Essure is a permanent birth control device that is inserted through the cervix by hysteroscopy. The device is placed in the fallopian tubes, where it causes occlusion by stimulating fibrosis. Patients can be followed up with plain-film X-rays, hysterosalpingography, and ultrasonography, although the devices can also be identified incidentally on CT and MRI. The follow-up of Essure is based on checking the criteria for appropriate positioning and correct functioning (tubal occlusion) and on diagnosing complications. The most common complications are perforation, migration (toward the uterine or peritoneal cavity), and occlusion failure. In hysterosalpingography, vascular intravasation is the most common cause of diagnostic error. Radiologists need to know how to recognize the device on different imaging techniques, how to check that it is correctly placed and functioning, and how to diagnose complications.
Essure es un dispositivo anticonceptivo permanente de inserción transcervical con histeroscopia. Se sitúa en las trompas a las que ocluye estimulando la fibrosis. Las técnicas para seguir a las pacientes son la radiografía simple, histerosalpingografía y ecografía, aunque los dispositivos se pueden identificar también incidentalmente con TC y RM. El seguimiento de Essure se basa en comprobar los criterios de posición adecuada y funcionamiento correcto (oclusión tubárica), y diagnosticar complicaciones. Las complicaciones más frecuentes son la perforación, migración (hacia la cavidad uterina o peritoneal), y fallo de la oclusión. La intravasación vascular es la causa más frecuente de error diagnóstico con la histerosalpingografía. Es importante que el radiólogo reconozca el dispositivo en las diferentes técnicas de imagen, los signos que indican que su situación y función son correctas y diagnosticar las complicaciones.
The Essure (Conceptus) system is one irreversible female anti-contraceptive method whose insertion through hysteroscopic approach does not require anesthesia and that can be used outpatiently. After two multicenter trials1,2 it was approved by the European health authorities in 2001 and by the U.S. Food and Drug Administration in November of 2002.3 This technique is way more cost-effective than laparoscopic tubal sterilization.4 The complications described during insertion and in the coming days are pain–usually mild or moderate and some times vaginal bleeding. Chronic pain and infections are rare.5,6 It is estimated that until 2010 around 450,000 Essure7 devices had been placed worldwide and the rate of pregnancies published is almost non-existent.8
After the insertion of Essure the image modalities used for assessment purposed are simple radiology, ultrasound and hysterosalpingography with different protocols. The goal in all cases is determining the correct positioning or function of this device. We reviewed the radiologic tests of 550 patients sterilized with Essure in our institution between September of 2008 and December of 2013. The control protocol on our institution consists of one simple X-ray in all patients and one hysterosalpingography in cases of difficult insertion or doubtful images in the simple X-ray. Ultrasound is used in cases in which there is suspicion of complications. Our goal is to show the radiologic characteristics of the permanente anti-contraceptive Essure, the diagnostic difficulties associated and signs that would lead us to be suspicious of complications.
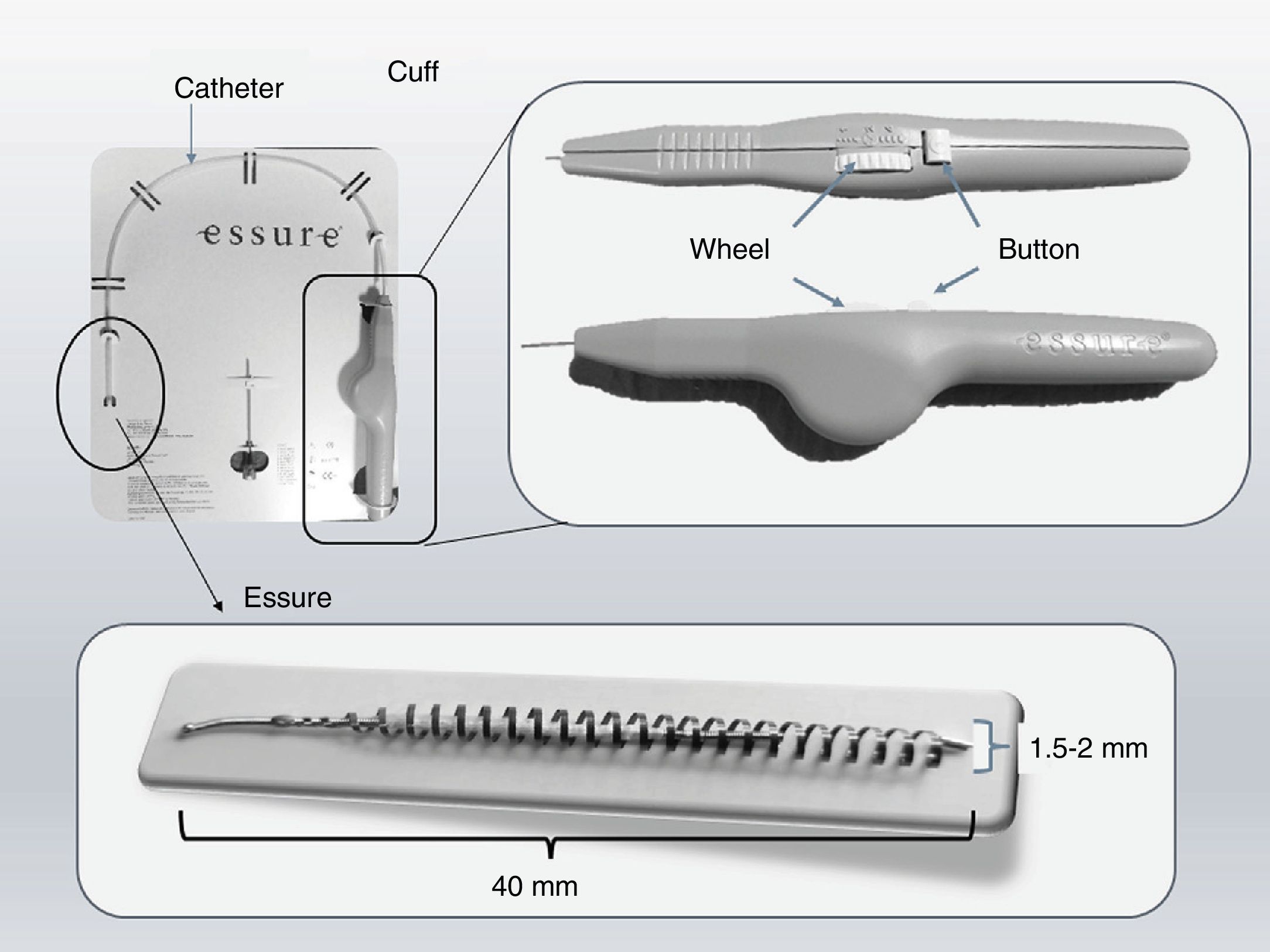
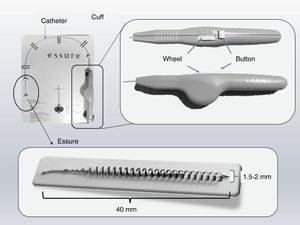
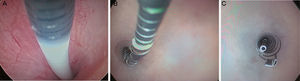
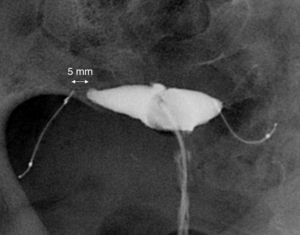
Essure deviceThe Essure device (Fig. 1) is a 40mm-long-0.8mm wide in diameter-flexible 26-coil system expanding up to 1.5–2mm of diameter once the insertion system has been released. Each micro-insert consists of two parts. The exterior self-expandable coil consists of a nickel-titanium alloy (nitinol) that when expanding anchors the device to the Fallopian tube. The interior component is stainless steel and contains and is surrounded by polyethylene terephthalate-dachron (PET) fibers. Each device has four radiopaque markers in the proximal and distal regions of the interior and exterior components.
Essure insertion system. It consists of a catheter in which one microinsert and a cuff are included. The cuff has a wheel with which once the catheter is located in the tube; when the wheel spins the Essure device is displaced and pressing the button releases the device that immediately expands. Essure device shown in expanded position.
Dacron fibers stimulate tissue growth and scarring. The mechanism of occlusion not only is the Fallopian tube lume occlusion but also the tissue barrier established approximately three months after the implantation due to the stimulation of tissue scarring through the device.6,9 This is why it is important to remind patients the need to maintain other kind of anti-contraceptive method during the first three months.
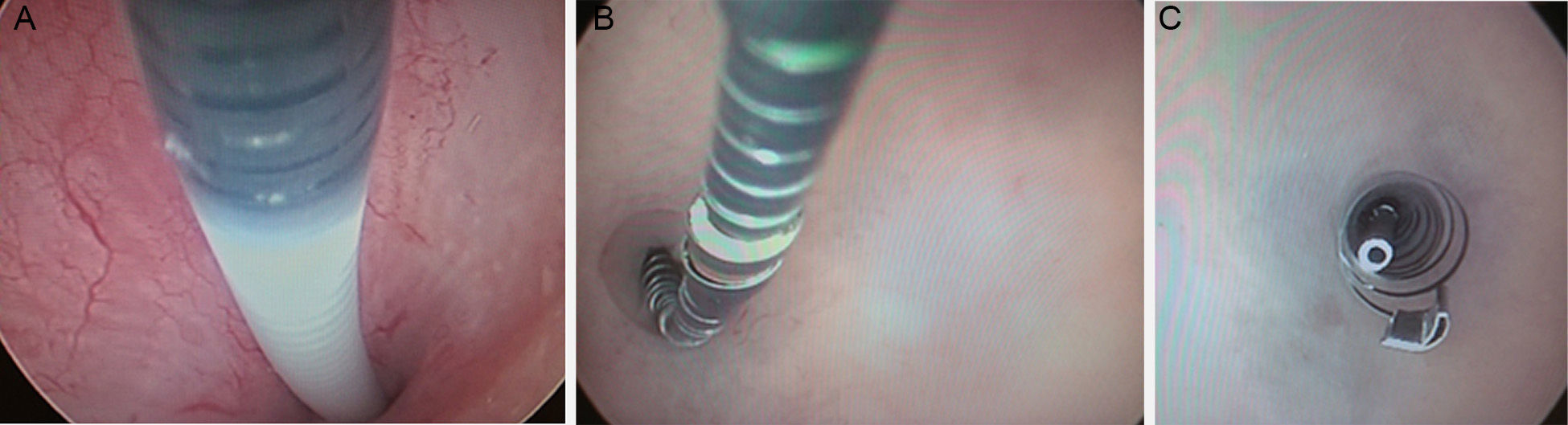
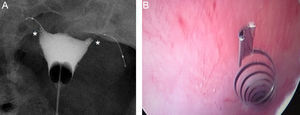
Essure insertionThe Essure device is inserted through the cervix by hysteroscopy. No previous preparation, local or general anesthesia are needed. The insertion takes place in the first stage of the cycle or under hormone anti-contraceptive therapy. After the cannulation of the Fallopian tube orifice with direct vision one device is released in its interior leaving preferably 3–5 coils in the uterine cavity (Fig. 2). This is how the proximal regions of the Essure device internal and external components remain in the uterine horn.
Essure insertion. (A) The catheter is introduced in the tube. (B) The device starts releasing the catheter while moving the wheel. (C) Once the Essure has been released and self-expanded a few coils from the exterior component and the end of the interior component in the uterine cavity can be seen.
The radiologic image modalities are performed to determine the position and function (tubal occlusion) of devices. In most European countries the follow-up takes place through a simple X-ray of ultrasound while the hysterosalpingography is used only in cases of hard insertion or doubtful images with other two modalities. In the United States the guidelines published by the Food and Drug Administration require performing hysterosalpingographies as part of the protocol before suppressing the anti-contraceptive.10
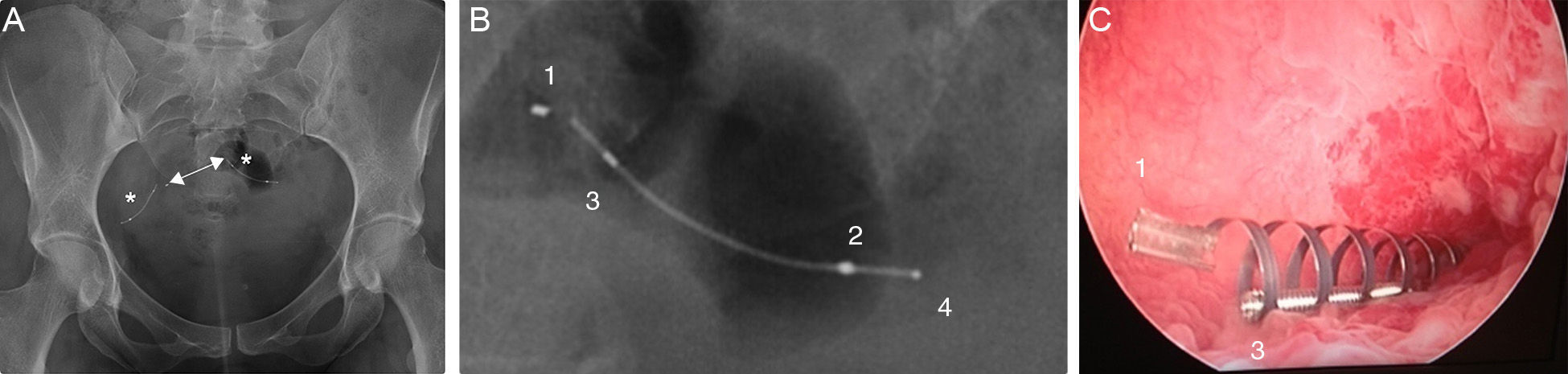
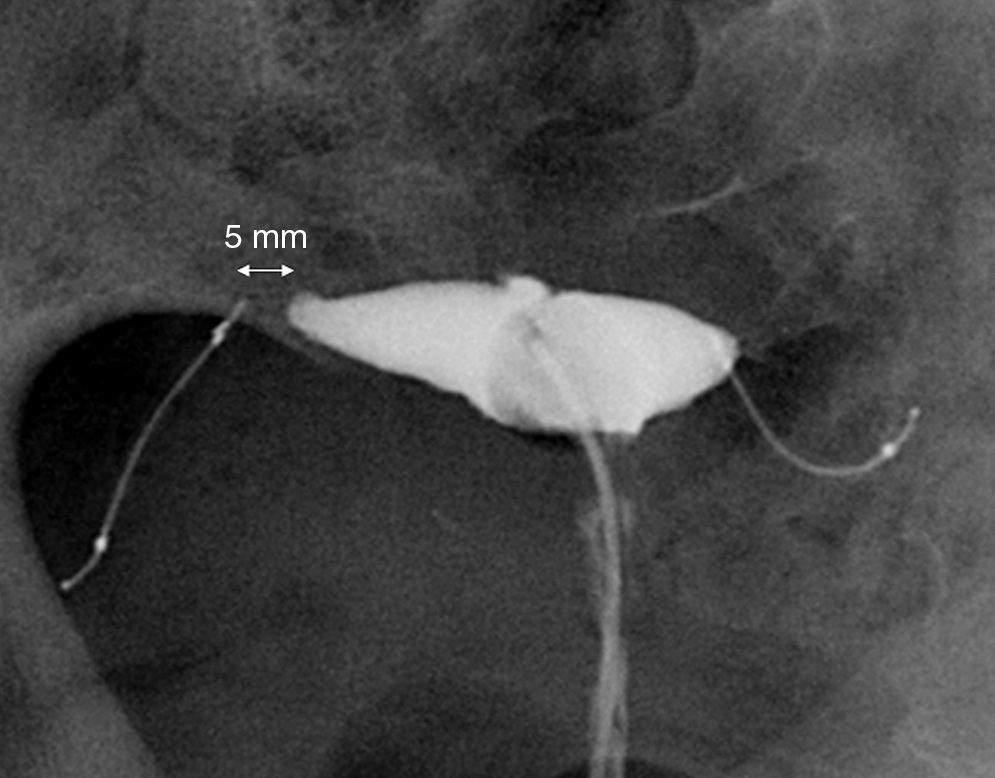
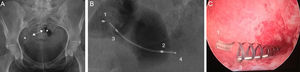
Simple radiographyThe simple radiography allows us to determine the position of the device only–there is no other data to assess tubal occlusion. Devices are shown in the radiography as two metallic linear structures located in the pelvic region usually in a symmetric disposition (Fig. 3). The four radiopaque markers located in the proximal and distal ends of the interior and exterior components of the microinsert allow us to spot their location through a simple radiography. The distance between the medial ends is usually under 2.5cm and the value considered as a normal limit is 4cm yet it can be higher or smaller depending on the variability of the location of the tubes with respect to the uterine cavity. The simple radiography is a not very reliable modality to determine the correct location of the Essure device.11
Anterior–posterior X-ray of pelvis. (A) Two Essure can be identified here (*) whose medial ends are 2cm away from each other (arrow). (B) Radiopaque markers of the Essure device. Proximal (1) and distal (2) interior components and proximal (3) and proximal and distal (4) exterior components. (C) Through the hysteroscopy at the uterine horn at the level of the tubal orifice the proximal region of both device components can be seen.
Hysterosalpingography allows us to assess both the position in the simple radiography prior to contrast and its correct functioning if tubal occlusion is confirmed. Hence the best time to do this study is three months after insertion when the tube occluding-fibrosis has already occurred. The setbacks of this modality are the use of a contrast agent and the dose of radiation as well as the use of this proceeding including the inconvenience of introducing contrast through the uterine cervix.
In patients carrying the Essure the hysterosalpingography should be performed using the “low flow-low pressure” technique since the tubal occlusion conditions the increase of intrauterine pressure. Also the injection of contrast needs to be slow to avoid tubal spasm. However it is also important that the radiologist makes sure that he has refilled the uterine cavity completely. This piece of information is confirmed by making sure that the uterine cavity has been refilled.
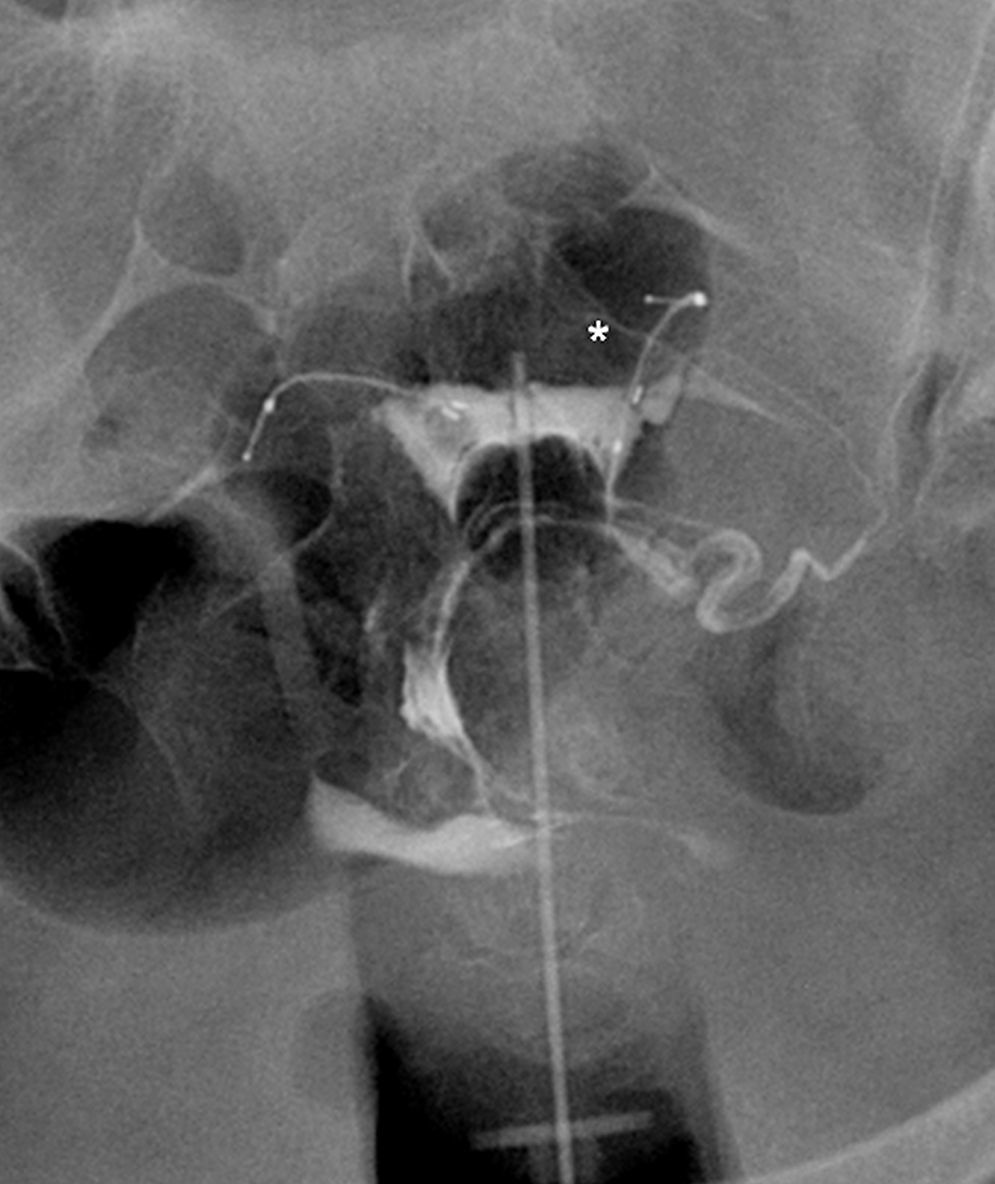
Even though the common technique in hysterosalpingography studies usually includes four projections,12 in patients carrying the Essure device six projections are recommended6: simple radiography before the introduction of contrast to confirm the presence and position of devices; anterior–posterior radiography with scarce uterine filling for the assessment of lesions in the cavity; anterior–posterior projection with complete uterine filling confirming we have reached the adequate uterine cavity distension; right and left oblique projections through which tubes will be seen if they are patent; anterior–posterior radiography after the contrast infusion system removal to see if there is contrast in the peritoneum. In these X-rays it is importante (Fig. 4) to pay attention to microinserts like radiopaque linear images, to its position with respect to the utero-tubal junction, that is, to the uterine neck filled up with contrast and finally to the existing contrast inside the tube and/or contrast passage to the peritoneum which is suggestive that the tube is patent. In some cases three months is not enough for the occlusion. In these patients we need to repeat the hysterosalpingography after another three months.
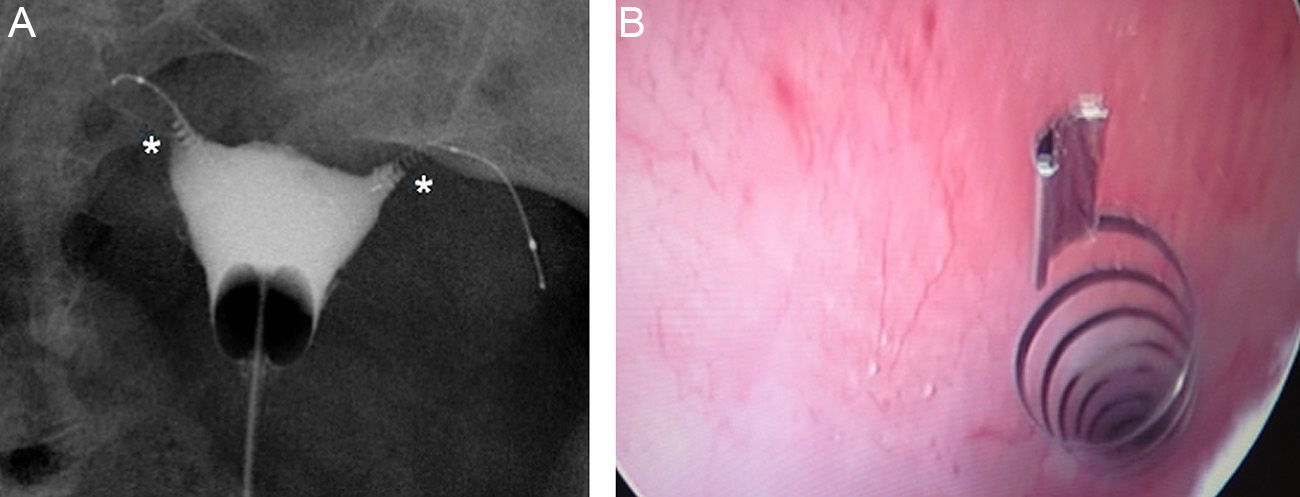
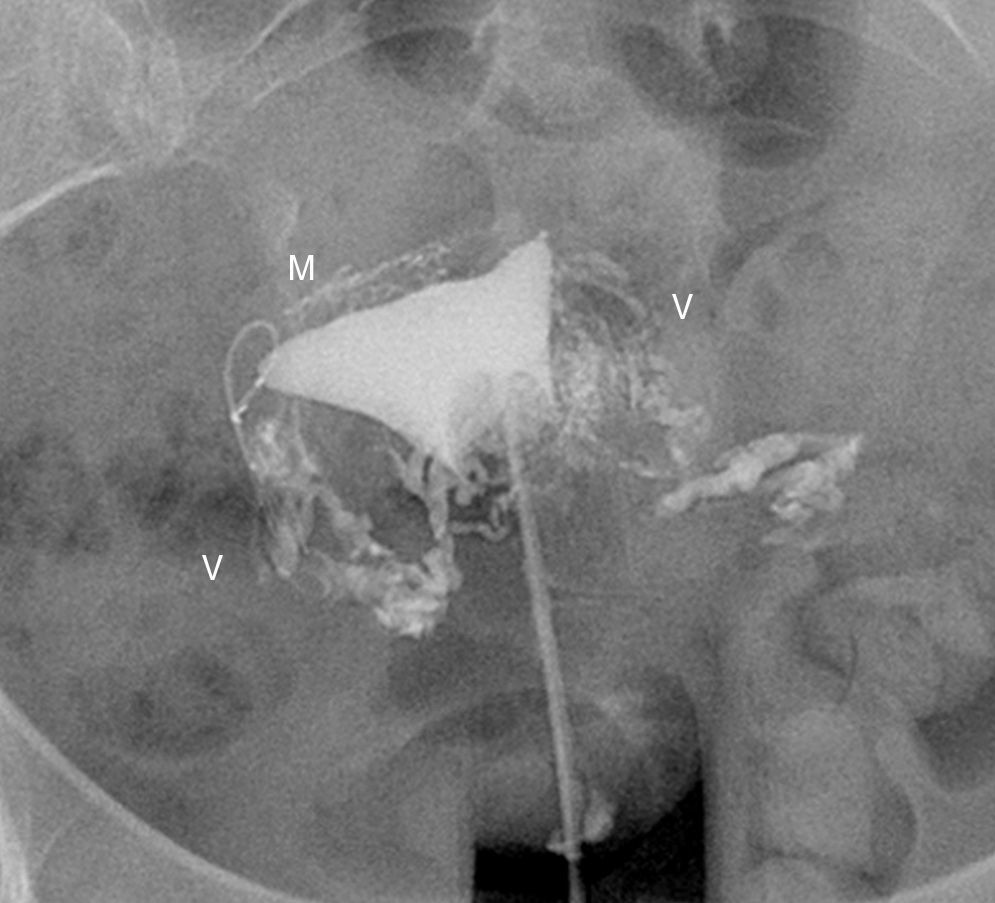
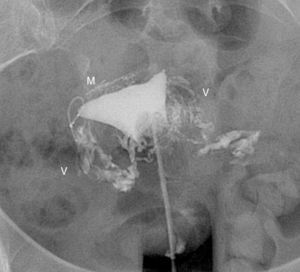
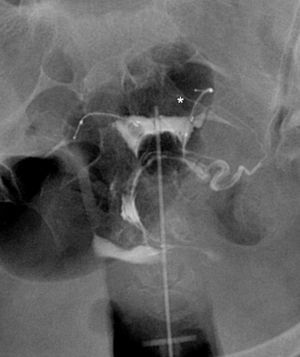
The commonest cause of error when interpreting the hysterosalpingography is venous or lymphatic extravasation (Fig. 5).13 This finding usually uncommon in hysterosalpingography studies is significantly more common in Essure carrier-patients probably due to an increase of intrauterine pressure due to tube occlusion.14 What allows differential diagnosis with tube patency is not see the tubes at the same time we are seeing the myometrial vessels forming a network around the uterine cavity and the pelvic veins that are parauterine and pelvic tubular structure with a fast contrast clearance. On the contrary, the contrast in the peritoneal cavity present when the tubes are patent lasts longer.
Hysterosalpingography. Left salpingectomy and right Essure in incorrect position (tube perforation) but without contrast passage to the tube. The contrast fills up the uterine cavity. Lineal fundus images indicating the passing of contrast to vessels of the myometrium (M) and parauterine serpiginose images, pelvic veins (V).
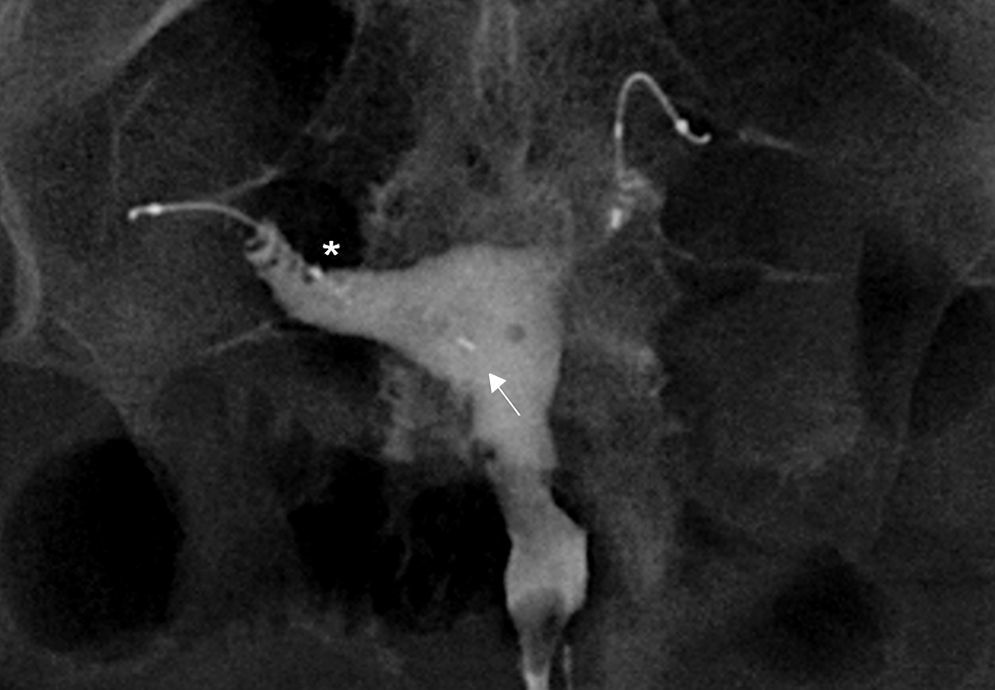
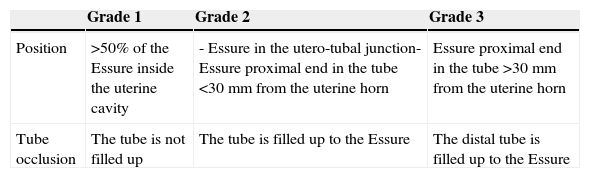
For the assessment of the Essure device we need to assess whether the position and tube occlusion are satisfactory (Table 1). Even though some authors categorize the result in different degrees for every parameter it can lead to confusion and this is why the categorization is not used systematically. The position is satisfactory (grade 2) (Fig. 6) when the microinsert crosses the utero-tubal junction <50% of the length of the interior component in the uterine cavity or an Essure device is located in the tube with the proximal end of the interior component inside the first 30mm of tube measured from the uterine horn. The position is considered unsatisfactory if the microinsert is very proximal with >50% of the length of the interior component in the uterine cavity (grade 1), and when the Essure device is distally located in the inside of the tube with the proximal end of the interior component >30mm from the uterine horn (grade 3). Even though the position is “not satisfactory” the Essure device can do its job causing tube occlusion.
Position and occlusion criteria of the Essure device.
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Position | >50% of the Essure inside the uterine cavity | - Essure in the utero-tubal junction- Essure proximal end in the tube <30mm from the uterine horn | Essure proximal end in the tube >30mm from the uterine horn |
| Tube occlusion | The tube is not filled up | The tube is filled up to the Essure | The distal tube is filled up to the Essure |
Satisfactory result: gray cells.
Some authors distinguish three different degrees of occlusion.15 They talk about satisfactory occlusion if there is filling in the tube (grade 1), or if the tube is filled up but not beyond the Essure device (grade 2). The occlusion is not satisfactory (grade 3) if the tube is filled up distally to the Essure device or the contrast passes to the peritoneum indicative of Essure malfunction.
In sum the important thing here is to highlight in this article the existence of this device, its position with respect to the utero-tubal junction and most important of all whether the tube is patent.
UltrasoundIt allows us to determine the position of the Essure device but not tube occlusion. It can be performed at any time from the device insertion. Some authors claim that the 3D ultrasound performed 3 months after insertion is useful to establish the correct or incorrect position of the device saving the hysterosalpingography for cases of inconclusive ultrasound.16 In Europe it is generally accepted that the correct position shown through transvaginal ultrasound instead of hysterosalpingography is comparable in terms of safety since there are no differences in the rate of pregnancies published.5
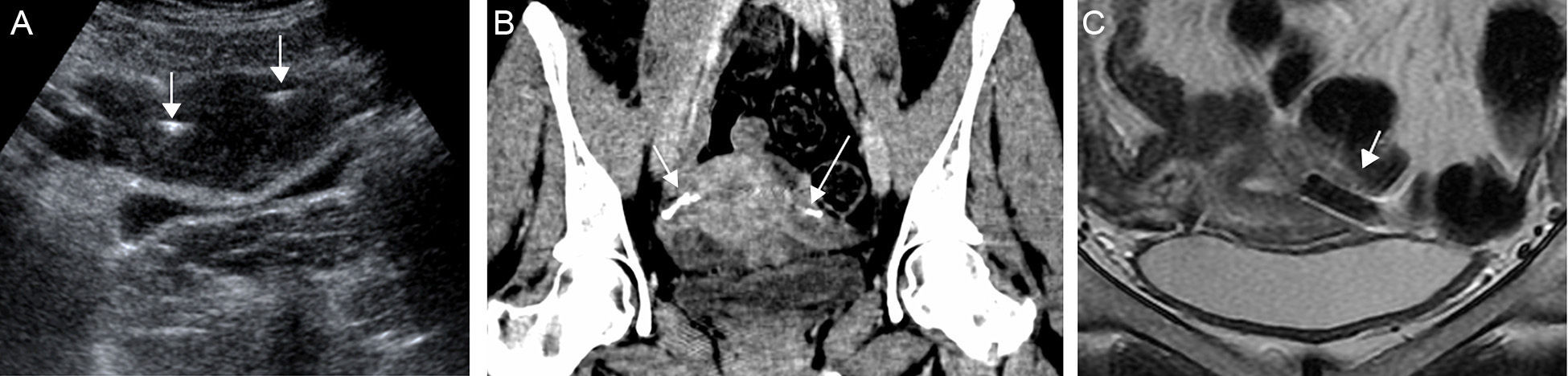
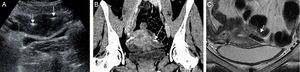
The study is usually done in the luteal stage since the endometrium is visible and the utero-tubal junction can be easily identified.17 The devices can be seen as hyperecogenic lines at both ends of the uterine cavity (Fig. 7A). If they are not identified in the utero-tubal junction it is assumed that the position is incorrect due to distal migration in the tube or the uterine cavity; however since the tube is not usually seen the location of the Essure device cannot be determined with absolute certainty through the ultrasound.
(A) Transabdominal uterine ultrasound. Hyperecogenic linear images (arrows) at both ends of the uterine cavity corresponding to two Essure devices in the utero-tubal junction. (B) CT with coronal reconstruction. The Essure (arrows) are structures with metal density at both ends of the uterine cavity. (C) Coronal T2-weighted FSE MRI. The signal void (arrow) characterizes the Essure in all sequences.
The computed tomography (CT) or the magnetic resonance (MR) are not used to determine the position of the Essure device and they are not good to determine tube occlusion.18 However since in some occasions the devices show up in CT or MRI studies it is useful to be able to recognize them. In the CT (Fig. 7B) the Essure devices are metallic lineal structures located in the tubes. In the MRIs the image shows signal void in all sequences (Fig. 7C).19
ComplicationsThe complications that can occur during the insertion process are tube or uterine perforation. Allergic reactions to nickel from the device are uncommon. Long term-complications are rare–above all bleeding and pain.7
The main sign of suspicion of a complication is the abnormal position of the Essure in the simple radiography. Clinical manifestations like vaginal pain or bleeding among periods or difficulties during the insertion process are pieces of information than lead us to discard complications.
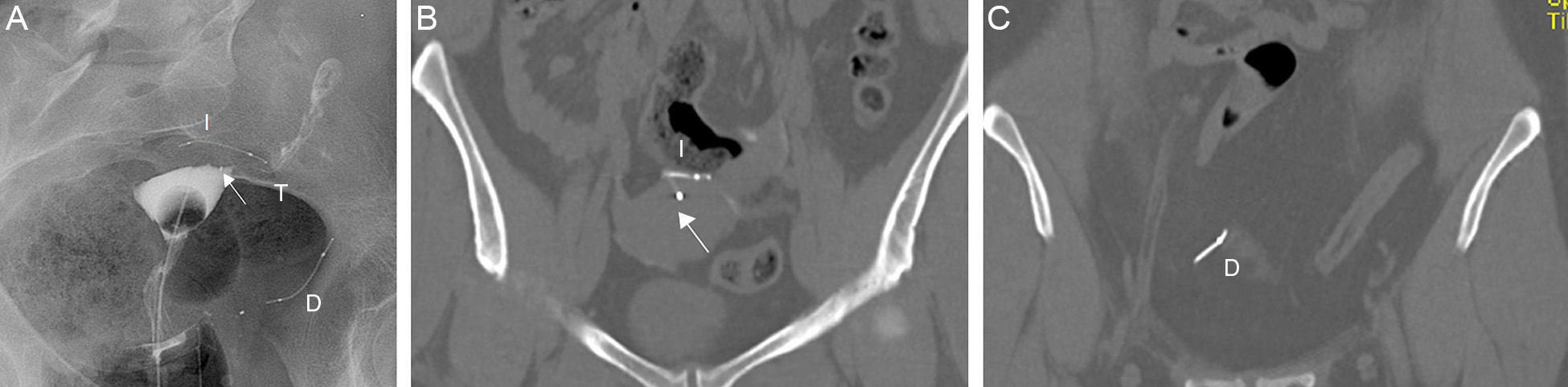
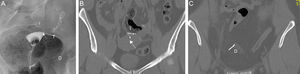
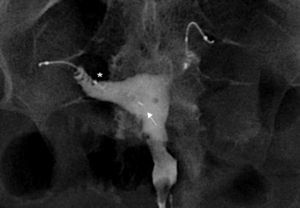
Complications can be associated with the position, structure or function of the Essure device.20 The abnormal position can be due to the migration of the device toward the uterine cavity (0.6–3%), or through the tube distal end toward the peritoneal cavity (0.1%)21 (Fig. 8). Some times the patient is asymptomatic though she might suffer from vaginal pain or bleeding. Also the position is incorrect in cases of tube or uterine horn perforations (1–2%)22 (Fig. 9). In these patients the ultrasound is usually common since the Essure device proximal region is in a correct position but the distal region is located outside the tube or the uterine horn.
Migration to the peritoneal cavity (A) Hysterosalpingography. Two Essure can be identified in anomalous position. The right one–in inferior position has migrated to the peritoneal cavity. (D) The medial marker of the left Essure (arrow) is in the uterine cavity and the left Essure (I) perforates the uterine wall. Patent left tube (T)–the right one is not patent. (B) CT with coronal reconstruction. Position of the left Essure (I) and the medial marker (arrow) in the uterine cavity. (C) CT with coronal reconstruction. Same data for the right Essure (D).
In some occasions structural anomalies can be seen in the Essure devices usually uncoiled coils and in rare occasions broken. In the X-rays the distance between proximal and distal markers of the internal and external component is greater than normal (Fig. 10).
The incorrect functioning of the Essure device occurs when the tube occlusion is not correct and there is risk of pregnancy. Most pregnancies with the Essure device occurred in patients who did not meet the follow-up protocols yet misinterpretation of the hysterosalpingography might have happened too. The hysterosalpingography allows us to detect the tube patency and when patency remains three months after insertion the right thing to do is to maintain the anti-contraception through other means and re-take the test after another three months since occasionally the time needed for the occlusion is greater.
ConclusionEssure is a feminine system of permanent anti-contraception commonly used since it is safe and easy to insert. The modalities used for the follow-up of these patients vary yet it is important to know the advantages of each and every modality as well as the findings in order to know what the correct position of the devices is and its complications.
Ethical responsibilitiesProtection of people and animalsAuthors confirm that no experiments have been performed on human beings or animals.
Data confidentialityAuthors confirm that there are no data from patients in this article.
Right to privacy and informed consentAuthors confirm that there are no data from patients in this article.
Authors- 1.
Manager of the integrity of the study: RMLR.
- 2.
Original Idea of the Study: RMLR, JAA.
- 3.
Study Design: RMLR, JAA.
- 4.
Data Mining: RMLR, JAA, PARM, FJSA, JCAM.
- 5.
Data Analysis and Interpretation: RMLR, JAA, PARM, FJSA, JCAM.
- 6.
Statistical Analysis: N/A.
- 7.
Reference Search: RMLR, PARM.
- 8.
Writing: RMLR.
- 9.
Critical review of the manuscript and intellectually relevant remarks: RMLR, JAA, PARM, FJSA, JCAM.
- 10.
Approval of final version: RMLR, JAA, PARM, FJSA, JCAM.
Authors declare no conflicts of interests.
Please cite this article as: Lorente Ramos RM, Azpeitia Armán J, Aparicio Rodríguez-Miñón P, Salazar Arquero FJ, Albillos Merino JC. Valoración radiológica del anticonceptivo permanente de inserción histeroscópica Essure. Radiología. 2015;57:193–200.