To assess the usefulness of iodine-125 (125I) seeds as an alternative to surgical clips for marking the location of nonpalpable malignant breast lesions for surgery.
Material and methodsWe included patients with histologically confirmed nonpalpable malignant lesions treated by surgery in 2015 or 2016. Patients were randomly assigned to presurgical marking with metallic clips (Group A) or with 125I seeds (Group B). In both groups, marking was guided by ultrasound and/or mammography depending on the radiologic characteristics of the lesion. During surgery, a gamma probe was used and afterward the presence of seeds in the surgical specimen was checked radiologically. In the histological analysis, the absence of tumour in the stain was considered free margins. We analysed the following variables: age, lesion characteristics (laterality, mean size on MRI and in the surgical specimen, radiological type), and presence/absence of free margins.
ResultsIn Group A (n = 53), the most common histologic subtypes were infiltrating ductal carcinoma (IDC, 84.9%) and luminal A (LA, 49.1%); the mean size of the lesions was 1.8 cm. In Group B (n = 45), the most common histologic subtypes were IDC (82.2%) and LA (46.5%); the mean size of the lesions was 1.5 cm.
In Group A, 13.2% had involved margins and 13.2% underwent a second surgical intervention. In Group, B 11.4% had involved margins and 7.5% underwent a second surgical intervention. The differences between groups were not significant (p = 0.7 for involved margins and p = 0.5 for reintervention). The volume of the surgical specimens was significantly lower in Group B than in Group A (128.68 cm3 vs. 189.37 cm3; p < 0.05).
ConclusionsUsing 125I seeds was feasible and enabled significantly smaller surgical specimens than using metallic clips.
Evaluar los resultados de la cirugía radioguiada mediante semillas de 125I como alternativa al arpón quirúrgico en pacientes con lesiones no palpables malignas de mama.
Material y métodosSe incluyeron pacientes con diagnóstico anatomopatológico de cáncer de mama, con lesiones no palpables, candidatas a tratamiento quirúrgico durante 2015–2016. Las pacientes fueron asignadas de manera aleatoria al marcaje prequirúrgico con arpón metálico (grupo A) o con semilla (grupo B). En ambos grupos, el procedimiento fue guiado mediante ecografía y/o mamografía en función de las características de la lesión radiológica. Durante la cirugía se utilizó una sonda gammadetectora y, posteriormente, se comprobó mediante radiología la presencia de las semillas en las piezas quirúrgicas. Se realizó el análisis histológico de las piezas, considerando márgenes libres la ausencia de tumour en la tinta. Las variables analizadas fueron la edad de las pacientes y varias características de la lesión (lateralidad, tamaño medido por resonancia magnética y en la pieza quirúrgica, tipo radiológico y presencia de márgenes quirúrgicos libres).
ResultadosEn el grupo A (n = 53), los subtipos histológicos más frecuentes fueron el carcinoma ductal infiltrante (CDI, 84,9%) y el luminal A (LA, 49,1%); el tamaño medio lesional (TML = 1,8 cm). En el grupo B (n = 45), los resultados fueron CDI = 82,2%, LA = 46,5%, TML = 1,5 cm. En el grupo A, la tasa de márgenes afectados fue del 13,2% y la tasa de reintervenciones, de un 13,2% (p = 0,7), y en el grupo B, la tasa de márgenes afectados fue 11,4% y la tasa de reintervenciones, del 7,5% (p = 0,5). Los volúmenes de las piezas quirúrgicas fueron significativamente menores en el grupo B (V = 128,68 cm3) que en el grupo A (V = 189,37 cm3) (p < 0,05).
ConclusionesLa utilización de semillas de 125I se ha mostrado como una técnica factible en la localización de lesiones no palpables de mama, mostrando diferencias significativas en el tamaño de las piezas quirúrgicas respecto al arpón.
Recent years have seen an increase in the incidence of non-palpable malignant breast lesions. This is due to both the implementation of the early breast cancer detection programme and to improvements in diagnostic imaging techniques (digital mammography, tomosynthesis, ultrasound with high frequency transducers, breast magnetic resonance imaging [MRI], etc.). This, in turn, has increased the number of lumpectomies, a technique that requires pre-surgical location of the lesion in order to perform conservative surgery.1–4
In our hospital, hookwires, placed on the day of the intervention using mammography or ultrasound guidance, are usually used to mark the location prior to surgery.5 This method is not without its drawbacks, including displacement of the hookwire before surgery,6 and the fact that the insertion point chosen by the radiologist to mark the lesion does not always coincide with the ideal skin incision site for the surgeon, following aesthetic and proximity criteria. However, the main drawback of preoperative wire localisation is the high rate of incomplete margin resection in non-palpable tumours, which require intraoperative extension of the margins, or even re-operation in some cases.1,7
In the interests of improving surgical outcomes in these patients, radioactive seed localisation using 125I seeds has emerged as an alternative to wire localisation. This technique consists of the preoperative implantation of titanium seeds marked with 125I to mark the location of non-palpable breast tumours.
Like macroaggregated albumin used in the ROLL (radioguided localization and removal of occult breast lesions) technique, 125I seeds are a type of radiotracer that does not migrate to the lymphatic system. Marking with seeds, which are radiopaque, has the advantage of allowing the clinician to correctly localise the tumour using mammography.
The objective of this study was to evaluate the results of preoperative marking of non-palpable malignant breast tumours using 125I seeds vs. hookwires.
Material and methodsThis was a prospective, longitudinal study in which patients with non-palpable lesions and a pathological diagnosis of breast cancer, who were candidates for conservative surgical treatment, were included between 2015 and 2016.
The patients were informed of the procedure and signed an informed consent form. The study was approved by the Ethics Committee of our hospital in accordance with the Declaration of Helsinki, and was granted the relevant licences by the Radioactive Substances Board.
In our centre, breast pathologies are treated in the gynaecology department. A total of four gynaecologists with 30, 19, 11 and 6 years of experience, respectively, participated in this study; three radiologists with 23, 11 and 9 years of experience, respectively; and two nuclear medicine physicians, with 30 and 9 years of experience, respectively.
The first 53 patients underwent preoperative wire localisation, before the introduction of radioactive seed localisation with 125I seeds, and formed the control group (group A), which was compared with the first 45 patients undergoing preoperative radioactive seed localisation (group B).
The inclusion criteria were: histological diagnosis of breast cancer with non-palpable lesions at the time of diagnosis or after treatment with neoadjuvant chemotherapy, candidates scheduled for conservative surgery by the Multidisciplinary Breast Committee.
The exclusion criteria for both groups were identical to avoid biases, and included: aged under 18, palpable lesions, extensive non-palpable lesions needing to be marked with more than one hookwire or seed, and patients with pre-invasive, non-infiltrating tumours.
The variables included in the study were age, laterality of the lesion (right or left breast), the size of the radiological lesion obtained on staging or reassessment MRI after neoadjuvant chemotherapy, the type of radiological lesion visualised on mammography and/or ultrasound (nodule, distortion, microcalcifications, asymmetric focal density, etc.), histological type and molecular subtype, the size of both the tumour and the surgical specimen determined by pathological anatomy, and free surgical margins, defined as absence of tumour tissue on staining.
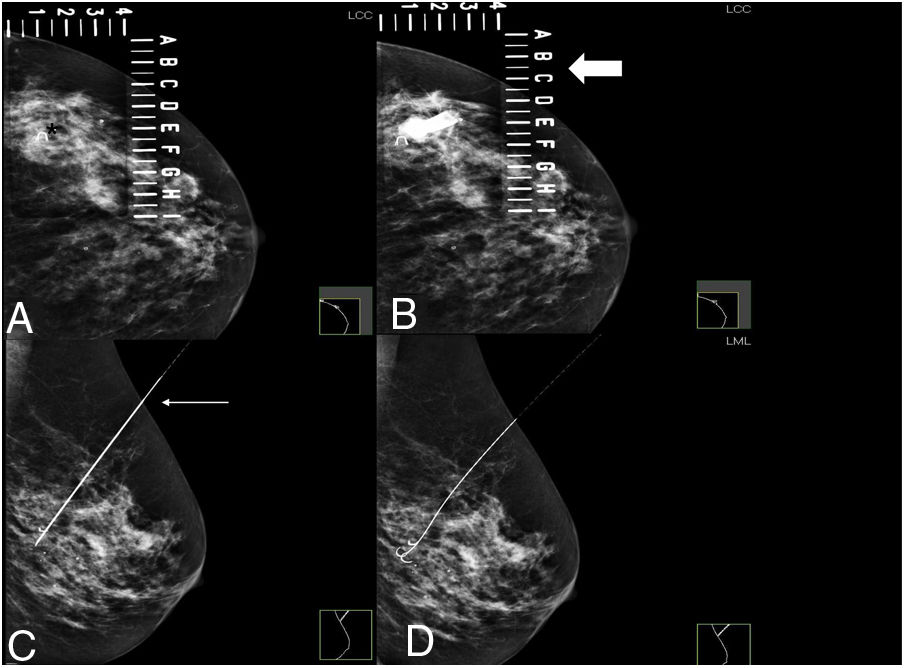
In patients undergoing wire localisation, the hookwire was placed on the day of surgery following the usual procedure with mammographic or ultrasound guidance, specifying in the radiological report the distance that the distal tip of the wire emerged from the border of the tumour. Ultrasound guidance was used whenever the lesion could be visualised using this technique. Otherwise (complete radiological response after neoadjuvant therapy in a previously marked lesion, infiltrating microcalcifications, or focal asymmetric density not visible on ultrasound) mammographic guidance was used. Correct localisation of the wire was verified by mammography (Figs. 1 and 2).
Preoperative wire localisation of BI-RADS 6 lesion with complete radiological response, marked at the beginning of treatment.
A. MI craniocaudal view to locate the wire marker (asterisk) inside the fenestrated plate.
B. Insertion of the metal wire at the coordinates established on the fenestrated plate (thick arrow) where the marker is located.
C. Lateral view of the left breast: to verify that the wire crosses the marker (thin arrow) and the depth at which it is located.
D. Lateral view of the left breast: opening of the correctly positioned wire across the metal marker.
In patients undergoing preoperative radioactive seed localization, the seed was placed up to one week before surgery.
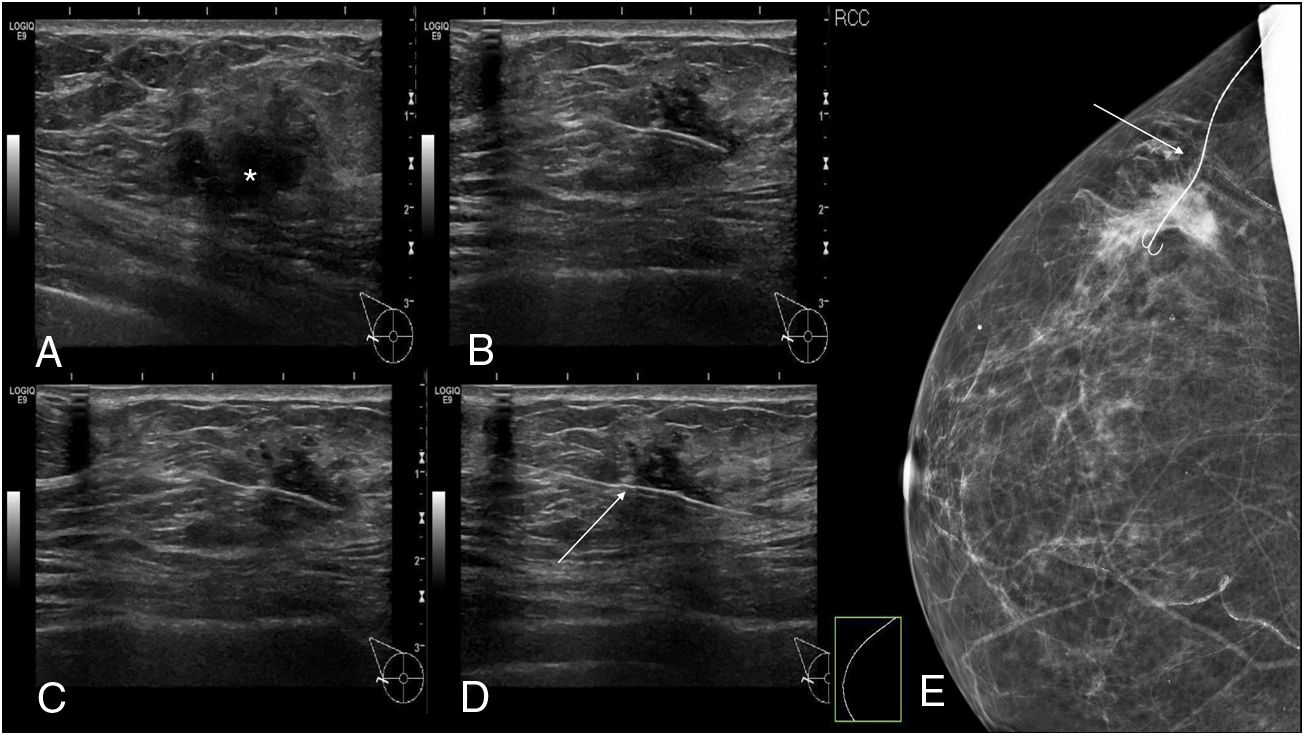
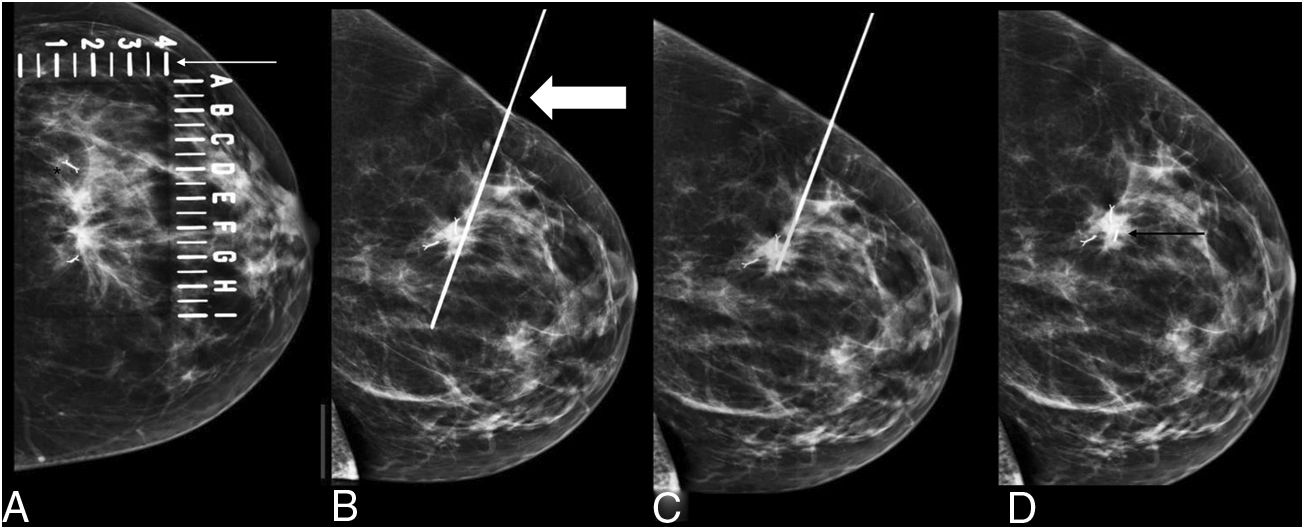
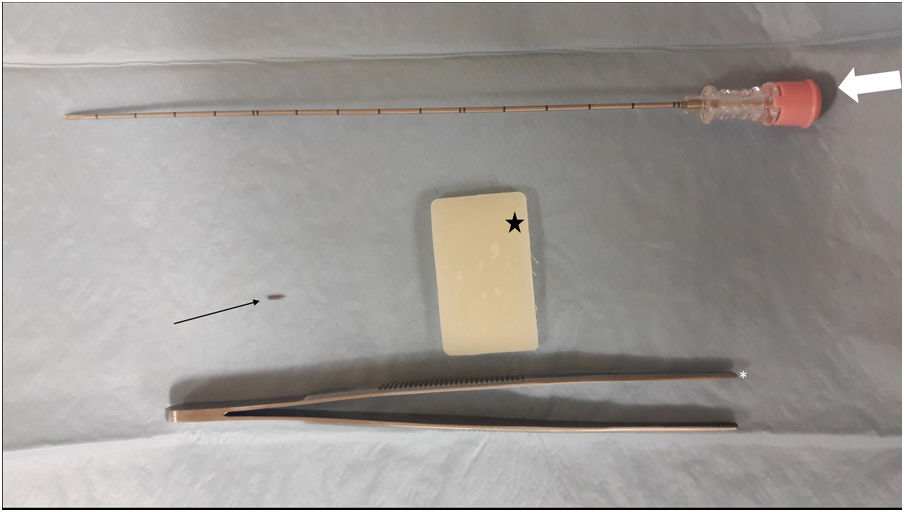
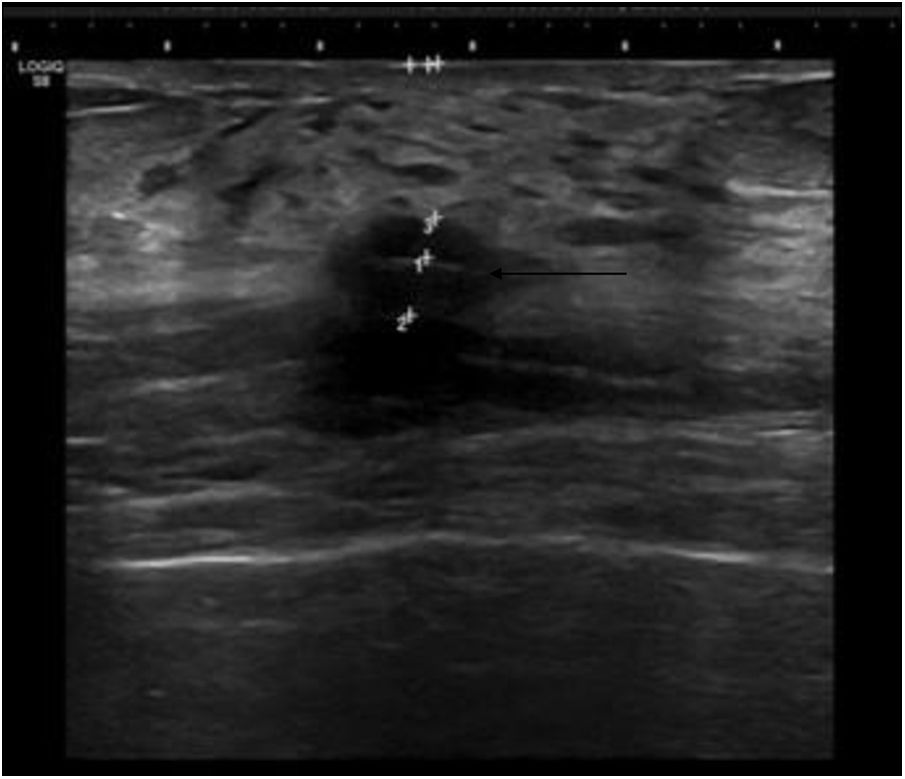
The procedure for implanting the seed consisted of locating the lesion by mammography (Fig. 3) or ultrasound (Fig. 4), the administration of local anaesthesia and the introduction of the seed through an 18 G bevelled needle correctly sealed with a bone wax plug at the tip to prevent premature deployment of the seed (Fig. 5). The radiological report specified a series of measurements, such as the distance from the seed to the skin and to the anterior and posterior edges of the lesion, to facilitate correct planning of the surgery (Fig. 6).
Preoperative seed localisation guided by mammography of malignant lesion, marked with two seeds, with greater partial radiological response, with a distortion in the breast parenchyma persisting in the right breast. It was decided to place a seed between the two markers. A) Marker localisation (black asterisk) guided by mammography with fenestrated plate (thin white arrow). B) Insertion of 18 G needle (thick white arrow) loaded with the seed in the craniocaudal view. C) Lateral view to locate the depth and verify the correct location, in this case, between the two markers. D) Implantation of the seed (thin black arrow).
Once in the operating room, a gamma detection probe with a 27-keV emission peak (125I) was used as a guide for the surgeon to remove the lesion based on aesthetic criteria.
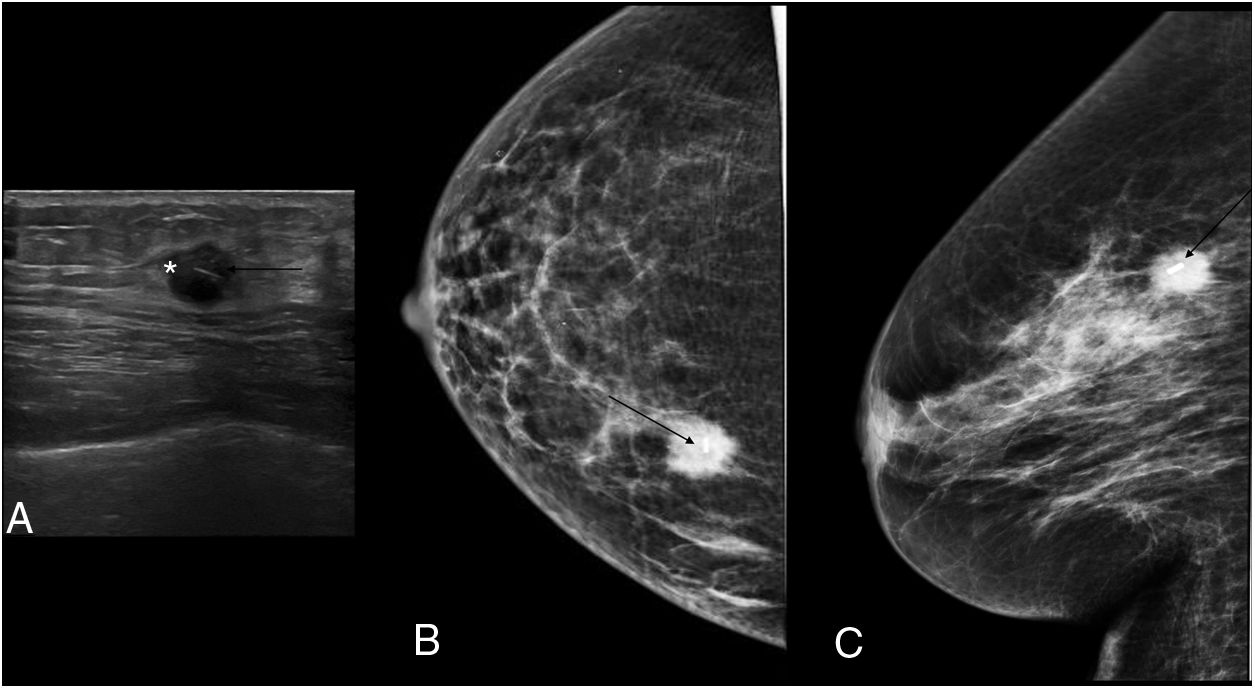
Once the lesion was removed, the presence of the seed in the surgical specimen was verified by direct visualisation in the operating room and also by mammography (Fig. 7).
Finally, the seeds were recovered by the nuclear medicine service, and appropriate measures were taken for disposal.
Statistical analysis of data was performed using IBM SPSS Statistics version 19 for Windows. Numerical variables were described as mean and standard deviation, or median and percentile in the case of non-normality; and qualitative variables were described as absolute and relative frequencies (number and percentage). Group A and B variables were compared using bivariate analysis, using the Student's t-test in numerical variables, or the Mann-Whitney U test in non-parametric cases. The variables were tested for normality using the Kolmogorov-Smirnov test. Qualitative variables were analysed using Pearson’s χ2 test or Fisher’s exact test. Statistical significance was set at p < 0.05.
ResultsA total of 98 patients were included in the study, 53 in group A and 45 in group B. The average age in both groups was 59 (group A: 59.9 ± 11.4 years; group B: 59.1 ± 12.3 years; p = 0.73).
The most common type of radiological lesions in both groups were nodules, with an average size determined by magnetic resonance of 18 mm (group A) and 15 mm (group B).
The most common histological type of the breast lesions was invasive ductal carcinoma (group A: 84.9%; group B: 82.2%) and the most common molecular subtype was luminal A (group A: 52%; group B: 46.5%). No statistically significant differences were found between any of these variables (Table 1).
Surgical wire vs. iodine-125 (125I) seeds for marking the location of nonpalpable malignant breast lesions, preliminary results.
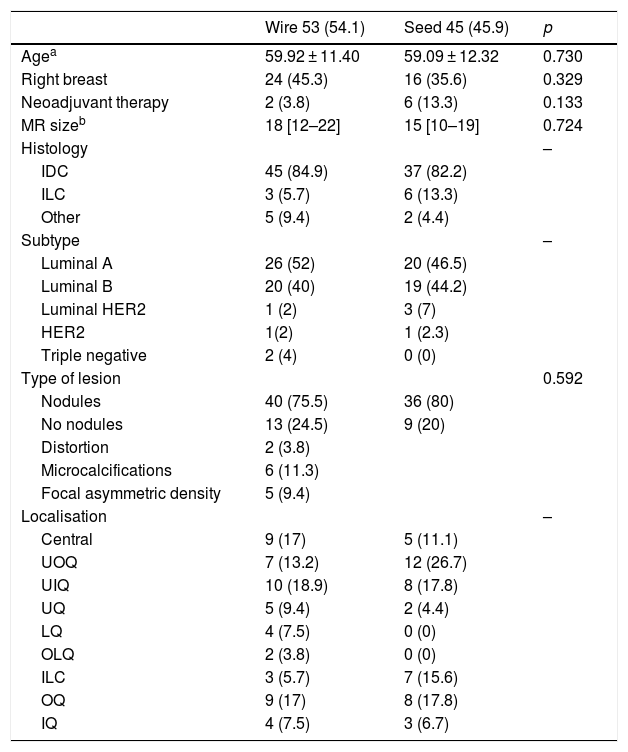
| Wire 53 (54.1) | Seed 45 (45.9) | p | |
|---|---|---|---|
| Agea | 59.92 ± 11.40 | 59.09 ± 12.32 | 0.730 |
| Right breast | 24 (45.3) | 16 (35.6) | 0.329 |
| Neoadjuvant therapy | 2 (3.8) | 6 (13.3) | 0.133 |
| MR sizeb | 18 [12–22] | 15 [10–19] | 0.724 |
| Histology | – | ||
| IDC | 45 (84.9) | 37 (82.2) | |
| ILC | 3 (5.7) | 6 (13.3) | |
| Other | 5 (9.4) | 2 (4.4) | |
| Subtype | – | ||
| Luminal A | 26 (52) | 20 (46.5) | |
| Luminal B | 20 (40) | 19 (44.2) | |
| Luminal HER2 | 1 (2) | 3 (7) | |
| HER2 | 1(2) | 1 (2.3) | |
| Triple negative | 2 (4) | 0 (0) | |
| Type of lesion | 0.592 | ||
| Nodules | 40 (75.5) | 36 (80) | |
| No nodules | 13 (24.5) | 9 (20) | |
| Distortion | 2 (3.8) | ||
| Microcalcifications | 6 (11.3) | ||
| Focal asymmetric density | 5 (9.4) | ||
| Localisation | – | ||
| Central | 9 (17) | 5 (11.1) | |
| UOQ | 7 (13.2) | 12 (26.7) | |
| UIQ | 10 (18.9) | 8 (17.8) | |
| UQ | 5 (9.4) | 2 (4.4) | |
| LQ | 4 (7.5) | 0 (0) | |
| OLQ | 2 (3.8) | 0 (0) | |
| ILC | 3 (5.7) | 7 (15.6) | |
| OQ | 9 (17) | 8 (17.8) | |
| IQ | 4 (7.5) | 3 (6.7) |
Numbers expressed as n (%).
IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; IQ: inner quadrants; LIQ: lower inner quadrant; LQ: lower quadrants; LOQ: lower outer quadrant; OQ: outer quadrants; UIQ: upper inner quadrant; UOQ: upper outer quadrant; UQ: upper quadrants.
The mean volume of the surgical specimens obtained in both groups showed statistically significant differences (group A: V = 189.37 cm3; group B: V = 128.68 cm3; p < 0.05).
The rate of margin involvement in group A was 13.2%, and 11.4% in group B, although this difference was not statistically significant (p = 0.784).
Seven patients in group A and three in group B underwent re-operation due to margin involvement (p = 0.507). Table 2 shows the values of these and other variables compared in both groups.
Measurements obtained in the hookwire group and in the seed group.
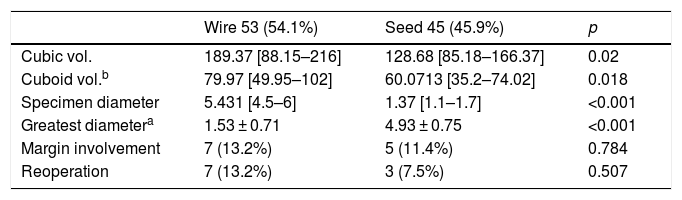
| Wire 53 (54.1%) | Seed 45 (45.9%) | p | |
|---|---|---|---|
| Cubic vol. | 189.37 [88.15–216] | 128.68 [85.18–166.37] | 0.02 |
| Cuboid vol.b | 79.97 [49.95–102] | 60.0713 [35.2–74.02] | 0.018 |
| Specimen diameter | 5.431 [4.5–6] | 1.37 [1.1–1.7] | <0.001 |
| Greatest diametera | 1.53 ± 0.71 | 4.93 ± 0.75 | <0.001 |
| Margin involvement | 7 (13.2%) | 5 (11.4%) | 0.784 |
| Reoperation | 7 (13.2%) | 3 (7.5%) | 0.507 |
Specimen diameter: diameter of the surgical specimen.
In one patient, seed implantation was unsatisfactory due to non-target lesion marking; the seed was located using a gamma detection probe and removed, and the target lesion was marked with a hookwire. This patient was excluded from the study.
DiscussionPreoperative wire localisation of non-palpable breast tumours has been the most widely used technique in recent years.2–18 One of the main drawbacks of this method is the high rate of margin involvement in the surgical specimen.9,10
Other technical difficulties for the surgeon include the possibility of migration of the wire and the difficulty in locating the tip, and, therefore, the lesion. When this occurs, extensive dissection is required to remove the entire tumour without margin involvement, resulting in the unnecessary loss of healthy tissue and a less aesthetic outcome.1,11
Radioactive seed localisation was first described by Gray et al. in 2001.1 The seed in question is a 4.5 × 0.8 mm titanium capsule, marked with radioactive 125I, which is inserted into the breast lesion.11,12 As this is a ROLL technique, resection is guided by a gamma detection probe, with the clear advantage that correct placement can be verified by mammography, since 125I seeds are radiopaque.13
The half-life of 125I is 59.4 days13, allowing implantation to take place well in advance of surgery, unlike wire localisation. In our study, however, the seed was placed within seven days of the intervention in order to facilitate planning and improve logistical issues between the departments involved.
Unlike wire localisation, seeds can be implanted in a far more central, delimited location within the breast and the tumour.14 Implantation is performed using localisation techniques similar to those used for wire localisation (mammography and ultrasound), and no additional training is required on the part of radiologists2,3; therefore, the technique can be performed in any adequately equipped breast radiology unit.
There are two main disadvantages of radio-guided surgery with 125I seeds with respect to preoperative wire localisation:
- 1
Exposure of the patient to radiation. Seeds show levels of radioactivity ranging from 0.1 to 0.3 mCi, which is considered safe for human exposure by the Nuclear Regulatory Commission.14 Only one seed was placed in our study patients, and remained inside the patient for a limited time (five to seven days) before surgical excision.8
- 2
Possible incorrect placement of the seed inside the breast. If a seed is not initially placed in the right position, it cannot generally be removed before the operation. A second incision must be made to locate and remove the seed, because unlike metal markers it cannot remain in the patient. Post-implantation seed migration is rare (<1% of cases).14 We observed fewer complications in our study patients compared to other authors.15
In addition to the above, some non-palpable breast lesions are large (extensive and/or dispersed microcalcifications, lesions larger than 2 cm). In these cases, it is important to bear in mind that radioactive seed localisation, like other preoperative localisation techniques, marks the centre of the lesion. However, if this is very large, the area of resection will extend beyond the location of the seed in order to avoid margin involvement. This problem can be solved using two markers (in this case radioactive seeds) to delimit the margins of the lesion.4
Outcomes of radio-guided surgery with 125I seeds are not inferior to wire marking in the localisation of non-palpable breast tumours.8–18
In our study, the volume of the specimens resected using seeds was significantly less than those marked with hookwires. These results are consistent with those reported by other working groups, such as Lovrics9 and Bas Pow,10 but differ from the results obtained by Ahmed and Douek.19 These authors found a statistically significant benefit of radio-guided surgery with 125I over wire localisation in non-palpable malignant lesions in terms of margin involvement, reoperation rates, and reduced operative time, with no statistically significant difference in the volume of surgical specimens in the treatment of non-palpable breast tumours located with both techniques.
In our study, although statistical significance was not reached, the rates of margin involvement and re-operation could have been lower with seed localisation compared to wire marking, but it should be taken into account that statistical significance possibly could not be reached due to the small sample size.10–17
The use of seeds is an important advance in the surgical treatment of non-palpable breast tumours in patients undergoing conservative breast surgery.20 Following introduction of this technique in our Breast Unit, breast surgeons, radiologists and pathologists agree that it is preferable and offers greater benefits than wire localisation for both medical staff and patients.
However, this technique is only possible in hospitals with a nuclear medicine department that supplies the seeds, and that can be present in the operating room at the time of the intervention to locate the seed, and, therefore, the lesion, using the gamma detection probe.19 This is why radiologists and nuclear medicine physicians must work together to implement this technique.
The main limitation of this study is its small sample size, a drawback that will probably be overcome in the future. As mentioned above, the small sample may have prevented us from obtaining statistically significant differences in some of the variables measured between the study groups.
ConclusionPreoperative radioactive seed localisation is an appropriate technique for marking non-palpable breast lesions, revealing significant differences in the size of surgical specimens with respect to wire localisation, although statistical significance between margin involvement was not observed, probably due to the small sample size.
Authorship- 1
Responsible for the integrity of the study: IMA, RSS, MCC.
- 2
Study conception: IMA, RSS, MCC, ÁCRA.
- 3
Study design: IMA, RSS, MCC.
- 4
Data collection: IMA, RSS, MCC, ADGJ.
- 5
Data analysis and interpretation: IMA, RSS, MCC, ADGJ.
- 6
Statistical processing: IMA, RSS, MCC, ADGJ.
- 7
Literature search: IMA, RSS, MCC, ADGJ.
- 8
Drafting of the article: IMA, MCC.
- 9
Critical review of the manuscript with intellectually relevant contributions: RSS, MCC, ÁCRA, SMM.
- 10
Approval of the final version: IMA, RSS, MCC, ADGJ. SMM.
The authors declare that they have no conflicts of interest.
Please cite this article as: Mendoza Arnau I, Sánchez Sánchez R, Culiáñez Casas M, Rebollo Aguirre ÁC, González Jiménez AD, Martínez Meca S. Arpón quirúrgico vs. semilla de iodo (125I) en la localización de lesiones no palpables malignas de mama. Resultados preliminares. Radiología. 2020;62:38–45.