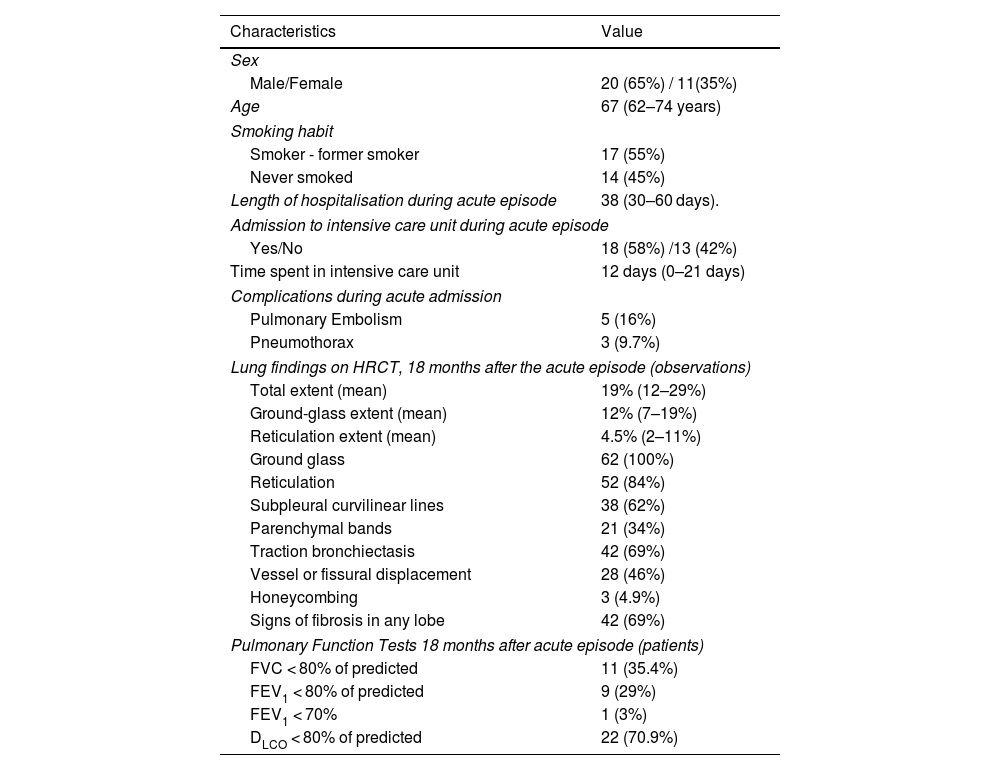
Of the 31 patients included in the study, 20 were male (65%). The mean age at admission for SARS-CoV-2-induced pneumonia was 67 years (range: 62–74 years); 17 patients were smokers or former smokers (55%). The mean length of hospitalisation was 38 days (range: 30–60 days). Eighteen patients required admission to the respiratory intensive care unit (58%) with mean length of stay of 12 days (range: 0–21 days). Complications during the acute episode included five patients who presented with pulmonary embolism (16%) and three with pneumothorax (9.7%) (Table 1).
Clinical characteristics of 31 patients and HRCT findings at 18 months in 62 observations.
| Characteristics | Value |
|---|---|
| Sex | |
| Male/Female | 20 (65%) / 11(35%) |
| Age | 67 (62–74 years) |
| Smoking habit | |
| Smoker - former smoker | 17 (55%) |
| Never smoked | 14 (45%) |
| Length of hospitalisation during acute episode | 38 (30–60 days). |
| Admission to intensive care unit during acute episode | |
| Yes/No | 18 (58%) /13 (42%) |
| Time spent in intensive care unit | 12 days (0–21 days) |
| Complications during acute admission | |
| Pulmonary Embolism | 5 (16%) |
| Pneumothorax | 3 (9.7%) |
| Lung findings on HRCT, 18 months after the acute episode (observations) | |
| Total extent (mean) | 19% (12–29%) |
| Ground-glass extent (mean) | 12% (7–19%) |
| Reticulation extent (mean) | 4.5% (2–11%) |
| Ground glass | 62 (100%) |
| Reticulation | 52 (84%) |
| Subpleural curvilinear lines | 38 (62%) |
| Parenchymal bands | 21 (34%) |
| Traction bronchiectasis | 42 (69%) |
| Vessel or fissural displacement | 28 (46%) |
| Honeycombing | 3 (4.9%) |
| Signs of fibrosis in any lobe | 42 (69%) |
| Pulmonary Function Tests 18 months after acute episode (patients) | |
| FVC < 80% of predicted | 11 (35.4%) |
| FEV1 < 80% of predicted | 9 (29%) |
| FEV1 < 70% | 1 (3%) |
| DLCO < 80% of predicted | 22 (70.9%) |
DLCO: diffusing capacity of lung for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
In qualitative variables, percentages of the total are given in brackets. Quantitative variables are expressed as a median, with the interquartile intervals in parenthesis.
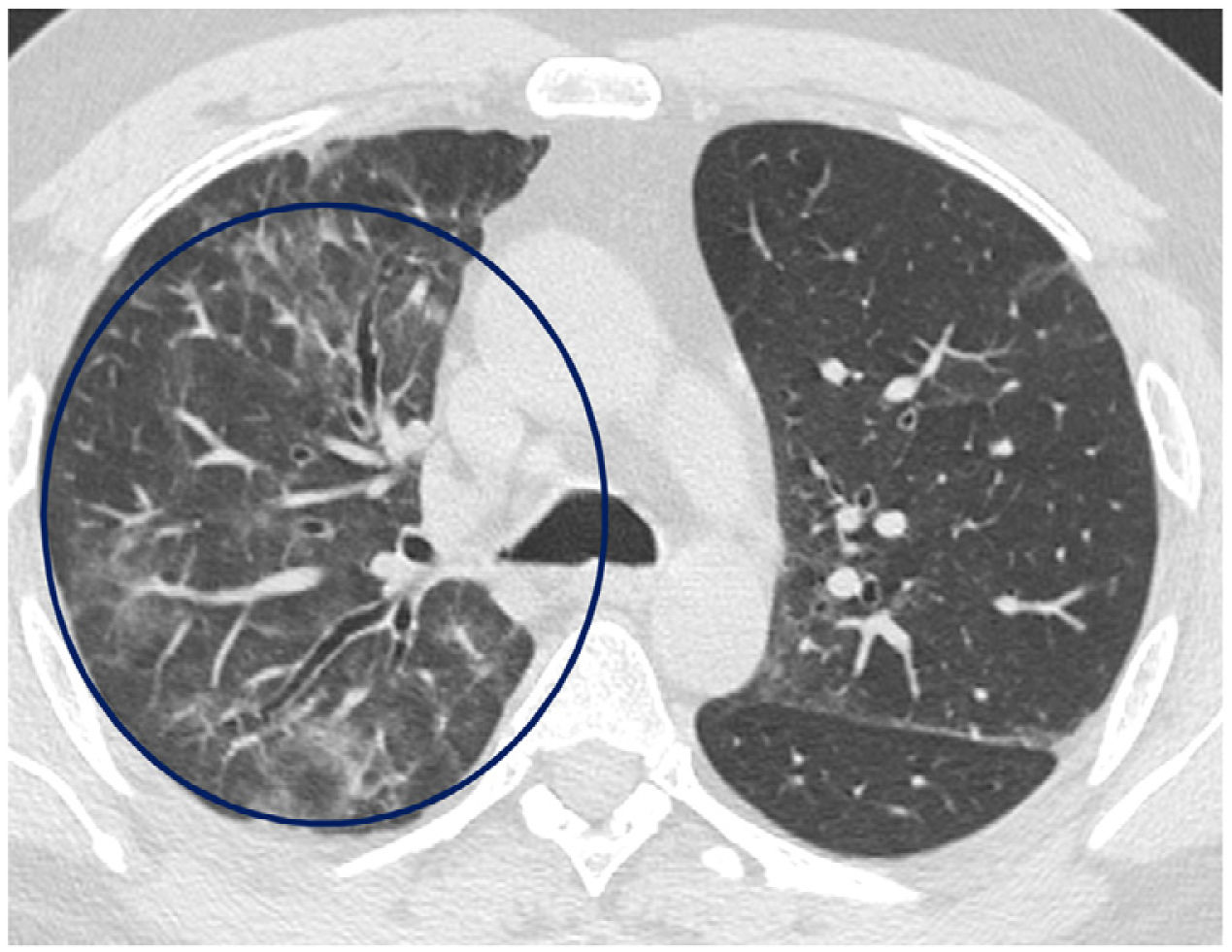
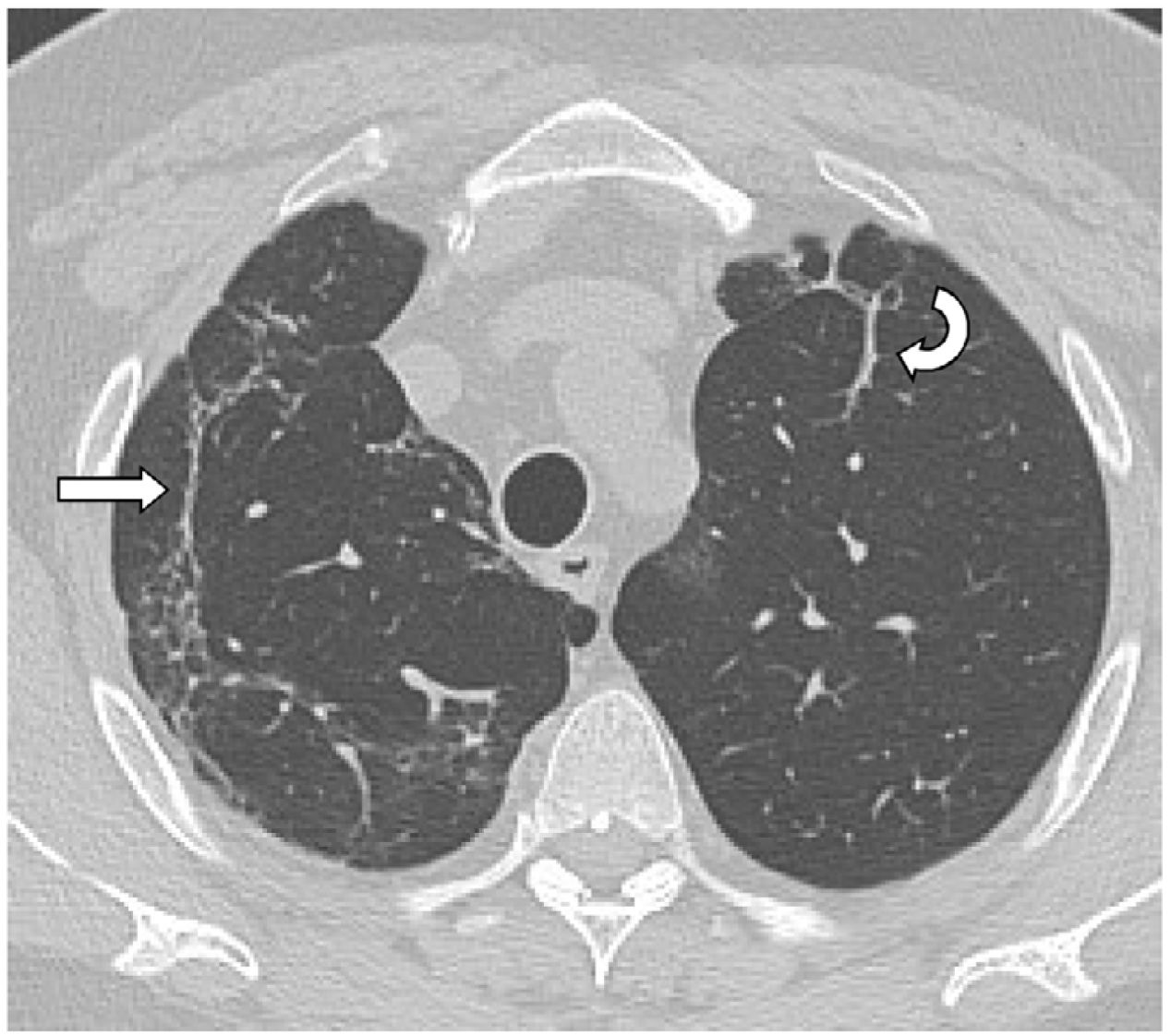
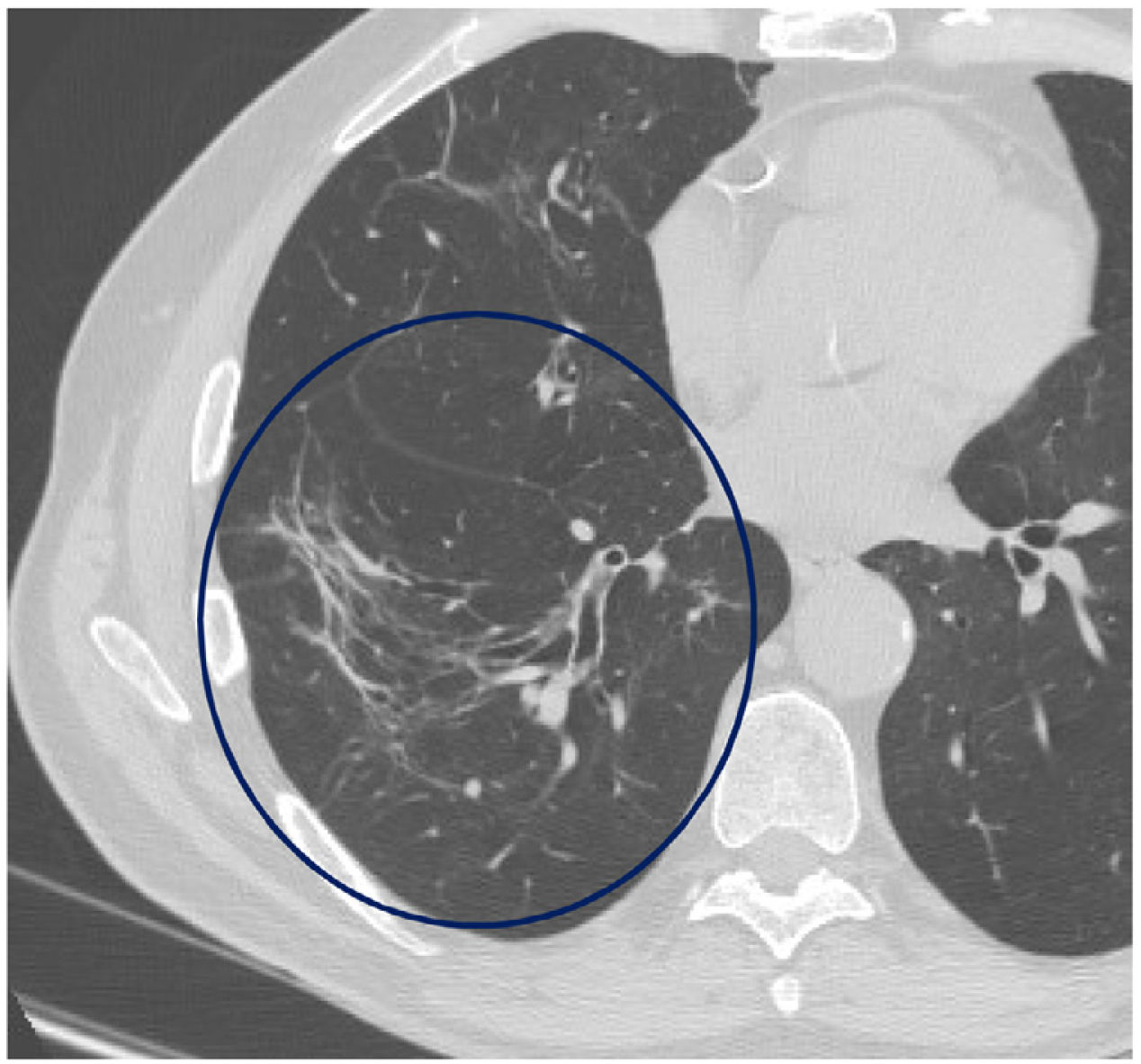
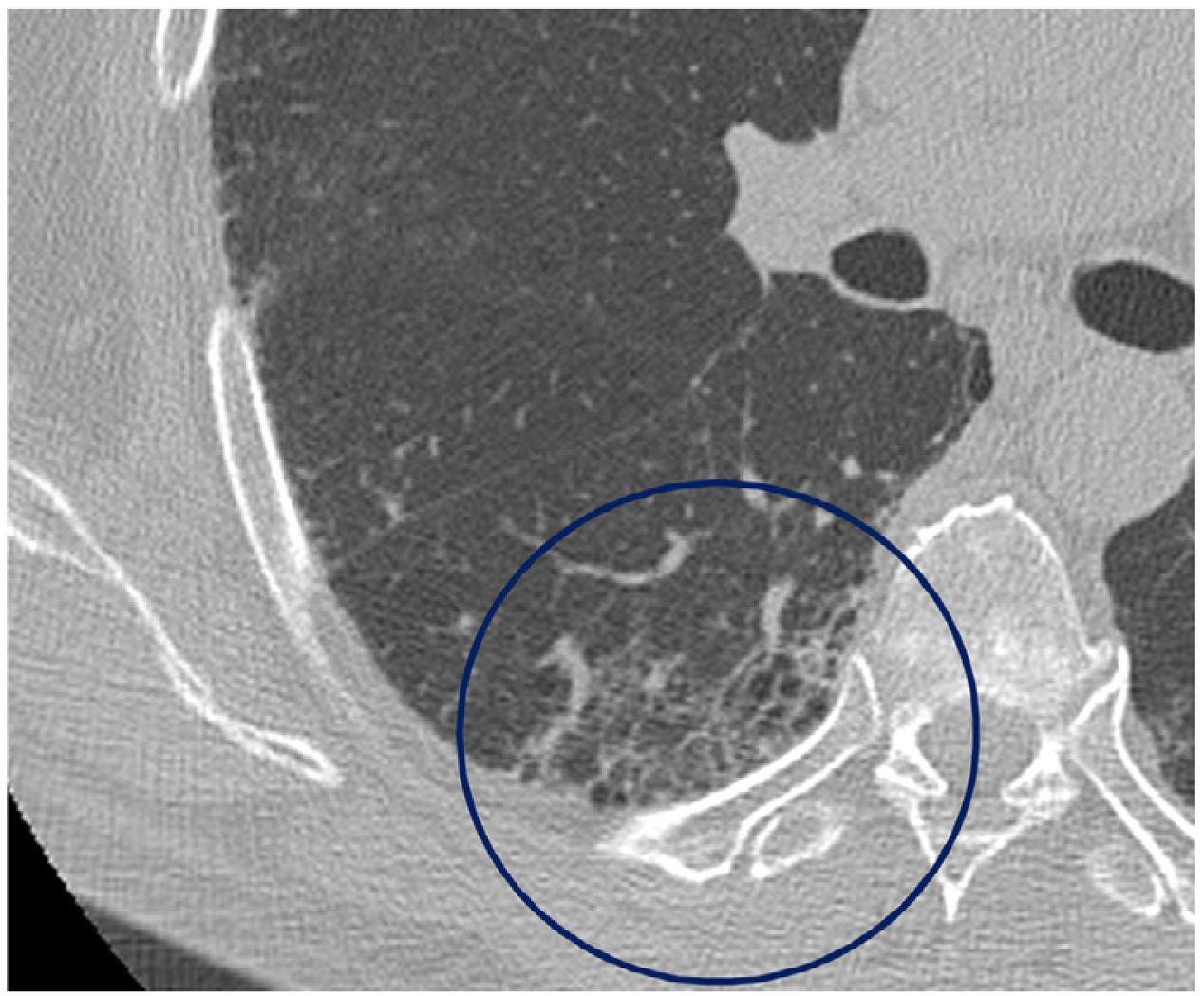
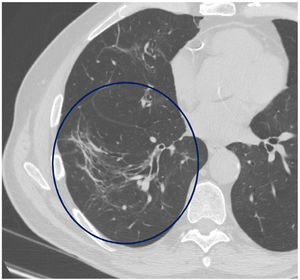
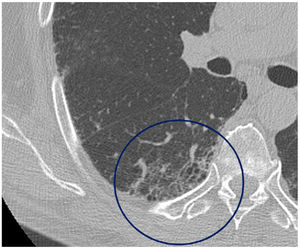
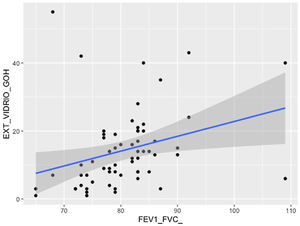
The mean time from the onset of SARS-CoV-2-induced pneumonia to chest HRCT was 20.34 months (18–24 months). We observed a mean total extent of radiological abnormalities on HRCT of 19%, with the proportion of ground-glass opacities being 12% (Fig. 2) and the extent of reticulation being 4.5%. We observed ground-glass opacities in 62 observations (100%), reticulation in 52 observations (84%), subpleural curvilinear lines in 38 observations (62%) (Fig. 3), parenchymal bands in 21 observations (34%), traction bronchiectasis in 42 observations (69%), distortion with vessel or fissure displacement in 28 observations (46%) (Fig. 4) and honeycombing in three of the observations (4.9%) (Fig. 5)
Fibrotic signs were present in one lobe in 69% of the observations. In 24% of the observations fibrotic signs were present in one or two lobes. Three lobes were affected in 15%, four lobes in 16% and all lobes in 13%.
The interobserver agreement (kappa index) for traction bronchiectasis, subpleural curvilinear lines, parenchymal bands and architectural distortion was: 0.61 [0.32–0.91]; 0.43 [0.09–0.75]; 0.29 [0.06–0.53] and 0.52 [0.22–0.83], respectively. The Spearman’s correlation (rho coefficient) was 0.84 for the total extent; 0.82 for the proportion of ground-glass; and 0.71 for the extent of reticulation.
At 18 months after the acute episode of pneumonia, PFTs showed mean values of: FVC 2.75 l (92% of the predicted value); FEV1 of 2.23 l (96% of the predicted value); FEV1/FVC ratio of 80% and DLCO of 71% of the predicted value. Eleven patients (35.4%) suffered decreased FVC% (< 80%). Nine patients (29%) had lower FEV1 (< 80%). One patient (3%) had a FEV1/FVC ratio (< 70%). In 20 patients (64%) these PFTs were normal. Abnormal DLCO% (< 80%) was observed in 22 patients (70.9%).
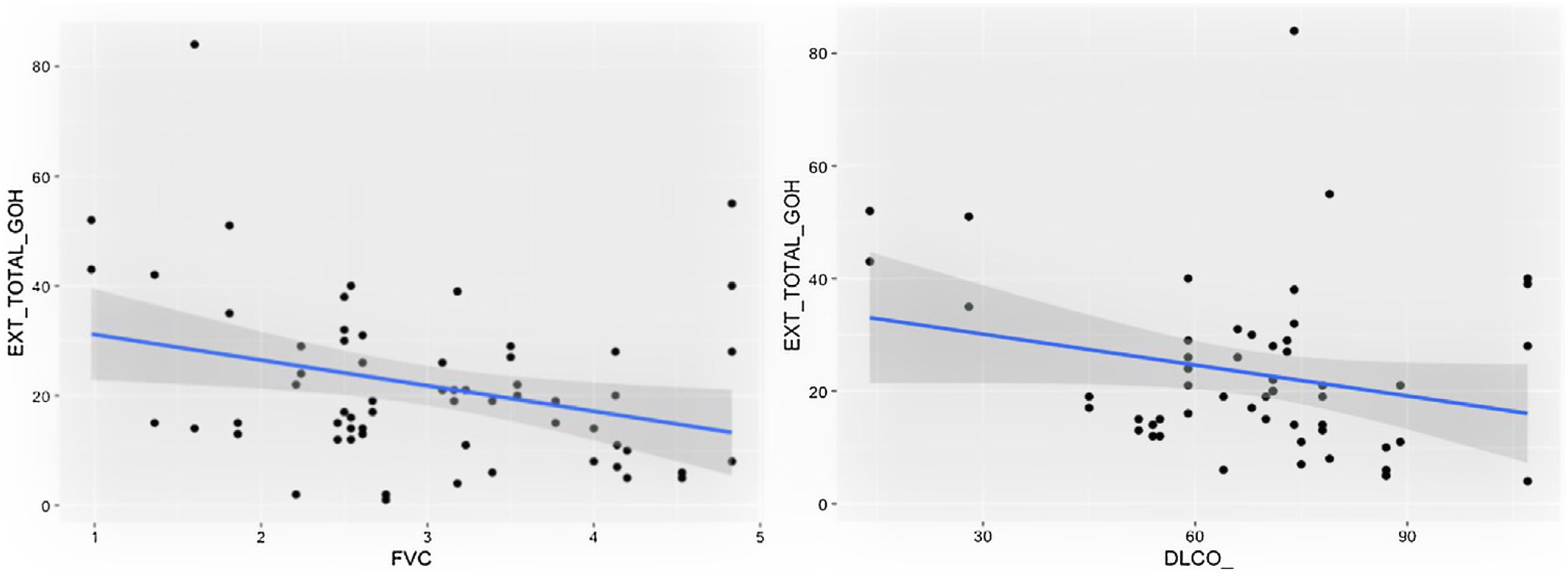
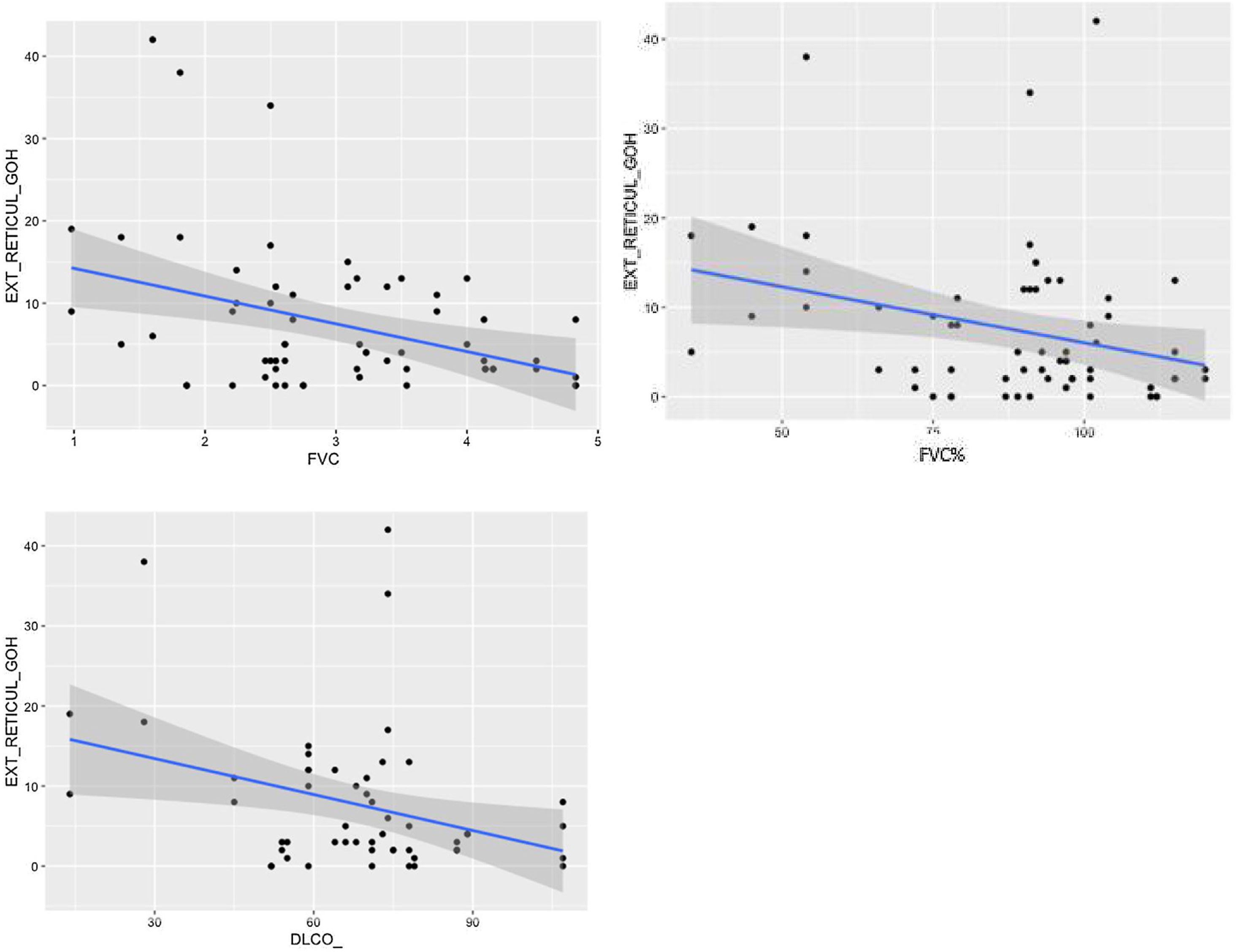
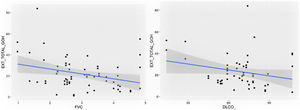
Comparison of radiological variables with clinical data and pulmonary function testsIn the univariate analysis, the total visual extent of HRCT abnormalities was found to be significantly associated (p < 0.05) to FEV1, FEV1% and FVC. A trend approaching significance (p = 0.051) was found between the total extent of lung abnormalities and the DLCO% value (Fig. 6).
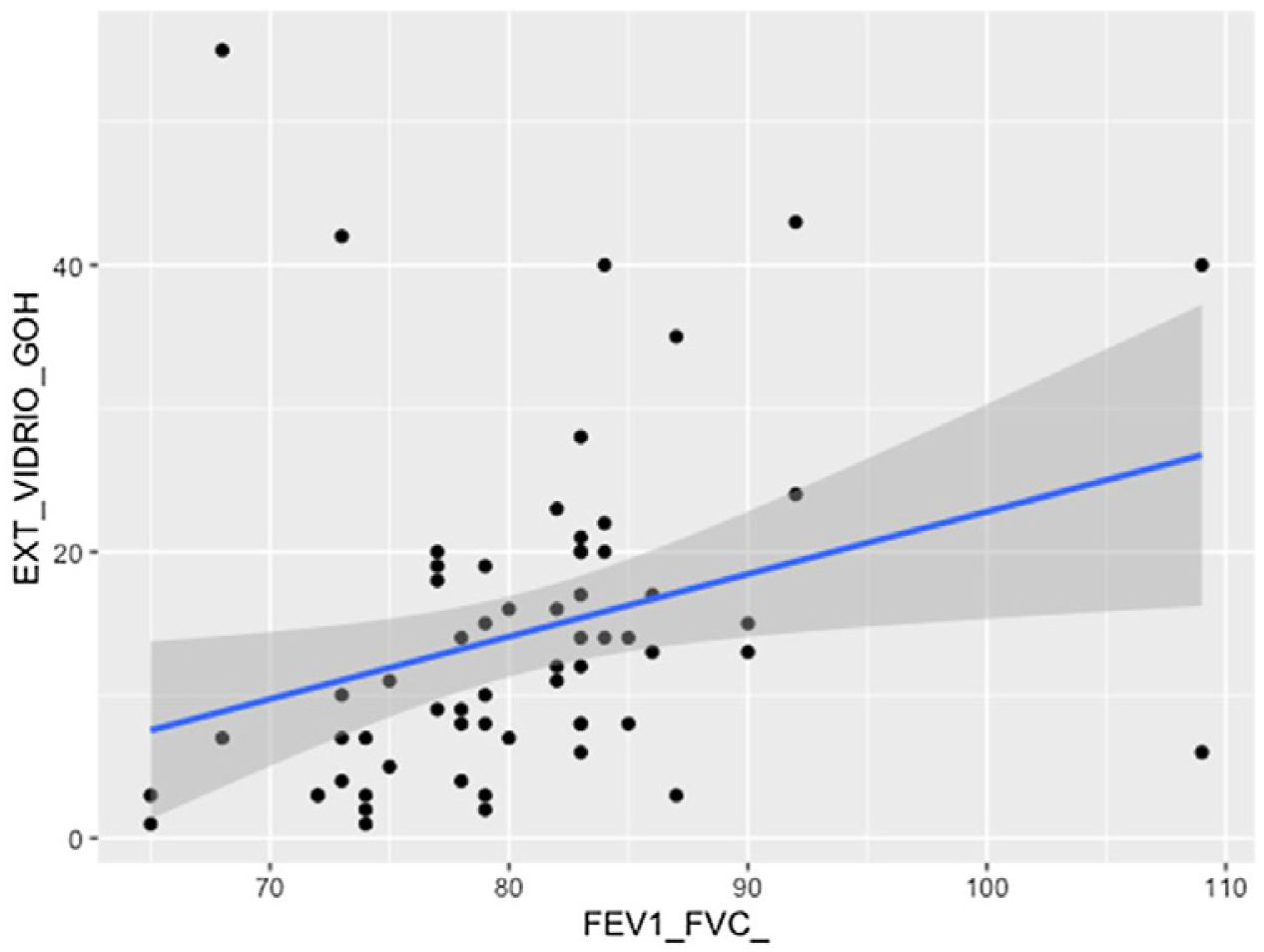
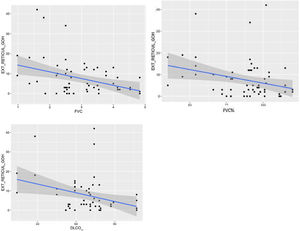
The visual extent of ground-glass opacities and the FEV1/FVC ratio was found to be associated (p < 0.01) (Fig. 7). The extent of reticulation demonstrated a statistically significant association (p < 0.05) to FVC, FVC%, FEV1, FEV1% and DLCO% (Fig. 8).
An association approaching statistical significance was found between vessel and fissural displacement and both FEV1% (p = 0.051) and predicted FVC% (p = 0.053).
There was a statistically significant trend (p = 0.07) between the total extent of abnormalities on HRCT and pneumothorax during initial admission for pneumonia.
DiscussionPulmonary abnormalities persist in the long term in some patients who have suffered SARS-CoV-2-induced pneumonia, the prevalence of which ranges from 30% to 39% in different publications. This depends on the number and type of patients included in the study, the reason for the hospital visits, the severity of the acute episode and at what point during follow-up the data are analysed.2,6,9,15,17 These interstitial lung sequelae after SARS-CoV-2-induced pneumonia are more pronounced in older men who have had a more severe episode of pneumonia.2,17
In our study, we analysed 90 patients who suffered acute SARS-COV-2-induced pneumonia requiring hospitalisation or admission to respiratory intensive care, who were referred to an ILD specialist clinic for follow-up by a specific post-COVID-19 respiratory medicine unit due to persistent interstitial lung abnormalities. Of these, 31 (34%) continued to show abnormalities on HRCT of the chest 18 months after the acute pneumonia episode and within the study period.
Most published studies present follow-up data from the first year after acute illness.2,6,17,19,20 Recently, Han et al.15 reported the results of SARS-CoV-2-induced pneumonia progression at two years in 144 patients. Thirty-nine per cent of the participants had interstitial lung abnormalities on HRCT of the chest (23% with fibrotic and 16% with non-fibrotic forms) and also showed a decrease in DLCO. The remaining 88 patients (61%) showed complete radiological resolution. Similar results were observed in our study, where 34% of the patients analysed presented with abnormalities 18 months after the acute episode, with said abnormalities having resolved in 63% of cases.
The radiological findings in our series consisted of ground-glass opacities present in all patients, with a mean extent of approximately 12%. Reticulation associated with ground-glass opacities or isolated reticulation appeared in 84% of the cases. These findings are the most common and coincide with those reported in other publications.2,9,15,17,19,20 We found subpleural curvilinear lines or parenchymal bands in 62% and 34% of cases, respectively, a finding also reported in other series, probably reflecting the progression to an organising pneumonia pattern.14,20
Certain abnormalities that may indicate fibrotic sequelae, defined by some authors as ‘post-COVID fibrosis’ or as fibrotic abnormalities,15,20 include findings such as traction bronchiectasis, coarse reticulation, architectural distortion and volume loss, and have been highly frequent in our study, since they affected at least one lobe in 69% of cases, and the entire lung in 13%. Han et al.15 demonstrated that 23% of patients who had suffered severe SARS-CoV-2-induced pneumonia showed fibrotic-type abnormalities at two years. In the meta-analysis published by Watanabe et al.,17 20.6% of patients showed fibrotic abnormalities. Honeycombing, however, was scarce in our series, as in previous publications which have made references to ‘fibrosis without honeycombing’.2,14,20 The higher frequency of these fibrotic manifestations in our study could be explained by the fact that of the 772 patients being seen at the post-COVID-19 respiratory medicine clinic, we selected the 90 patients (11%) who had been referred to the ILD-specific clinic as a result of persistent respiratory symptoms and radiological abnormalities during follow-up. Our data indicate that a small number of patients, approximately 10%, with a history of moderate/severe SARS-COV-2-induced pneumonia present with long-term radiological abnormalities after the acute episode. Some authors propose that these post-COVID-19 abnormalities should be included within fibrotic imaging patterns.14,15
Few studies describe histopathological findings in patients with long-term follow-up after SARS-CoV-2-induced pneumonia. Bronchiolocentric interstitial fibrosis with architectural distortion and extracellular matrix deposition has been confirmed by transbronchial biopsies performed between 4 and 15 months after the acute phase.12 Three groups of histological findings have been described in transbronchial cryobiopsies of patients with persistent symptoms and residual radiological abnormalities: chronic fibrosis (suggestive of interstitial disease prior to SARS-CoV-2 infection), acute-subacute damage (including organising pneumonia, fibrotic non-specific interstitial pneumonia and diffuse alveolar damage) and vascular abnormalities (diffuse vascular dilation, distortion or enlargement in an otherwise normal lung parenchyma).23 No invasive diagnostic procedures were performed on the patients included in our study to obtain histology specimens during follow-up.
In our series we found that the total extent of the abnormalities (ground-glass opacities and reticulation) correlated with FVC, FVC%, FEV1 and FEV1% values. We want to highlight the statistically significant association between the extent of reticulation (a parameter that can be considered fibrotic) and FVC, FVC%, FEV1, FEV1% and DLCO% values. Other radiological manifestations of fibrotic character (vessel and fissural displacement) were linked to lower predicted FVC% and FEV1% values in the spirometry tests. This is similar to that found in patients with fibrotic ILD, although in our study only 11 patients (35%) had an FVC of less than 80%. The lung function parameter that most frequently continued to show abnormalities at 18 months was a decreased DLCO% in 70% of cases. The mean DLCO% value was 71% of predicted (59%–78%) (mild decrease in DLCO), in line with other publications.15,17 These PFT data suggest that the long-term evolution for a third of SARS-CoV-2-induced pneumonia patients involves interstitial pulmonary abnormalities or sequelae with little impact on respiratory function. A multicentre study analysing the data from 284 patients hospitalised 12 months prior for SARS-CoV-2-induced pneumonia found alterations to DLCO value in 39.8% and fibrotic-type imaging abnormalities in 22.7% of patients.24
Faverio et al.,25 in a multicentre study with follow-up at 12 months of 287 patients hospitalised for SARS-CoV-2-induced pneumonia, found a mild decrease in DLCO and interstitial pulmonary abnormalities in between 46% and 80% of cases, which increased in frequency as severity of the acute episode increased. The main interstitial abnormalities were ground-glass opacities and reticulation, findings very similar to those in our study.
Considering the radiological progression in our patients 18 months after the initial pneumonia episode, it is likely that we are dealing with irreversible sequelae, with little functional impairment. In the case of the pandemic caused by SARS-CoV-1, which was characterised by acute pulmonary findings very similar to those of SARS-CoV-2, improvement in morphological abnormalities occurred in the first year and remained stable for the following 14 years.26 Although there is still no consensus on the follow-up of these patients, in our opinion, and according to some publications, a multidisciplinary approach is recommended.13 Patients with persistent interstitial abnormalities can be managed through specialist ILD clinics where there are already established multidisciplinary teams, mainly formed by expert pulmonologists, pathologists and radiologists. Follow-up after SARS-CoV-2-induced pneumonia should be tailored according to the severity of the initial acute episode, symptoms and persistent clinical signs. The basic workup includes a chest radiograph and PFTs. In patients with suspected pulmonary sequelae, an HRCT of the chest, including expiratory slices, is recommended, and in case of suspected pulmonary embolism, an CT pulmonary angiogram or a dual-energy CT scan.3,9,14
There are limitations in our study: it is a series with a small number of patients, and therefore a statistical analysis has been performed with two readings of each image in order to increase the sample size. Although cases suspected of having ILD prior to the episode of acute pneumonia were excluded from the study, this is difficult to establish on some occasions when there were no previous imaging studies available for the patients. We have not analysed data such as mosaic pattern or air trapping, or vascular abnormalities that have been described as highly prevalent in some series.27 A visual method of analysis of CT scans described by Goh et al.21 has been used, which is not specific for post-COVID-19 abnormalities, and the limitations of qualitative measurement methods must also be considered. In the near future, automatic quantification methods will provide more objective and reliable information for imaging studies.
ConclusionsLong-term lung abnormalities persist in approximately 11% of patients with SARS-CoV-2-induced pneumonia who required hospitalisation and/or admission to respiratory intensive care. Of these patients, 34% continue to show interstitial pulmonary abnormalities beyond 18 months following the acute pneumonia. Ground-glass opacities are the most frequent abnormality seen on HRCT. Fibrotic abnormalities appear in more than 50% of cases. However, persistent interstitial abnormalities have little impact on respiratory function, with 70% of patients presenting a mild decrease in carbon monoxide diffusion capacity.
Author contributionsClaudia Valenzuela: study concept, design and manuscript review.
Luisa de la Fuente: case entry into database.
Susana Hernández: image interpretation and manuscript review.
María José Olivera: data collection, image interpretation.
Carlos Molina: database development and data collection.
Nuria Montes: statistical study.
Carmen Benavides: data collection and manuscript review.
Paloma Caballero: study concept, design and manuscript draft.
Conflict of interestsClaudia Valenzuela: fees for participating in talks and consulting for Boehringer Ingelheim, Hoffman-La Roche, Ltd. not linked to this publication. The other authors have no conflict of interests to declare.