The Spanish Society of Emergency Radiology (SERAU), the Spanish Society of Neuroradiology (SENR), the Spanish Society of Neurology through its Cerebrovascular Diseases Study Group (GEECV-SEN) and the Spanish Society of Medical Radiology (SERAM) have met to draft this consensus document that will review the use of computed tomography in the stroke code patients, focusing on its indications, the technique for its correct acquisition and the possible interpretation mistakes.
La Sociedad Española de Radiología de Urgencias (SERAU), la Sociedad Española de Neuroradiología (SENR), la Sociedad Española de Neurología a través de su Grupo de Estudio de Enfermedades cerebrovasculares (GEECV-SEN) y la Sociedad Española de Radiología Médica (SERAM) se han reunido para redactar este documento de consenso que repasará el uso de la tomografía computarizada en el código ictus, centrándose en sus indicaciones, la técnica para su correcta adquisición y las posibles causas de error en su interpretación.
Neuroimaging is an indispensable tool in stroke code procedure. Computed tomography (CT) is the most useful of the existing techniques, due to its availability in most emergency departments and its speed of acquisition. This makes it possible to obtain an accurate diagnosis with the speed that this pathology requires, so that the most appropriate treatment can be started in a timely fashion (Fig. 1).1
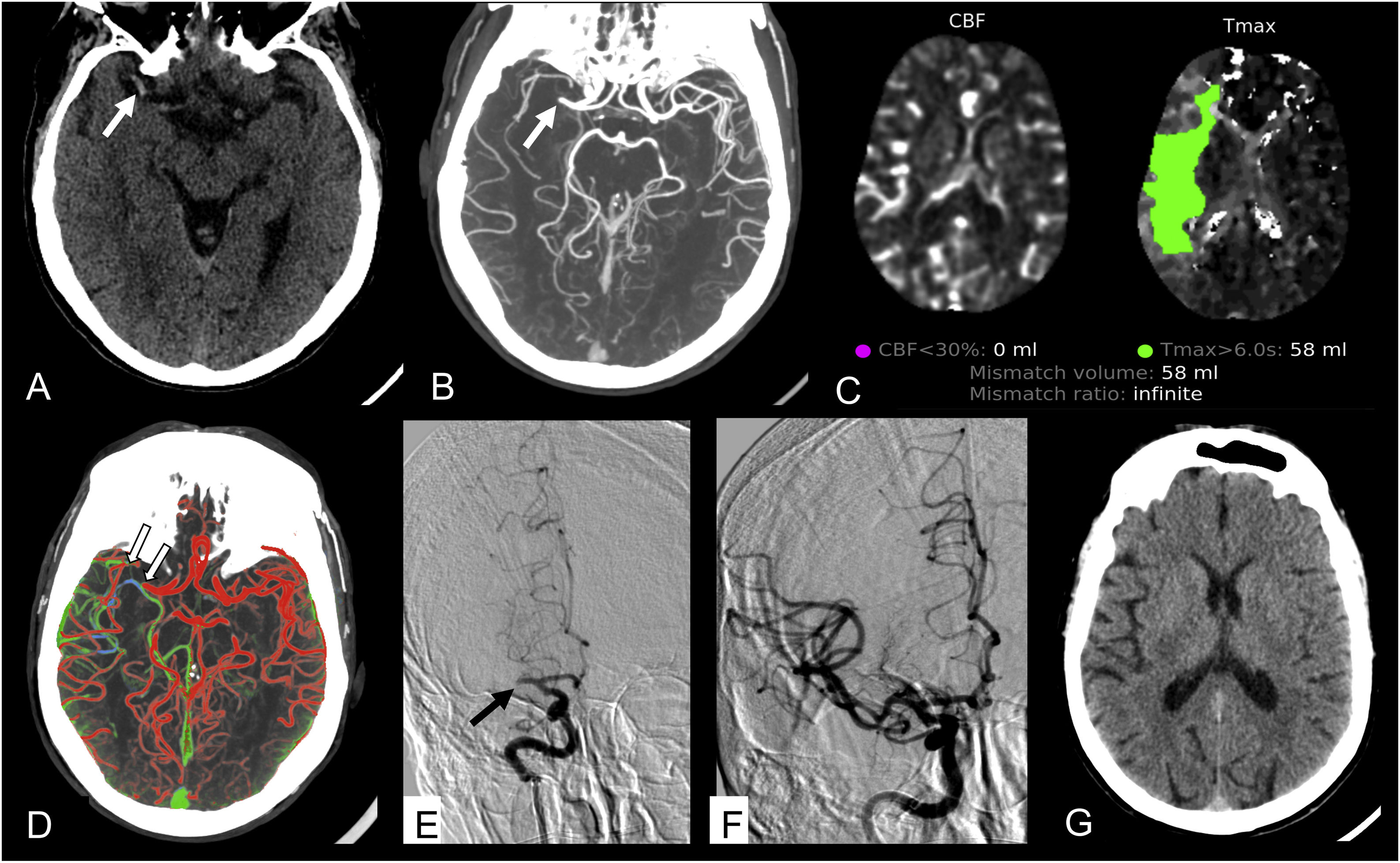
Male, aged 62, with cardiovascular risk factors, who presented with more than 12 h of left hemiparesis. De novo atrial fibrillation on admission to the emergency department. A) NCCT scan. Shows the right middle cerebral artery (MCA) hyperdense (arrow). There were no parenchymal lesions. ASPECTS 10. B) CTA. Repletion defect in M1 segment of the right MCA (arrow). C) CT perfusion. Shows a critically hypoperfused territory of 58 mL with no core, equivalent to a 100% penumbra area. D) Multiphase CT shows good collateral flow in the right hemisphere (grade 4), where there are vessels of similar quantity and calibre to the contralateral hemisphere with predominantly retrograde filling in arterial (red) and early venous (green) phases. The clot is marked as a repletion defect (arrows). E) CTA prior to mechanical thrombectomy shows a complete M1 occlusion of the right MCA (arrow). F) CTA after mechanical thrombectomy. The right MCA is completely recanalised, with a persistent M3 distal frontal defect (not shown), TICI 2B revascularisation. G) Follow-up NCCT scan at 24 h. No residual lesions.
Different CT techniques can acquire images of both the brain parenchyma and the vascular tree, while also providing information on the state of regional cerebral blood flow (CBF) and of tissue perfusion in the case of ischaemic stroke.2 Thus, the primary objective of brain CT is to distinguish between ischaemic and haemorrhagic stroke and to rule out other stroke-mimicking lesions. CT angiography (CTA) studies require the administration of intravenous contrast and are used to identify arterial occlusions in the event of ischaemic stroke. In the case of haemorrhagic stroke, CTA is used to identify vascular malformations, aneurysms or cerebral venous sinus thrombosis. Multiphase CTA (mCTA) studies are used to assess the collateral circulation status.3
Finally, CT perfusion (CTP) studies map the timing of the contrast passage and the concentration reached in the cerebral vascular tree, which are directly related to cerebral perfusion, enabling estimation of the viability or not of the ischaemic tissue.4,5
In order to get the best results from the different CT tools included in the stroke code, practitioners should be familiar with their indications, what useful information they can provide in each case, the technical aspects that should be considered for their correct acquisition and possible causes of error in their interpretation. In order to answer these questions, the Spanish Society of Emergency Radiology (SERAU), the Spanish Society of Neuroradiology (SENR) and its Cerebrovascular Disease Study Group (GEECV-SEN) and the Spanish Society of Medical Radiology (SERAM) have met to write this consensus document.
Clinical indications for CTHead CT with/without contrastA non-contrast CT (NCCT) of the head is the first imaging technique to be used to diagnose a stroke patient. It should be performed immediately on all patients who arrive at the emergency department with acute focal neurological deficits.6 The same urgent priority given to these patients in terms of clinical care should also be assigned to the neuroimaging process, which should be performed as soon as the patient’s clinical condition is stabilised on arrival.
This is necessary in order to differentiate between ischaemic and haemorrhagic strokes, rule out other stroke-mimicking lesions and start appropriate treatment as soon as possible.7 A plain head CT is recommended within 20 min of the patient's arrival at the emergency department.8 This recommendation also applies to patients with transient ischaemic attack. The fact that symptoms resolve can be due to a clinical change secondary to another modification such as blood pressure rather than a complete clinical resolution.
Head CT is performed without contrast, generally reserving the use of contrast for the diagnosis of stroke-mimicking lesions (such as tumours or encephalitis) when the plain head CT findings require greater definition for the final diagnosis.
CT angiographyIn the stroke code protocol, CTA is stipulated for the non-invasive assessment of the cerebral vascular tree in order to detect occlusions, stenosis and other vascular abnormalities in stroke patients. In ischaemic stroke, urgent CTA is indicated so that should large vessel occlusion be detected, treatment with mechanical thrombectomy can be recommended for candidate patients.8,9 However, the detection of occlusion in medium vessels (including the anterior cerebral artery, M2-M3-M4 segments of the middle cerebral artery, and posterior cerebral artery) is of great importance in the clinical management and functional prognosis of the patient.10
It is important to note, however, that if there is an indication of arterial occlusion, intravenous thrombolytic treatment can be administered before it is confirmed by diagnosis. In fact, thrombolysis should not be delayed, but carried out immediately after the plain head CT without the need to wait for the CTA result.11
With these general considerations in mind, CTA should be performed immediately after plain head CT in all patients with moderate to severe ischaemic stroke, or when there are signs suggesting injury to the cortex, such as aphasia, since the likelihood of a large vessel occlusion is higher in these cases.
This includes patients with high-risk transient ischaemic attacks.12 In fact, CTA is now recommended for all acute ischaemic stroke patients in order to obtain a more accurate diagnosis from the outset. This test has been incorporated into routine stroke code management protocols in most hospitals that treat these patients given the increasing familiarity of radiologists and radiographers with this type of study, as well as the characteristics of contemporary CT scanners which make image acquisition quicker and simpler without delaying therapeutic decision making.
This general recommendation should be reconsidered for patients for whom specific treatments are ruled out a priori, such as those with pre-existing poor clinical or functional status, prolonged evolution time, or strong suspicion of stroke-mimicking conditions.
In the case of haemorrhagic strokes, urgent CTA is indicated when the characteristics of the haemorrhage suggest an underlying secondary aetiology such as the rupture of a vascular malformation or an aneurysm. It should be indicated in all cases of subarachnoid haemorrhage and in parenchymal haemorrhages in young patients, especially if there is no history of hypertension or if the haemorrhage occurs in the lobes of the brain. Likewise, a CT cerebral venography should be performed if there is suspicion of cerebral venous thrombosis.
Multiphase CT angiography and CT perfusionAlthough these two CT techniques are distinct and are dealt with separately in the section on acquisition recommendations, the information they provide is complementary and their clinical indications are clearly related, so they are discussed together in this section.
Both techniques provide information on the perfusion status of the tissue at risk when arterial occlusion is present and therefore offer indirect markers of tissue viability in patients with ischaemic stroke, enabling the size of the penumbra area to be estimated. They are therefore used to identify those ischaemic stroke patients who may benefit from extended-window reperfusion therapy.
Neither CTP nor mCTA provide any information that seriously impacts treatment indications within the treatment windows established by the main studies on the implementation of these treatments (4.5 h for intravenous thrombolysis and 6 h for mechanical thrombectomy)13 and are therefore not recommended.8,11,14
Furthermore, these techniques should not be used to rule out reperfusion therapy in patients who otherwise meet criteria, especially in early stages after the onset of symptoms, because just as they can reliably identify potentially salvageable tissue in extended windows, there is evidence to suggest that not all tissue identified as core actually represents necrosis and irreversible damage in the early stages of ischaemia.15
However, the information provided by these techniques can help select patients who may benefit from reperfusion therapy from among those whose onset of symptoms is unknown, or when the treatment windows have passed, up to 24 h following onset. Therefore, all potential candidates who meet these criteria should be screened for reperfusion therapy. Tissue survival is a dynamic and variable process that depends on the individual susceptibility of each subject; it is directly proportional to the efficiency of the collateral circulation and inversely proportional to the severity of the ischaemia.
Thus, the concept of the treatment window has evolved from a static and purely temporal approach to one that is more closely related to the pathophysiology of cerebral ischaemia. This means that decisions on whether treatment is an option are based on whether or not a sufficient amount of tissue that is not irreversibly damaged (penumbra area) can be identified, regardless of the time elapsed since the onset of symptoms.
This is made possible by identifying areas in which regional CBF is preserved above the threshold of irreversible membrane failure, using techniques that can measure cerebral perfusion (such as CTP),16 or by identifying the membrane failure itself, which is possible using diffusion-weighted and FLAIR magnetic resonance imaging (MRI), which, in addition, provide an estimate of the time elapsed since the onset of symptoms.17,18
Several studies demonstrate the usefulness of these techniques in clinical practice. In this paper, we will not discuss the studies which support the use of MRI to evaluate the possibility of using extended-window thrombolysis.19,20 Instead, given the purpose of the article, we mention only those using the relevant CT techniques. In CTP, irreversibly damaged tissue, or infarct core, is defined as tissue in which regional CBF falls below 30% of healthy tissue, while critically hypoperfused tissue is defined as tissue in which contrast reaches its maximum concentration in a maximum time (Tmax) of more than six seconds.21
A mismatch between the two volumes indicates how much tissue is penumbra and therefore potentially salvageable, that is: tissue which is so critically hypoperfused that it produces symptoms but which is not irreversibly damaged, thanks to sufficient regional CBF. The EXTEND study demonstrates the benefit of intravenous thrombolysis for patients who can be treated within 4.5–9 h from symptom onset and who have a penumbra core mismatch ratio > 1.2, absolute difference > 10 mL and core volume < 70 mL. The use of automated measurement systems is recommended.21
A recent meta-analysis that also included MRI studies confirms the benefit of intravenous thrombolysis for patients with unknown time of onset, provided that the existence of penumbra tissue can be demonstrated using the above criteria.22 The DAWN and DEFUSE-3 studies also demonstrated the benefits of using perfusion imaging and MRI to select patients for late-window mechanical thrombectomy, albeit with different criteria.
The DAWN study (6–24 h after symptom onset) uses the clinical imaging mismatch criterion to determine the existence of penumbra tissue, and thus, in patients over 80 years of age, it considered there to be salvageable tissue—and, therefore possible benefits from using thrombectomy—when the NIHSS score > 10 and the core < 21 mL; and in those under 80 years of age when the NIHSS score > 10, and the core < 31 mL; or the NIHSS score > 20 and the core < 51 mL.23 The DEFUSE-3 study (6–16 h after symptom onset) establishes the benefit of thrombectomy when core ≤ 70 mL, penumbra tissue ≥ 15 mL and the ratio of hypoperfused tissue to core > 1.8.24
Multiphase CTA is also useful for selecting patients for mechanical thrombectomy treatment in extended windows when good collateral flow can be demonstrated (grade 4 or 5),25 and a comparative study suggests that this technique might be a better predictor than CTP or MRI.3,26,27 Based on these data, current therapeutic guidelines recommend the use of these techniques to select patients for extended-window reperfusion therapy. 8,11,14
In addition to these indications, CTP may be useful for the diagnosis of stroke mimics, given that in these cases there will be no perfusion deficit detected in a given vascular territory.
Patient preparationIn order to shorten the diagnostic process, the following steps should be taken before or upon arrival of the patient for the CT scan:
- □
Before the study begins, all metallic items should be removed from the patient's head and neck, including but not excluding dentures, glasses and hairpins.28
- □
An 18–20 gauge peripheral IV or larger, should ideally be inserted in the right upper limb of patient,29 allowing for an intravenous contrast injection rate of at least 4 mL/s,28 as most authors recommend an intravenous contrast injection rate of 5 mL/s.30
- □
In agitated patients, sedation may be considered in discussion with the neurology and anaesthesia teams.
- □
Patient positioning: patient in the supine position. It is very important that the patient's head is immobilised and in a symmetrical position.
- 1.
Rule out haemorrhagic stroke or any lesion that mimics a stroke, such as tumour, arteriovenous malformation or a central nervous system infection.
- 2.
Detect early signs of ischaemia and quantify them using the Alberta Stroke Program Early CT Score (ASPECTS) scale.31
We recommend that at the start of the stroke code study, a general mapping of the area should be performed from the aortic arch to the vertex of the skull, to be used for the subsequent programming of CTA of the cerebral arteries.30
For head NCCT, the acquisition area should include the area from the base of the skull to the vertex, and can be acquired using sequential or helical scanning. Traditionally, sequential acquisition has been preferred for devices with less than 64 detector channels with the scan angle set parallel to the orbitomeatal or supra-orbital line.30 However, multidetector scanners (MDCT), especially those with more than 64 detector channels, have significantly improved helical scanning, both in terms of image quality and a reduction of artefacts. Scanners with fewer detector rows may still provide higher quality images and less artefacts with sequential acquisition, which is why we advise each user to evaluate the most appropriate acquisition according to the particular scanner they are using.
Slice thickness should be 2 mm or less, and images should be reconstructed with a minimum slice thickness of 5 mm, as this reflects the thickness used in some approved programmes for the selection of patients with acute ischaemic stroke for endovascular treatment (for example, RAPID).
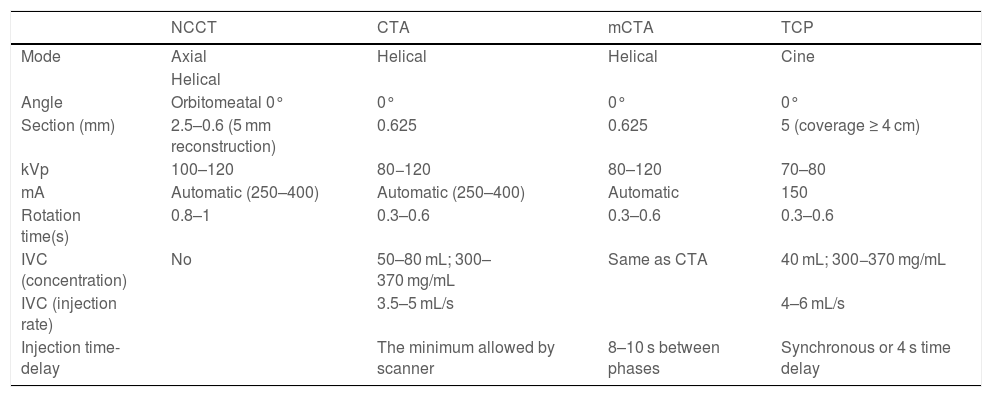
Dual-energy acquisitions can provide better contrast when it comes to identifying subtle hypodensities, and allow for better visualisation of haemorrhagic transformation.32 The acquisition parameters are summarised in Table 1.28,32–36
Acquisition parameters.26,29–33
| NCCT | CTA | mCTA | TCP | |
|---|---|---|---|---|
| Mode | Axial | Helical | Helical | Cine |
| Helical | ||||
| Angle | Orbitomeatal 0° | 0° | 0° | 0° |
| Section (mm) | 2.5–0.6 (5 mm reconstruction) | 0.625 | 0.625 | 5 (coverage ≥ 4 cm) |
| kVp | 100–120 | 80−120 | 80–120 | 70–80 |
| mA | Automatic (250–400) | Automatic (250–400) | Automatic | 150 |
| Rotation time(s) | 0.8–1 | 0.3–0.6 | 0.3–0.6 | 0.3–0.6 |
| IVC (concentration) | No | 50–80 mL; 300–370 mg/mL | Same as CTA | 40 mL; 300−370 mg/mL |
| IVC (injection rate) | 3.5–5 mL/s | 4–6 mL/s | ||
| Injection time-delay | The minimum allowed by scanner | 8–10 s between phases | Synchronous or 4 s time delay |
- 1.
Detection of occlusion or significant stenosis of major vessels such as the M1, M2 or M3 segments of the middle cerebral artery; the A1 and A2 segments of the anterior cerebral artery; the P1–P2 segment of the posterior cerebral artery; the vertebral arteries; the common carotid arteries; and the internal carotid arteries, which are suitable for emergency endovascular treatment.
- 2.
Detection of vascular injury which explains the aetiology of the stroke, such as dissection, vasculitis, dural arteriovenous fistula.
- 3.
Assessment of vascular anatomy—mapping for treatment.
- 4.
Estimate of collateral score.
- 5.
Detection of incidental vascular pathology that may condition treatment, such as vascular malformations or aneurysms.
CTA uses the automatic bolus tracking technique.28 The study should be performed from the aortic arch to the vertex with helical acquisition without the need to tilt the gantry.30 A thin slice thickness of 1.5 mm or less, typically 0.625 mm, should be used with a lower pitch, and images reconstructed with a slice thickness of at least 1.25 mm.28 In CTA studies, the standard protocol to achieve good contrast of vascular structures recommends the use of 100–120 kV. Low kV (70 kV) protocols with a lower contrast dose can be used in scanners that enable lower kV.37
It is recommended that the region of interest (ROI) be placed on the ascending aorta.28 If the contrast bolus is injected through the left upper limb, the artefacts that can result upon repletion of the left brachiocephalic venous trunk or from the respiratory movements of the patient can be mitigated, when using scanners with at least 64 detector rows, by placing the ROI on the descending aorta (less prone to artefacts caused by respiratory movements), thereby protecting the quality of the image.
Several authors recommend setting the tracking threshold at 80 HU,28 although this has to be adapted to the speed of acquisition of each scanner and may differ among models produced by different manufacturers.
Iodine concentration in the intravenous contrast bolus should be between 300 and 370 mg/mL for angiographic studies. In scanners with at least 32 detector channels, 60–80 cc of contrast is sufficient, followed by injection of 20 cc saline at an injection rate of 2 mL/s.28,30 If a multiphase CT scan will immediately follow the CTA of the cerebral arteries study, it is advisable to use at least 80 cc of intravenous contrast.38 The acquisition parameters are summarised in Table 1.
CT angiography before or after CT perfusionSince both CTA and CTP use intravenous contrast, there are often concerns about interference between the two techniques resulting from contrast accumulation.39
Some authors argue that the main drawback of performing a CTA study before a CTP is the potential impact on the mathematical calculations of the perfusion maps caused by the prior administration of intravenous contrast.40 This problem could be solved with a 30-s pause to allow the contrast concentration to stabilise before starting the CTP.41
A potential drawback of performing CTP before the CTA is venous contamination, which could be solved by waiting three minutes for contrast washout.39 Other studies conclude that the order in which they are performed does not influence the results at a technical level.28,39
Given that acquisition protocols are highly variable, and that it has been very difficult for us to make a recommendation on this point, we have decided to set out the possible advantages of both options so that the practitioner can decide according to the technical characteristics of their scanner and healthcare environment.
On the one hand, the possible advantage of performing CTP before the CTA is that the three phases of the contrast-enhanced cerebral arteries study can be acquired in the same acquisition:
- □
Arterial phase: enhancement by the contrast administered for the CTA.
- □
Delayed phase for the evaluation of the brain parenchyma: enhanced by the contrast previously administered for the CTP. This contrast uptake by the parenchyma increases the difference between grey and white matter density, thereby resulting in higher inter-rater agreement of the ASPECTS score, and a better prediction of the final infarct volume compared to that predicted from the early ischaemic changes on the NCCT scan. To this end, we should assign the ASPECTS score based on CTA Source Images (CTA-SI) using axial multi-planar reconstruction (MPR) with a 5 mm thickness.42–46
- □
Venous phase: enhanced by the contrast administered previously for the CTP, allowing us to rule out cerebral venous thrombosis without having to perform another acquisition.
On the other hand, below are some possible benefits of performing the CTA prior to the CTP39:
- □
The area to be studied in the CTP can be properly targeted thanks to information acquired from the previous scan. CTP scans most commonly focus on the level of the basal ganglia by default. In patients with more distal obstructions (M2, M3), it is necessary to align the slices more closely with the cranium. In perfusion scanners with limited coverage, it is possible that by automatically selecting the basal ganglia as the area to be studied, our territory of interest may not be included.
- □
The CTA study provides information on the haemodynamic status of the patient, which helps in the interpretation of the perfusion study, as the bolus takes longer to reach the maximum arterial curve in patients with poor cardiac output, which can invalidate the CTP.
The literature does not provide evidence on the influence of performing a CTP prior to an mCTA so we do not know if any artefacts are produced in this study as a result.
Multiphase CT angiographyObjectives25,35,47- 1.
Characterisation of leptomeningeal collaterals.
- 2.
Increase sensitivity in the detection of large vessel occlusions, especially in distal branches (M2–M3).
- 3.
Assess the length and permeability of the clot.
- 4.
Assess venous drainage, increasing the sensitivity of detection of venous anomalies or alternative diagnoses, such as sinus thrombosis.
Multiphase CTA is a technique that studies the filling of the intracranial vessels using the same bolus of intravenous contrast in three helical acquisitions at different times: arterial phase, early venous phase and late venous phase.25
The technique includes:25,35
- □
A conventional CTA of the cerebral arteries, with bolus tracking, 100−120 kV, and a slice thickness of 0.625 mm. An 80 mL intravenous contrast bolus with a concentration of at least 320 cc/mL is administered with a recommended injection rate of 5 mL/s, followed by 50 mL of saline.
- □
A second acquisition four seconds after the end of the previous phase (enough time to reposition the table), from the base of the skull to the vertex, using the same imaging technique.
- □
A third acquisition four seconds after the end of the previous phase (enough time to reposition the table), from the base of the skull to the vertex, using the same imaging technique.
These recommendations apply to scanners with 64 detector rows or more. For scanners with fewer rows, it is possible that the repositioning time between one phase and the next may take longer than eight seconds, with the result that the image quality may not be optimal for interpretation. Some articles extend the delay between the first and second phase to 10 s.38 The acquisition parameters are summarised in Table 1.
CT perfusion in strokeObjectives- □
Detect the presence of penumbra tissue that has the potential to be salvaged.
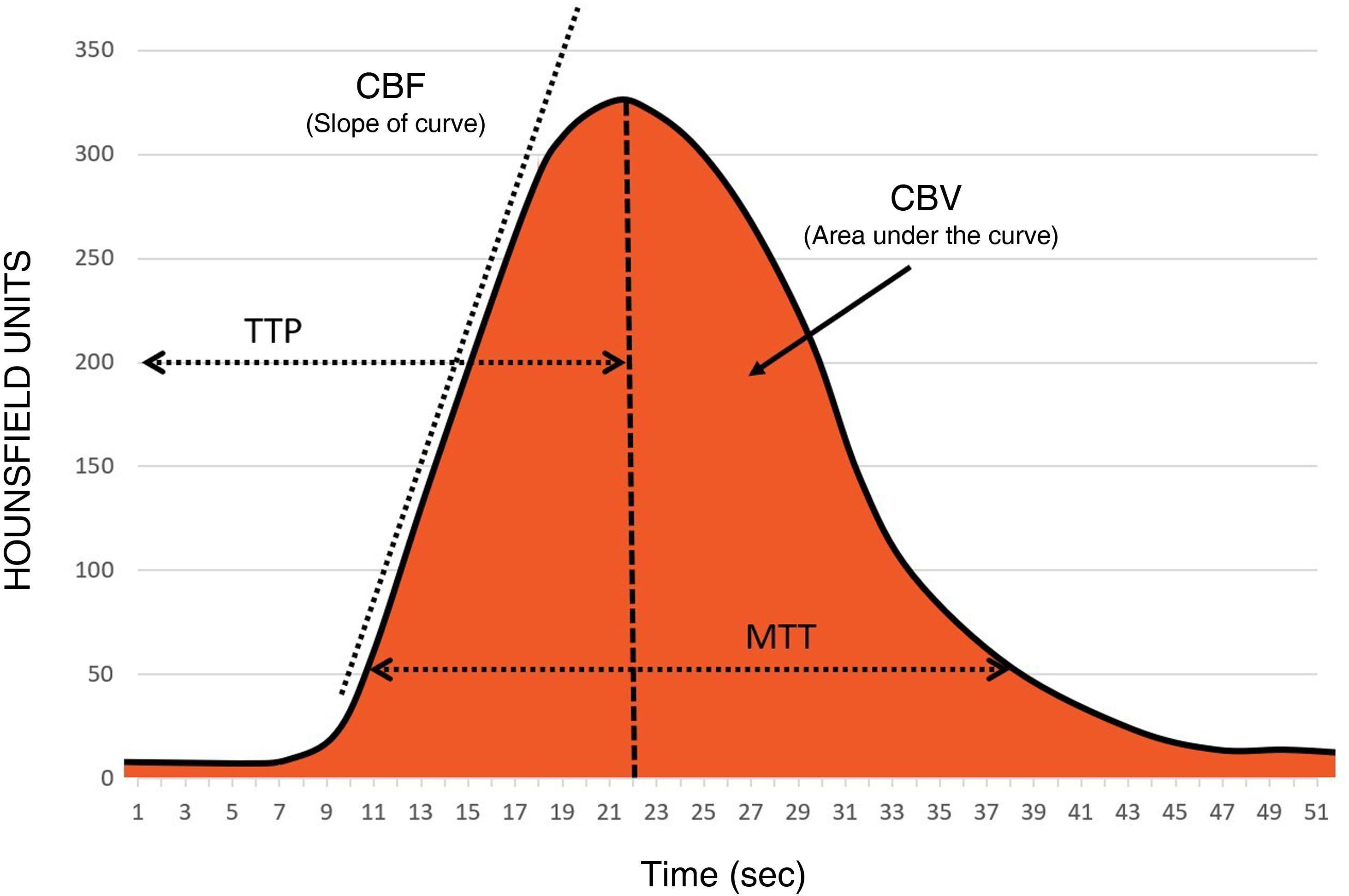
In order to fully understand the importance of the technical aspects involved in the acquisition, we will first review the significance of the different perfusion maps (Fig. 2):
- □
Time to Peak (TTP): value in seconds from the start of the contrast injection to the maximum contrast concentration.
- □
Mean Transit Time (MTT): value in seconds of the average time it takes for the contrast to pass through a region of the brain.
- □
Time-to-Maximum (Tmax): a perfusion parameter that reflects the time delay of the contrast bolus arriving in the proximal large vessel arterial circulation (arterial input function [AIF]) and the brain parenchyma, calculated by deconvoluting the AIF.
- □
Cerebral blood volume (CBV): the total volume of blood circulating in a region of the brain, measured in mL/100 g of brain parenchyma. It is represented by the area under the concentration-time curve in Fig. 2.
- □
Cerebral blood flow (CBF): the volume of blood circulating in a region of the brain per unit of time; the unit employed is mL/min/100 g of brain parenchyma. It is shown as the ascending slope of the curve in Fig. 2.
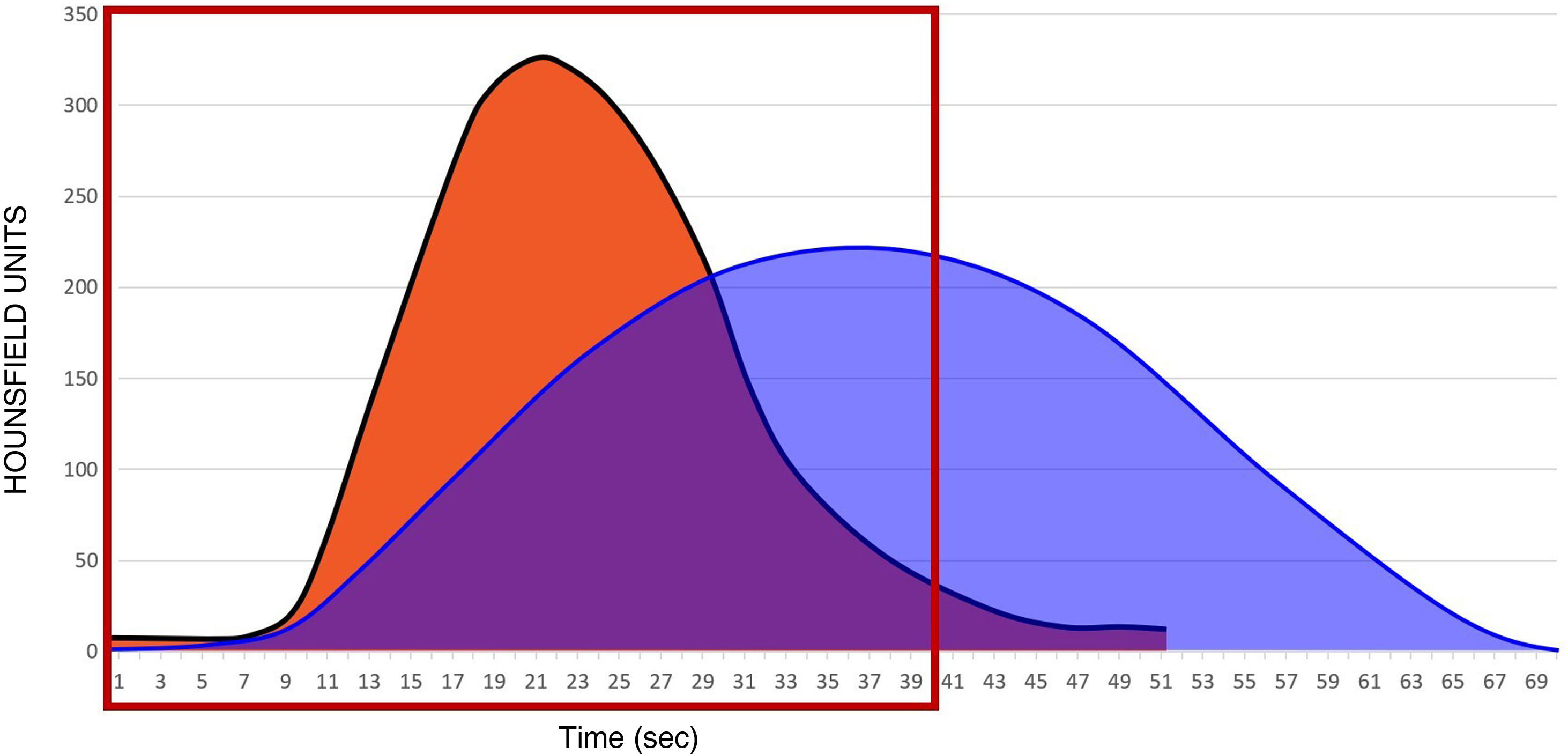
Fig. 3 shows the perfusion curve in an area with a patent cerebral artery (orange curve) and the perfusion curve of an area with an occluded cerebral artery (blue curve). When an artery distal to the Circle of Willis is occluded, (for example the M1 segment of the middle cerebral artery), the only way that blood flow can return to the irrigated area is via leptomeningeal (collateral) connections from the anterior and posterior cerebral arteries ipsilateral to the distal territory of the middle cerebral artery. As a consequence, the contrast arrival curve is less steep and more extended in time (blue curve in Fig. 3).
The slope of the curve (CBF) indicates the speed of contrast arrival. It generally takes about 10 s to reach maximum contrast concentration in an open artery (orange curve in Fig. 3). However, in an occluded artery, with repermeabilisation by collaterals, it can take twice as long (blue curve in Fig. 3). In addition, the maximum contrast concentration in a territory that corresponds to an occluded artery will also be lower than in a territory that corresponds to a patent artery.
AcquisitionThe following technical aspects should be considered: slice thickness, duration, temporal sampling, volume and speed of contrast injection, anatomical coverage, radiation and order or timing of the multimodality protocol. The acquisition parameters are summarised in Table 1.
- □
Slice thickness. The recommended slice thickness is 5 mm, with an increment of 5 mm.
- □
Duration. CTP is performed by way of the dynamic acquisition of a bolus of iodinated contrast through the cerebral circulation. Continuous cine mode images are obtained over 60–70 s for the same brain area. If the scan is too short, the passage of the contrast bolus is not captured completely, resulting in inaccurate perfusion maps and unreliable estimates of lesion volume. When the scan is too long, it increases the radiation dose by capturing images of the tail end of the bolus passage, which does not provide useful information. This duration varies between patients; in > 90% of patients, correct image acquisition is achieved with a 60-second scan duration.
- □
Temporal sampling. Data sampling should be fast during the ascending curve (one image per second for 37 s). To ensure good quality perfusion maps, sampling can be slower (one image every three seconds, for 33 s) on the descending curve, thus limiting the radiation dose in the part of the curve that does not provide relevant information.
- □
Volume and speed of contrast injection. Dynamic administration of a 50 mL bolus of contrast at high flow rate (4−6 mL/s), followed by 40 cc of saline at an injection rate of 2 mL/s.28 The injection of contrast should be synchronised with the start of the acquisition. It is important to obtain some baseline acquisitions (5–10 s) to properly calculate perfusion maps. The CTP acquisition should be started four seconds after the injection of contrast begins.
- □
Anatomical coverage. The minimum anatomical coverage size should be 4 cm, and the desired area of study selected at the level of the basal ganglia, which contains the territory where the three main arteries coincide: the anterior, middle and posterior cerebral arteries. However, with such limited coverage, the full extent of the ischaemic lesion may not be captured. At least 8 cm of z-axis coverage, extending rostrally from above the orbits is recommended. Z-axis coverage is primarily determined by the CT detector width, which varies from 4 to 16 cm on most modern scanners. One option to increase coverage is to obtain two individual CTP scans at adjacent levels, each requiring its own contrast injection. In this case, the CT table moves between scans, but remains stable during each individual scan. Another option is to move the table during a single CTP acquisition.28,41
- □
Radiation. A drawback of CT is the radiation dose, which should be based on the principle of as-low-as-reasonably-achievable (ALARA). Depending on the scanner, different dose reduction systems are available, and these should be included and adapted in the perfusion protocols.
After acquisition, the image is processed to generate the perfusion maps. The contrast medium passes through the brain tissue, causing a transient hyperattenuation that is directly proportional to the amount of contrast in the vessels and blood in the region. Time attenuation curves are generated by selecting an arterial ROI in an unaffected vessel perpendicular to the acquisition plane (contralateral anterior or middle cerebral artery) to estimate the AIF. A venous ROI is placed over the superior sagittal sinus to compensate for the partial volume effect to ensure that an accurate estimate of perfusion parameters can be achieved.28
It is important to analyse the AIF and venous output function (VOF) curves visually during processing and monitor their quality, ensuring they have a similar shape: the AIF precedes the VOF by a few seconds, there is a short pre-contrast baseline (5−10 s), and the ascending and descending slope of the VOF should be fully included.
The dynamic data acquired are used to create different colour-coded perfusion maps: CBV, MTT, TTP and CBF. These parameters are quantified according to the equation CBF = CBV/MTT. MTT is calculated using a mathematical technique called deconvolution which is applied to the time-attenuation curve of each pixel with respect to the arterial curve (AIF). CBV is calculated by dividing the area under the curve in a parenchymal pixel by the area under the curve in an arterial pixel.28
Errors in image acquisitionErrors in image acquisition can occur, resulting in incorrect perfusion maps. These errors include:
- □
Patient movement: An AIF curve with multiple peaks indicates head movement.
- □
Inadequate selection of coverage. In acquisitions with a coverage of 4 cm, some of the affected territory may not be covered.
- □
Inadequate selection due to delay in contrast arrival. If the contrast arrives late, the AIF and VOF curves are not fully captured; if the acquisition is terminated during the descending slope, it may produce false estimates of CBV, Tmax, MTT and CBF.
- □
Short duration of acquisitions (Fig. 3). Acquisitions that take less than 30–40 s (red box) may not capture all the volume information (area under the blue curve) corresponding to the occluded territory, leading to an overestimation of the territory with a low CBV (theoretically unrecoverable tissue).
- □
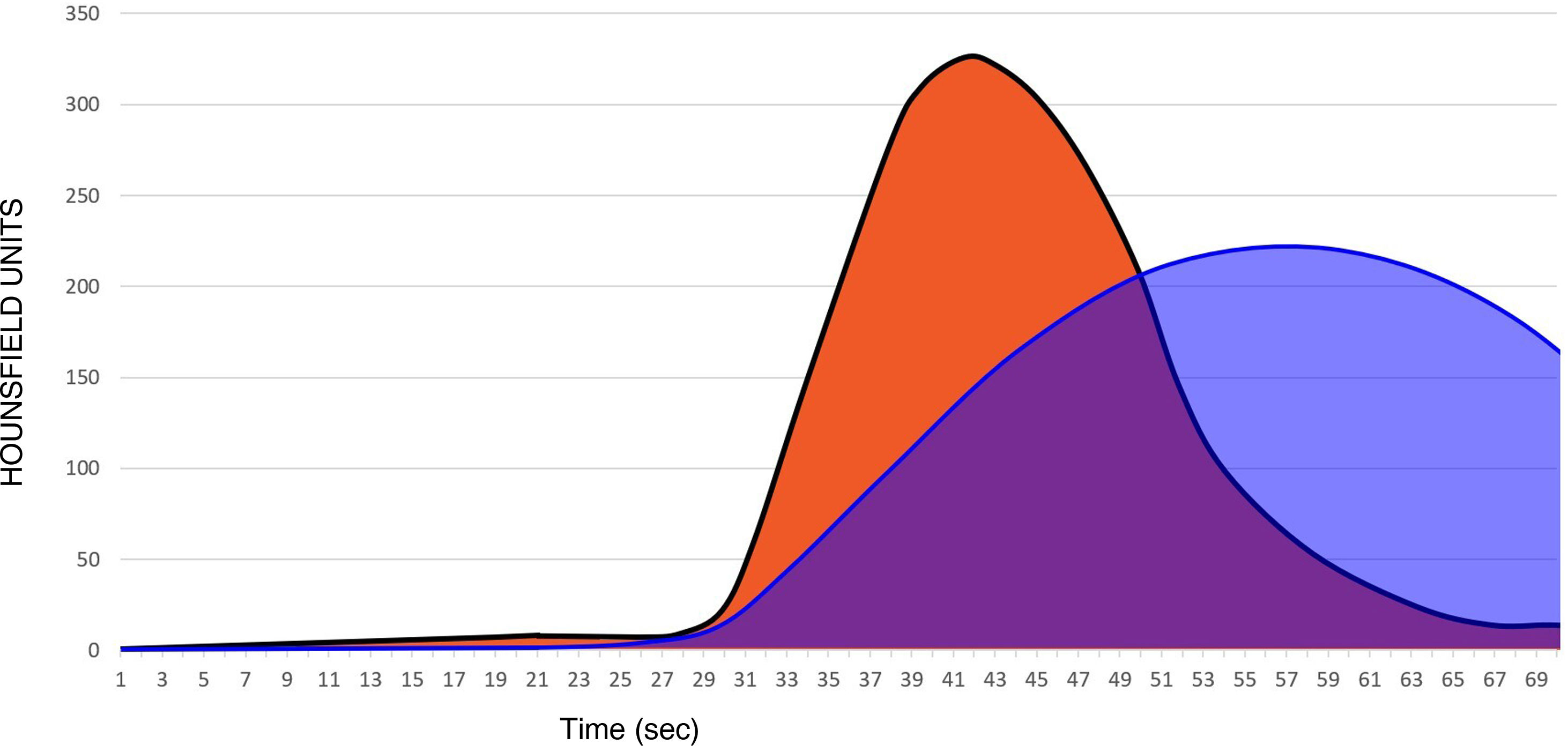
Low ejection fraction (Fig. 4). A delay in the arrival of contrast from the heart to the brain means that, even if an adequate acquisition protocol is in place (60–70 s), it is possible that it will not be possible to collect all the information for the area under the curve. Therefore, in this situation, it is important to consider the way the acquisition was performed in order not to overestimate the area under the curve with low CBV.
- □
Acquisition of CTP after CTA. If CTP is acquired after CTA, artefacts may be seen in the perfusion maps, resulting in erroneously low CBV values, leading us to make the mistake of overestimating the territory with low CBV.
Regarding the qualitative assessment of perfusion maps, it is important that the term ‘significant mismatch’ is defined clearly within the local service as it is a concept that can have multiple definitions, an uncertainty that can potentially lead to serious miscommunication between neurologists and neurointerventionalists. We recommend, in accordance with the resources available in each centre, reaching a consensus on the definition of the term ‘significant mismatch’ among all the professionals involved in the stroke code process in order to minimise communication errors among team members.
Other conditions that can cause misinterpretations or clinically mimic infarction are vasospasm, posterior reversible encephalopathy syndrome (PRES), chronic infarction, the postictal phase of epilepsy, the aura phase of migraine, cerebral hyperperfusion syndrome after carotid stent placement or carotid endarterectomy, lacunar infarcts or borderline infarcts.48,49
ContraindicationsThe only absolute contraindication for performing the test is a severe allergy to iodinated contrast. Given the severity of the clinical situation, relative contraindications (renal insufficiency, pregnancy, etc.) do not preclude a contrast study for patients with acute ischaemic stroke and suspected arterial occlusion. If the patient has a severe allergy to iodinated contrast, the appropriateness of the contrast study should be assessed and enquiries made to determine whether the anaesthesia team can be present to manage any severe reaction that may occur on injecting the contrast.
FundingThis project has not received any funding.
Conflict of interestThe authors declare that there is no conflict of interest.
Author contributions- 1.
Research coordinators: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
- 2.
Development of study concept: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
- 3.
Study design: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
- 4.
Data collection: N/A.
- 5.
Data analysis and interpretation: N/A.
- 6.
Data Processing: N/A.
- 7.
Literature search: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
- 8.
Writing of article: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
- 9.
Critical review of the manuscript with intellectually relevant contributions: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ
- 10.
Approval of the final version: ALR, LIS, MAL, DAMR, AVB, MCR, LOZ.
The authors would like to thank Iniciativa Angels, and in particular Belén Velázquez and Alicia Arjona, for their involvement in the project, proposing, coordinating and facilitating this consensus document.