To evaluate the diagnostic capacity of ultrasonography (US) for differentiating between malignant and benign thyroid nodules and its usefulness in obviating unnecessary invasive procedures.
Patients and methodsFrom January 2012 through December 2014, a total of 321 fine-needle aspiration biopsy (FNAB) procedures were done in 302 patients selected according to the criteria recommended by the American Association of Clinical Endocrinology guidelines and the American Thyroid Association guidelines. We analyzed the following characteristics on US: location, size, morphology, contour, consistency, echostructure, echogenicity, calcifications, and vascularization. We used univariate and multivariate analyses to investigate the relationship between the US findings and thyroid cancer.
ResultsThe prevalence of malignancy in our study population was 5.92%. The US findings that were significantly associated with a greater probability of malignancy were microcalcifications, central vascularization, and hypoechogenicity. The US findings that were associated with a lower risk of malignancy were areas of colloid degeneration and nodule heterogeneity.
ConclusionOur results suggest that decisions about whether to perform FNAB should be based on the presence of suspicious US findings found with our statistic model rather than on the size of the nodule. Thus, unnecessary FNAB procedures on nodules without suspicious US characteristics can be avoided.
Evaluar la capacidad diagnóstica de la ecografía para diferenciar nódulos tiroideos benignos y malignos, y su utilidad para evitar procedimientos invasivos innecesarios.
Pacientes y métodosDe enero de 2012 a diciembre de 2014 se realizaron 321 procedimientos de punción-aspiración con aguja fina (PAAF) a 302 pacientes remitidos por el Servicio de Endocrinología del Centro Medico de Especialidades, seleccionados bajo los criterios de la Guía de la Asociación Americana de Endocrinología Clínica y de la Guía de la Asociación Americana del Tiroides. Se analizaron las siguientes características ecográficas: localización, tamaño, morfología, contorno, consistencia, ecoestructura, ecogenicidad, calcificaciones y vascularización. Se realizó un análisis univariante y multivariante para investigar la relación entre los hallazgos ecográficos y el cáncer de tiroides.
ResultadosLa prevalencia de malignidad en nuestra población de estudio es del 5.92%. Los hallazgos ecográficos que son estadísticamente significativos y están asociados a una mayor probabilidad de malignidad son la presencia de microcalcificaciones, la vascularización central y la hipoecogenicidad. Los hallazgos asociados a un menor riesgo son la presencia de halo, la existencia de áreas de degeneración coloide y la heterogeneidad de los nódulos.
ConclusiónSegún nuestro estudio, la indicación de realizar PAAF no debería basarse en el tamaño del nódulo, sino en la presencia de hallazgos ecográficos sospechosos de malignidad de acuerdo con las estimaciones de nuestro modelo estadístico. De esta forma sugerimos evitar realizar PAAF innecesarias en nódulos que no presenten dichas características.
Neck ultrasound is the diagnostic modality of choice and it is widely used in thyroid conditions. It is estimated that its use could show the existence of thyroid nodules in up to 67 per cent of the population of which approximately 5 per cent1 would be malignant. Ultrasound plays an essential role in thyroid evaluation, since it allows us to calculate its size distinguishing between (single or multiple) nodules and diffuse affectation with or without nodules. However though attempts have been made to determine what ultrasound characteristics distinguish malignant from benign nodules nowadays there is no consensus on which lesions should be biopsied. There are several practice guidelines for thyroid nodule management that suggest different strategies, but it is necessary to unify criteria.2 Given this problem it must be established what ultrasound findings indicate that the nodule is malignant or benign with an acceptable predictive value so in order to be able to decide and prioritize what nodules will undergo fine needle aspiration (FNA), because yet despite the fact that this is the best modality to distinguish benignity from malignancy, given its high sensitivity and specificity, it is an invasive procedure that does not provide immediate information and is subject to the uncertainties and errors of both sampling and analysis.1,3,4
The goals of this study are to analyze the diagnostic capacity of ultrasound to distinguish benign from malignant thyroid nodules, determine which individual or associated ultrasound findings can be considered high-risk factors of malignancy, and assess its utility to avoid unnecessary invasive procedures.
Material and methodsWe expressly declare our adherence to the Helsinki declaration and that the necessary written informed consents have been obtained to carry out all patients’ examinations.
PatientsThis retrospective, observational study included 302 patients evaluated between January 2012 and December 2014, with a total of 321 thyroid nodules diagnosed, of which 263 corresponded to women, with an average age of 57 years (range: 21–87 years), and 58 corresponded to men, whose average age was 59 years (range: 23–79 years). The study included all patients referred by the Endocrinology Unit of the Specialties Medical Center, during the above-mentioned three year-period, for the performance of thyroid FNPA (fine needle puncture aspirations) selected following the criteria of the American Association of Clinical Endocrinology Guidelines and the last iteration of the American Thyroid Guidelines5–7 which recommend performing biopsies:
- •
Nodules larger than 1cm with a high-suspicion ultrasound pattern, whose estimated malignancy risk is greater than 70–90 per cent, including solid hypoechoic nodules or with a partial cystic component, with one or more of the following ultrasound characteristics: irregular (infiltrating, spiculated or microlobulated) borders, microcalcifications, taller-than-wider shape, ring calcifications interrupted by a small component of hypoechoic soft parts and evidence of extra-thyroid expansion.
- •
Nodules larger than 1cm with an ultrasound pattern of immediate suspicion, whose estimated malignancy risk is 10–20 per cent, including solid hypoechoic nodules with smooth borders without microcalcifications, extra-thoracic expansion or taller-than-wider shape.
- •
Nodules larger than 1.5cm with a low-suspicion ultrasound pattern, whose estimated malignancy risk is 5–10%, including solid or partially cystic isoechoic or hypoechoic nodules with eccentric solid areas, without microcalcifications, irregular borders or extra-thyroid expansion, or taller-than-wider shape.
- •
Nodules larger than 2cm with a very low-suspicion ultrasound pattern, whose estimated malignancy risk is less than 3%, including partially cystic or spongiform nodules, without ultrasound characteristics described in low-, intermediate- and high-suspicion patterns.
- •
All nodules, regardless of their size, that have shown a significant growth in ultrasound controls or from patients with family antecedents or high-risk factors.
All patients whose nodules to be punctioned resulted ecographically anechoic and therefore of liquid nature were excluded from the study.
In case of multiple nodules, a FNPA is performed on those with ultrasound characteristics suspicious of malignancy, because if we were to puncture only the dominant nodule we might leave some cancer undiagnosed.8 Ultrasound (B-mode and color Doppler) was performed on all patients as well as an elastography and ultrasound-guided FNPA. The pathologist performed the cytological diagnosis based on the Bethesda-2007 classification.9 In the cases in which a type I or non-diagnostic or unsatisfactory cytology was obtained, the FNPA was repeated, and if the same result was obtained, ultrasound control was performed after 6 months, with a new assessment. All the patients whose second FNPA was null were excluded from the study. When the FNPA revealed benign or type II cytology (benign follicular nodule, lymphocytic thyroiditis and granulomatous thyroiditis), with a malignancy risk of 0–3 per cent, clinical follow-up was performed. In categories III or undetermined (atypia of undetermined significance and follicular lesion of undetermined significance), with a malignancy risk of 15–30 per cent, type IV or proliferation/follicular neoplasm, type V or suspicious of malignancy (papillary carcinoma, medullary carcinoma, metastasis and lymphoma), with a malignancy risk of 60–75 per cent, and type VI or malignant (papillary carcinoma, poorly differentiated carcinoma, medullary carcinoma, anaplastic carcinoma, squamous cells carcinoma, carcinoma with mixed characteristics, metastatic carcinoma, non-Hodgkin lymphoma) surgery was performed with partial or total thyroidectomy and subsequent pathologic analysis of the surgical piece.
Conventional thyroid ultrasoundThe thyroid ultrasounds were performed by two radiologists with 35 and 12 years of experience in ultrasound, using an ultrasound machine Siemens Acuson Antares (Siemens Medical Solutions, Mountain View, CA, USA) equipped with an 8–12MHz multifrequency linear transducer with color Doppler and elastography. All the FNPA were performed by a single radiologist with 25 years of experience doing this procedure. A 21–22 gauge needle was used connected to a 20 cc syringe and an aspiration mechanism (Cameco Medical Ltd London NW6 2BP), viewing the nodule through ultrasound.
The following parameters were analyzed to characterize each nodule studied:
- 1.
Nodule location: the nodules can be located in the right thyroid lobe, the left thyroid lobe and the isthmus.
- 2.
Presence or absence of other thyroid nodules: it can be a single nodule or one nodule in the context of multinodular goiter.
- 3.
Size: nodules are classified into two groups depending on their size, with the axis greater than 20mm and ≥20mm.
- 4.
Morphology: nodules can be round, wider than taller and taller than wider.
- 5.
Contour: nodules surrounded by a hypoechoic halo, well-delimited nodules and nodules with poorly-defined borders.
- 6.
Consistency: solid and mixed nodules (those with abundant areas of colloid degeneration) were included in the study, and lesions of solely cystic nature were excluded.
- 7.
Echostructure: homogeneous when the structure is uniform and heterogeneous if they show different echogenicity.
- 8.
Echogenicity: the echogenicity of each nodule was compared to that of the surrounding thyroid parenchyma. Four types were distinguished: hyperechoic, if it is more echogenic than the thyroid tissue; isoechoic, when it is similar; hypoechoic, if it is generally less echogenic; and mixed when there are hypoechoic, isoechoic and hyperechoic areas in the same nodule, without predominance of any type.
- 9.
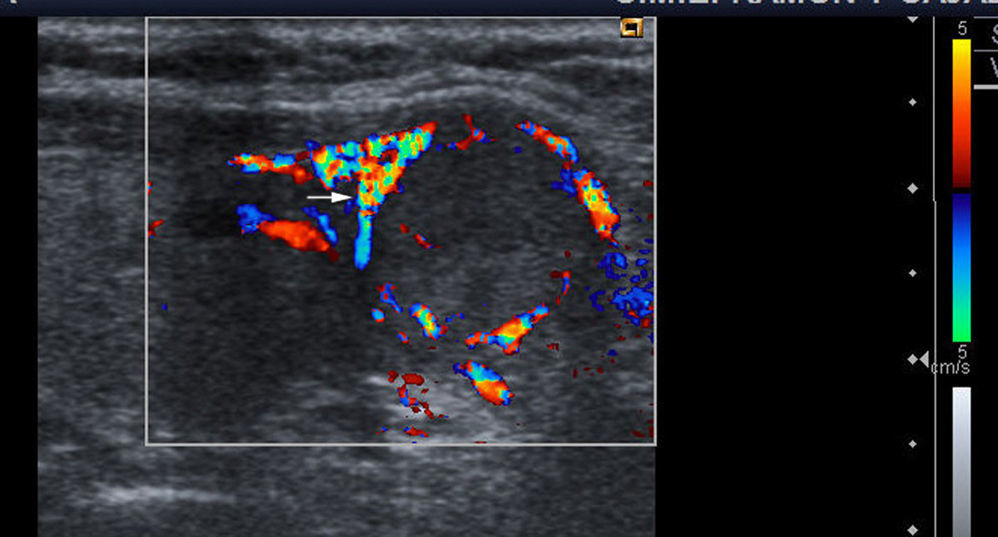
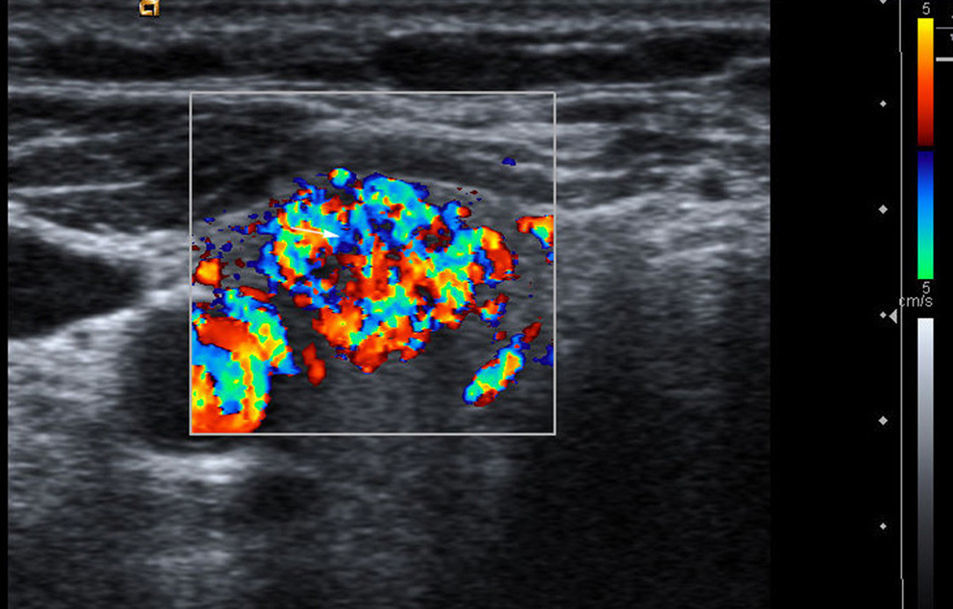
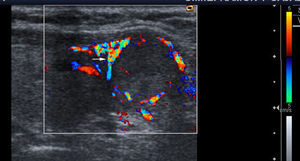
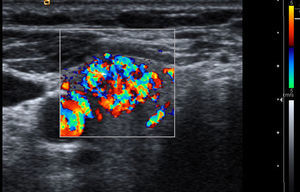
Vascularization: three patterns are defined with color Doppler. Type I: if it shows scarce vascular flow; type II: if there is predominantly peripheral vascularization (Fig. 1); and type III: when central intranodular flow predominates (Fig. 2).
- 10.
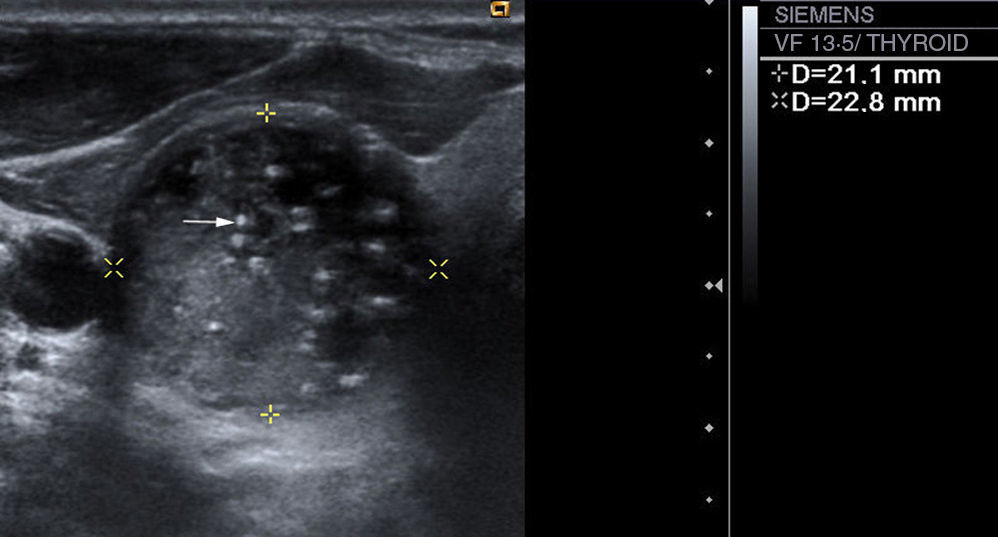
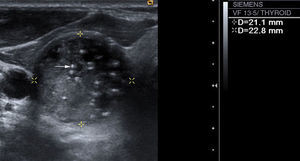
Presence or absence of intranodular calcifications: in case there are calcifications, we distinguish between microcalcifications (Fig. 3), described as hyperechogenic images with a diameter of ≤2mm with or without posterior acoustic shadows, and coarse calcifications (the rest).
We analyzed the probability for a thyroid nodule to be malignant based on each of the B-mode and color Doppler ultrasound signs that were described in the previous section. To this end, we used dichotomic variables to show the presence or absence of the ultrasound sign analyzed, and we estimated a logistic regression model that provides a coefficient β that relates every variable with the probability that the nodule analyzed is malignant, and the associated p value. In addition, the odds ratios (OR) and a measurement of the model total adjustment (pseudo-R2) are analyzed.
We studied the significance of each explicative variable in separate (single-variant analysis) and jointly (multivariant analysis). Explanatory variables considered in the multivariant model are those that in the univariant analysis are significant. This way the following dichotomic explicative variables in multivariant analysis can be considered: (1) whether it is a multiple nodule or not; (2) whether a halo is present or not; (3) whether the nodule is heterogeneous or not; (4) whether it shows microcalcifications or not; (5) whether there are colloid degeneration areas or not; (6) whether there is hypoechogenicity or not; and (7) whether vascularization is central or not.
In our empirical analysis, we put special emphasis on the capacity of the estimated models to correctly classify nodules as malignant or benign. In order to test the effectiveness of the model in nodule classification, we built a classification table in which the true value of each nodule under observation (1 or malignant, 0 or benign) is crossed with the prediction that the model considered assigns to such nodule. This prediction is made based on an arbitrary value of P0, so that a nodule is classified as malignant or benign if the probability that the model assigns to it, Pestimated, is greater or lower than P0, respectively. Based on the classification table, we estimated the sensitivity and the specificity of each model, as well as the probability for the disease to be present when the result of the diagnostic test is positive for the disease, or positive predictive value (PPV), and the probability for the disease to be absent when the result of the diagnostic test is negative for the disease, or negative predictive value (NPV). By repetition of this process for every possible value of P0 we obtain pairs of sensitivity and specificity that are represented in the ROC curve. Finally, the area under the ROC curve is calculated for each model, interpreted as the probability of correctly classifying a randomly-selected patient, and it is represented for the multivariant case.
Stata software was used for the statistical calculations.
ResultsThe study included 321 thyroid nodules, of which 201 obtained a cytological result of benignity (Bethesda II) in the FNPA, and no further diagnostic tests were performed. We received a non-diagnostic result on 32 occasions (Bethesda I) from the two punctures performed, and no significant changes were observed in all these cases in the ultrasound control performed after 6 months; therefore, an annual evolutionary control was subsequently indicated. These 32 nodules were eliminated from the analysis not to alter the results. The remaining 88 nodules obtained Bethesda III, IV, V and VI results in the FNPA through a cytological analysis of the sample and all of them underwent surgery. Among them, 3 nodules were classified as Bethesda III in the FNPA and their final diagnosis was thyroid nodular hyperplasia post-surgery; 67 nodules were classified as Bethesda IV after the FNPA, of which only 6 were cancer in the final diagnosis; 9 nodules obtained results of suspicion of malignancy in the FNPA and of these surgery confirmed malignancy in 4; and 9 nodules were classified as Bethesda VI or malignant in the FNPA and all of them were confirmed post-surgery. Overall, 19 nodules received a final diagnosis of malignancy, which represents 5.92 per cent of the total, with the following histological diagnoses: 12 nodules with papillary carcinoma, 3 with follicular variant of papillary carcinoma, 2 with follicular carcinoma and 1 with poorly differentiated carcinoma. The remaining 69 thyroid nodules operated obtained a histological diagnosis of benignity: 54 nodules corresponded to nodular hyperplasia, 6 follicular adenomas, 5 Hashimoto's thyroiditis, 4 non-Hashimoto chronic thyroiditis and 2Hürtle cell adenomas.
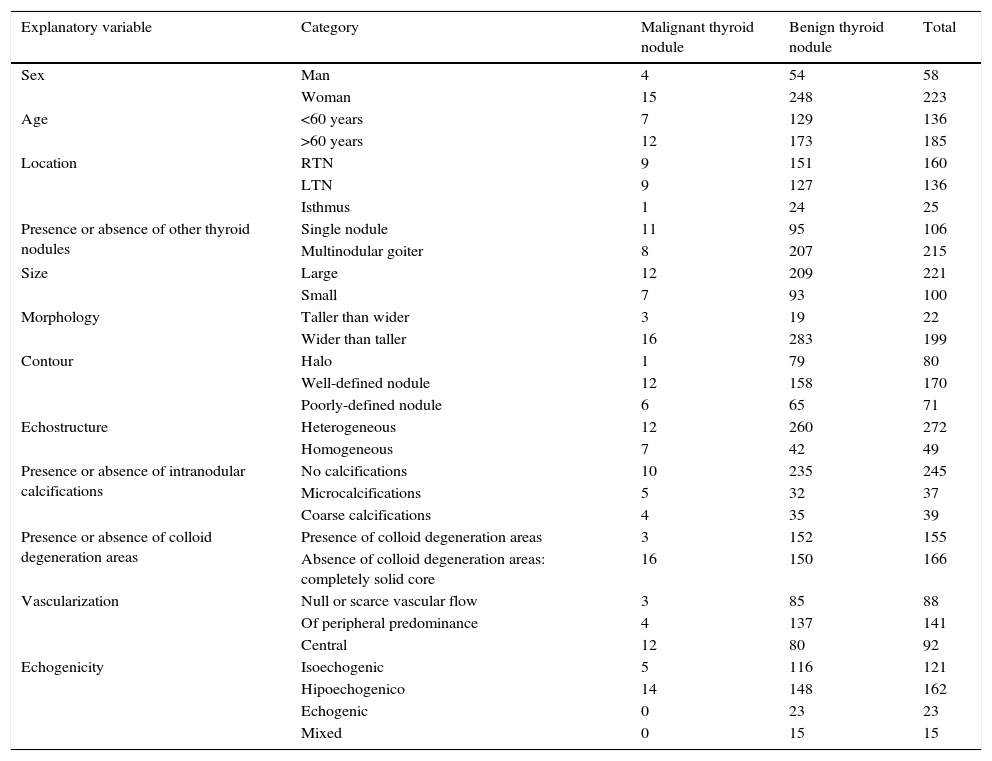
The absolute frequency of the patients’ characteristics (sex and age) is detailed in Table 1 as well as the ultrasound parameters selected for the sample total, and also based on the malignancy or benignity of the thyroid nodules analyzed.
Absolute frequencies of explanatory variables based on the diagnosis of the nodules.
| Explanatory variable | Category | Malignant thyroid nodule | Benign thyroid nodule | Total |
|---|---|---|---|---|
| Sex | Man | 4 | 54 | 58 |
| Woman | 15 | 248 | 223 | |
| Age | <60 years | 7 | 129 | 136 |
| >60 years | 12 | 173 | 185 | |
| Location | RTN | 9 | 151 | 160 |
| LTN | 9 | 127 | 136 | |
| Isthmus | 1 | 24 | 25 | |
| Presence or absence of other thyroid nodules | Single nodule | 11 | 95 | 106 |
| Multinodular goiter | 8 | 207 | 215 | |
| Size | Large | 12 | 209 | 221 |
| Small | 7 | 93 | 100 | |
| Morphology | Taller than wider | 3 | 19 | 22 |
| Wider than taller | 16 | 283 | 199 | |
| Contour | Halo | 1 | 79 | 80 |
| Well-defined nodule | 12 | 158 | 170 | |
| Poorly-defined nodule | 6 | 65 | 71 | |
| Echostructure | Heterogeneous | 12 | 260 | 272 |
| Homogeneous | 7 | 42 | 49 | |
| Presence or absence of intranodular calcifications | No calcifications | 10 | 235 | 245 |
| Microcalcifications | 5 | 32 | 37 | |
| Coarse calcifications | 4 | 35 | 39 | |
| Presence or absence of colloid degeneration areas | Presence of colloid degeneration areas | 3 | 152 | 155 |
| Absence of colloid degeneration areas: completely solid core | 16 | 150 | 166 | |
| Vascularization | Null or scarce vascular flow | 3 | 85 | 88 |
| Of peripheral predominance | 4 | 137 | 141 | |
| Central | 12 | 80 | 92 | |
| Echogenicity | Isoechogenic | 5 | 116 | 121 |
| Hipoechogenico | 14 | 148 | 162 | |
| Echogenic | 0 | 23 | 23 | |
| Mixed | 0 | 15 | 15 |
RTN, right thyroid nodule; LTN, left thyroid nodule.
Table 1 shows that malignancy is more frequent in nodules with microcalcifications, central vascularization and one anterior-posterior diameter greater than the transversal one (taller than wider), and less common in nodules in the context of multinodular goiter, in heterogeneous nodules, in nodules with colloid degeneration areas and in nodules surrounded by a hypoechoic halo.
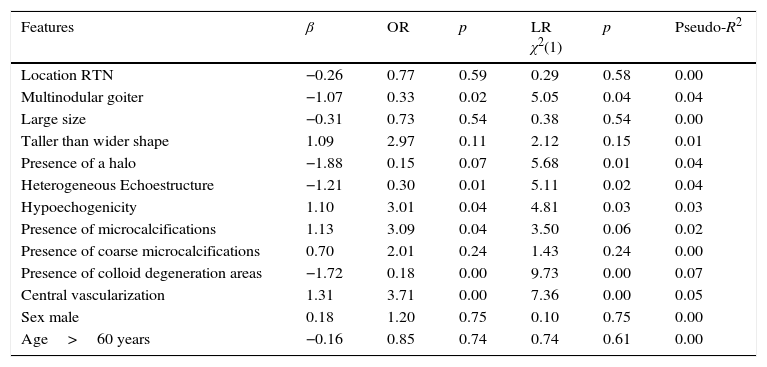
Univariant statistical analysis of thyroid nodule malignancyTable 2 shows the results obtained for the univariant logistic regression analysis.
Results from the logistic regression analysis for the detection of malignant thyroid nodules.
| Features | β | OR | p | LR χ2(1) | p | Pseudo-R2 |
|---|---|---|---|---|---|---|
| Location RTN | −0.26 | 0.77 | 0.59 | 0.29 | 0.58 | 0.00 |
| Multinodular goiter | −1.07 | 0.33 | 0.02 | 5.05 | 0.04 | 0.04 |
| Large size | −0.31 | 0.73 | 0.54 | 0.38 | 0.54 | 0.00 |
| Taller than wider shape | 1.09 | 2.97 | 0.11 | 2.12 | 0.15 | 0.01 |
| Presence of a halo | −1.88 | 0.15 | 0.07 | 5.68 | 0.01 | 0.04 |
| Heterogeneous Echoestructure | −1.21 | 0.30 | 0.01 | 5.11 | 0.02 | 0.04 |
| Hypoechogenicity | 1.10 | 3.01 | 0.04 | 4.81 | 0.03 | 0.03 |
| Presence of microcalcifications | 1.13 | 3.09 | 0.04 | 3.50 | 0.06 | 0.02 |
| Presence of coarse microcalcifications | 0.70 | 2.01 | 0.24 | 1.43 | 0.24 | 0.00 |
| Presence of colloid degeneration areas | −1.72 | 0.18 | 0.00 | 9.73 | 0.00 | 0.07 |
| Central vascularization | 1.31 | 3.71 | 0.00 | 7.36 | 0.00 | 0.05 |
| Sex male | 0.18 | 1.20 | 0.75 | 0.10 | 0.75 | 0.00 |
| Age>60 years | −0.16 | 0.85 | 0.74 | 0.74 | 0.61 | 0.00 |
LR: likelihood ratio; RTN: right thyroid nodule; OR: odds ratio.
In our sample, neither the patient's age or sex is a significant factor of malignancy. We did not find nodule location, size or morphology to be significant either.
The ultrasound findings that are statistically significant (p<0.05) and associated with a greater probability of malignancy, are the presence of microcalcifications, central vascularization and hypoechogenicity. The ultrasound findings that are significant and associated with less probability of malignancy, and therefore indicative of benignity, are the presence of a halo and colloid degeneration areas and the heterogeneity of the nodules.
The OR reaches its greatest values for central vascularization and the presence of microcalcifications. This is how a patient whose thyroid nodule is characterized by having central vascularization or microcalcifications increases his risk compared to other patient who does not show these characteristics in a factor equal to 3.71 and 3.09, respectively. On the contrary, if a nodule is surrounded by a halo or shows colloid degeneration areas the risk is reduced in a factor of 6.66 and 5.55, respectively.
Yet despite the fact that our univariant analysis indicates that there are variables significantly related with thyroid nodule malignancy, the study of the predictive capacity of the models estimated shows that no variable considered in isolation allows us to predict accurately malignancy of a thyroid nodule, because the goodness of the adjustment models measured through the pseudo-R2 is low and it is difficult to obtain high sensitivity and specificity values jointly. Table 2 shows that pseudo-R2 does is not beyond 10 per cent in any models. The ROC curve shows poor predictive capacity of explanatory variables: the area under the ROC curve for all models is very close to 0.5 (“random” classification of thyroid nodules) and never beyond 0.7 – far from the best possible model (area under the ROC curve=1). No model has a 100 per cent sensitivity and a 0 per cent specificity.
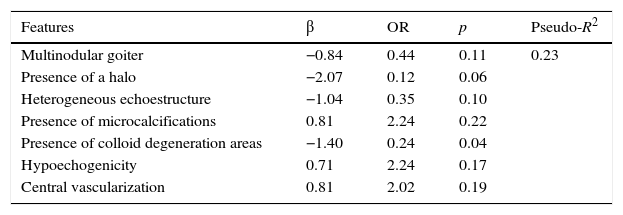
Multivariant analysis of thyroid nodule malignancyTable 3 shows the results of the multivariant logistic regression model estimation. The multivariant model estimated preserves the signs of the coefficients estimated in the univariant analysis. That is, the probability of malignancy of the thyroid nodule is positively associated with the presence of microcalcifications, central vascularization and hypoechogenicity, and negatively associated with the presence of halo, colloid degeneration areas and with the heterogeneity of the nodules.
Results from the logistic regression analysis for the detection of malignant thyroid nodules using different explanatory variables.
| Features | β | OR | p | Pseudo-R2 |
|---|---|---|---|---|
| Multinodular goiter | −0.84 | 0.44 | 0.11 | 0.23 |
| Presence of a halo | −2.07 | 0.12 | 0.06 | |
| Heterogeneous echoestructure | −1.04 | 0.35 | 0.10 | |
| Presence of microcalcifications | 0.81 | 2.24 | 0.22 | |
| Presence of colloid degeneration areas | −1.40 | 0.24 | 0.04 | |
| Hypoechogenicity | 0.71 | 2.24 | 0.17 | |
| Central vascularization | 0.81 | 2.02 | 0.19 |
OR: odds ratio.
The variables that provide a greater OR are central vascularization and the presence of microcalcifications. On the contrary the variables that have less OR are hypoechogenicity, the presence of a halo and the presence of areas of colloid degeneration.
Based on the logistic regression model estimation, the OR of a “maximum-risk” patient whose thyroid nodule shows microcalcifications, central vascularization, hypoechogenicity, absence of a halo and an absence of colloid degeneration areas is 2.50. On the other hand the OR of a “minimum-risk” patient showing absence of microcalcifications, absence of central vascularization and hypoechogenicity, combined with the presence of halo and colloid degeneration areas is 0.001.
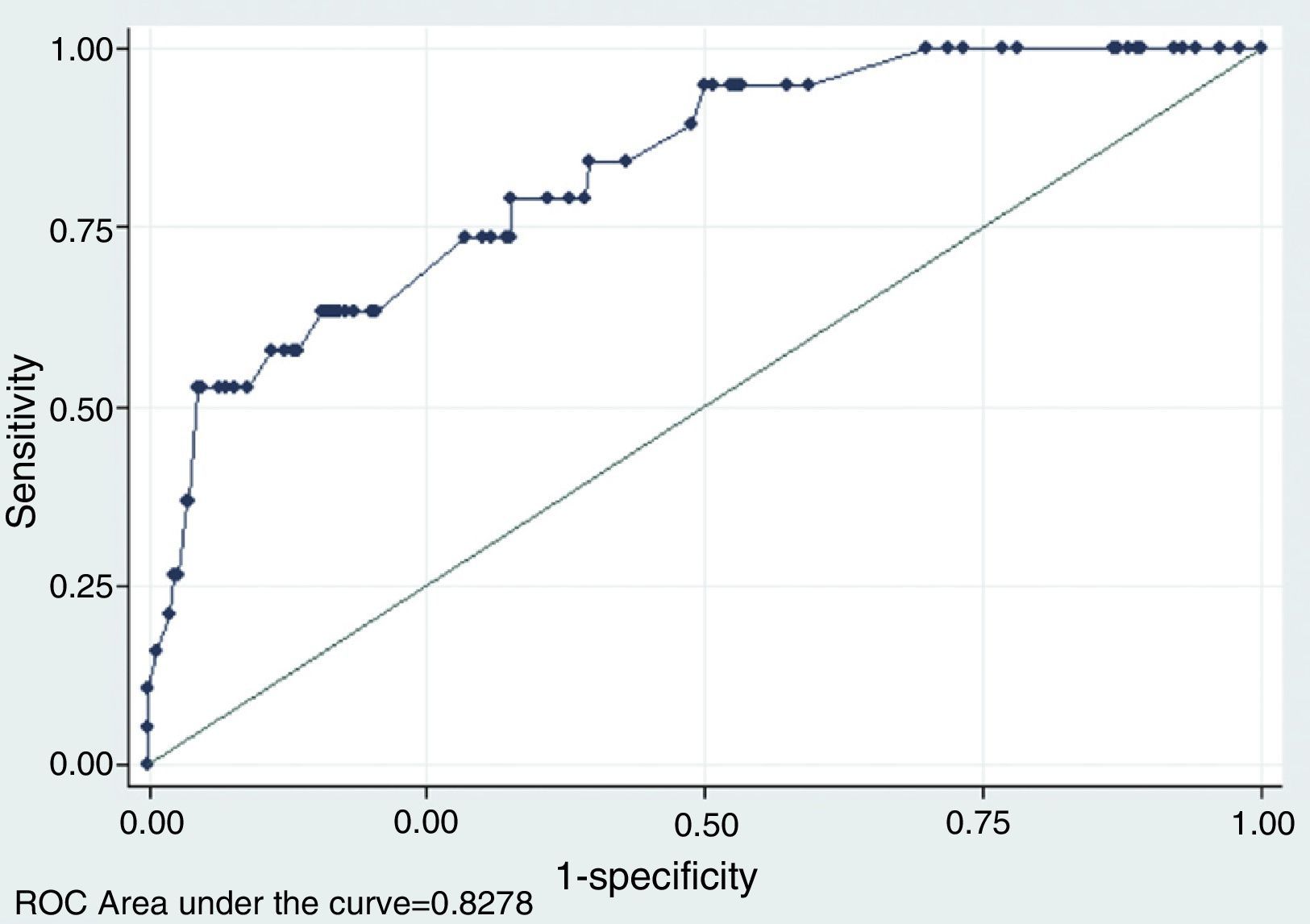
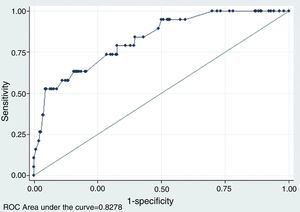
As expected, the predictive capability analysis of the multivariant model improves considerably with respect to the univariant analysis in terms of model adjustment and predictive capability. Pseudo-R2 increases significantly until it slightly goes beyond 23 per cent, so the significance contrast of the model is accepted (p=0). Taking as a reference different cut-off points of the probability of malignancy estimated, the multivariant model improves considerably the results of the univariant analysis, above all in terms of the possibility of obtaining, for a given sensitivity a higher specificity. Fig. 4 shows the ROC curve obtained from the model, whose area under the curve is 0.82, much greater than the values obtained in the univariant analysis – which in turn provides an additional story line about how good the model really is.
ROC curve using different explanatory variables for the odds of thyroid nodule malignancy. This figure shows the ROC curve related to the multivariant logistics model that explains the odds of thyroid nodule malignany based on the following ultrasound signs in mode B and color Doppler: (1) if the nodule is multiple or not; (2) if presence of a halo or not; (3) if the nodule is heterogeneous or not; (4) if there are microcalcifications or not; (5) if presence of colloid degeneration areas or not; (6) if presence of hypoechogenicity or not; and (7) if vascularization is central or not.
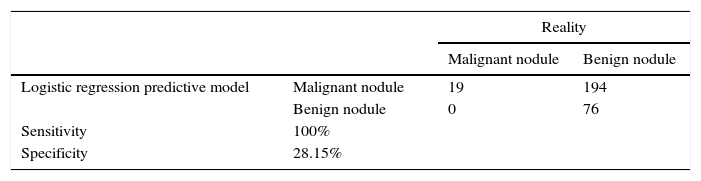
Based on the goal of this work, we showed the results of a possible strategy that can be implemented from the model estimation and based on the premise that maximum sensitivity is desirable so that no neoplasm goes undiagnosed. Table 4 shows that this conservative strategy is capable of diagnosing malignant nodules correctly and at the same time it allows us to diagnose at least 79 benign nodules correctly. The sensitivity of the model is 100 per cent and the specificity 28.15 per cent. Table 5 details the relative frequency of the variables used in the logistic regression model observed in the 79 benign nodules that have been categorized as such based on the logistic regression model.
Classification of nodules based on the strategy implementeda
| Reality | |||
|---|---|---|---|
| Malignant nodule | Benign nodule | ||
| Logistic regression predictive model | Malignant nodule | 19 | 194 |
| Benign nodule | 0 | 76 | |
| Sensitivity | 100% | ||
| Specificity | 28.15% | ||
Relative frequencies of explanatory variables of nodules classified as benign based on the strategy implemented.a
| Features | Percentage |
|---|---|
| Multinodular goiter | 76 |
| Presence of a halo | 69 |
| Heterogeneous echoestructure | 91 |
| Presence of microcalcifications | 0 |
| Presence of colloid degeneration areas | 68 |
| Hypoechogenicity | 18 |
| Central vascularization | 10 |
The results obtained in our study and the subsequent statistical analyses partly coincide with the empirical evidence from previous studies though there are also discrepancies with some of the existing medical literature. In our sample, almost 6 per cent of the patients had thyroid cancer, which is a percentage similar to that described by Papini et al.10 and Iannuccilli et al.11 but considerably less than that reported in most papers,1,12–15 that goes to nearly 14 per cent.
In our study, the ultrasound findings that are statistically significant and are associated with a greater probability of malignancy are hypoechogenicity, the presence of microcalcifications and central vascularization patterns. On the other hand, the ultrasound findings that are statistically significant and are associated with a greater probability of benignity are heterogeneity, the presence of colloid areas and the existence of a hypoechoic halo. Our results coincide with some of the previous empirical evidence.
Iannuccilli et al.11 claim that the only statistically significant criterion is the presence of microcalcifications, and Papini et al.10 believe that the ultrasound findings that have predictive value of malignancy, in keeping with a logistic regression analysis, are intranodular vascular patterns, microcalcifications and irregular or lobulated borders of the nodule, but they do not consider that the presence of a hypoechoic halo is indicative of benignity.
Kwak et al.16 find a significant association between malignancy and the presence of solid components, microcalcifications, irregular or microlobulated borders, hypoechogenicity and an anterior–posterior diameter that is greater than the transversal one.
Frates et al.13 claim that the ultrasound finding with the greatest sensitivity of all is the solid composition of a nodule, but it has a very low PPV, and that the finding with the greatest PPV is microcalcifications.
For Moon et al.15, the ultrasound findings suspicious of statistically significant malignancy are taller-than-wider shape, spiculated borders, marked hypoechogenicity, the presence of microcalcifications and macrocalcifications; and benignity, one spongiform appearance of the nodule and isoechogenicity. These authors believe that the shape of the nodule is very important and they claim that taller-than-wider shape is very specific of malignancy, because these malignant nodules grow through normal tissue planes, while benign nodules do so parallel to normal tissue planes.
For Rago and Vitti,17 the ultrasound findings associated with malignancy are microcalcifications, hypoechogenicity, irregular borders, the absence of a halo, solid appearance, intranodular vascularization and taller-than-wider shape, variants that individually have very little predictive value, but that when they appear simultaneously in the same nodule, the specificity of the ultrasound goes up even though sensitivity remains unacceptably low.
Kim et al.18, after analyzing 155 non-palpable thyroid nodules, coincide with us when they say that no statistically significant differences are found with respect to size in malignant and benign nodules. They propose, in the way of indications to perform FNPA, the presence of at least one of the four ultrasound criteria that based on their study, are indicative of malignancy: microcalcifications, irregular or microlobulated borders, marked internal hypoechogenicity of the nodule and taller-than-wider shape. Unlike us, they do not included central vascularization increase as a suspicious finding.
It is important to point out, as limitations of our study that the different ultrasound findings considered individually do not allow us to obtain jointly high sensitivity and specificity in the diagnosis of thyroid cancer. With the premise that maximum sensitivity is desirable so that no neoplasm goes undiagnosed, univariant models would reach a 100% sensitivity at the expense of a 0% specificity. This way, univariant models would not reduce the number of FNPA performed. Quite the contrary, multivariant model estimation has allowed us to reach a 100% sensitivity in the analysis within the sample and a 28.15% specificity. Thus, our model would save us from doing FNPA on 76 nodules of the 270 benign ones, which is an improvement with respect to the current situation, in which FNPA were performed on all the benign nodules. Also the solidity of our results should be confirmed in an analysis outside the sample, so our patient base needs to be broadened.
Consequently, we observed that our study coincides with most studies of the scientific literature when it comes to determining what ultrasound findings are suspicious of malignancy being the most significant of them all the presence of microcalcifications, central vascularization and hypoechogenicity, as well as ultrasound findings associated to benignity among which the most significant are the presence of colloid areas, the presence of a halo, the existence of multinodular goiter and nodule heterogeneity.
From the results of the logistic regression model estimated we wish to state that the joint presence of several ultrasound findings is necessary to get to a better malignancy diagnosis of a thyroid nodule that in turn allows us to not avoid performing FNPA in the largest number of cases possible. This result follows in the footsteps of the actual criteria by the American Association of Clinical Endocrinology Guidelines and the last version of the American Thyroid to biopsy thyroid nodules.
In sum the FNPA is not indicated in nodules that do not have microcalcifications, central vascularization or hypoechogenicity or in those showing a halo and colloid degeneration areas because their probability of malignancy is very low.
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Author's contribution- 1.
Manager of the integrity of the study: CFU.
- 2.
Study Idea: CFU, JPB.
- 3.
Study Design: CFU.
- 4.
Data Mining: CFU, JPB.
- 5.
Data Analysis and Interpretation: CFU, JPB, RLH.
- 6.
Statistical Analysis: CFU, RLH.
- 7.
Reference: CFU, JPB, CPL.
- 8.
Writing: CFU, JPB, RLH, CPL.
- 9.
Critical review of the manuscript with intellectually relevant remarks: JPB, CPL.
- 10.
Approval of final version: CFU, JPB, RLH, CPL.
The authors declare no conflict of interests.
Please cite this article as: Franco Uliaque C, Pardo Berdún FJ, Laborda Herrero R, Pérez Lórenz C. Utilidad de la ecografía en la evaluación de los nódulos tiroideos. Radiología. 2016;58:380–388.