The 2021 World Health Organization classification of CNS tumours was greeted with enthusiasm as well as an initial potential overwhelm. However, with time and experience, our understanding of its key aspects has notably improved. Using our collective expertise gained in neuro-oncology units in hospitals in different countries, we have compiled a practical guide for radiologists that clarifies the classification criteria for diffuse gliomas in adults. Its format is clear and concise to facilitate its incorporation into everyday clinical practice. The document includes a historical overview of the classifications and highlights the most important recent additions. It describes the main types in detail with an emphasis on their appearance on imaging. The authors also address the most debated issues in recent years. It will better prepare radiologists to conduct accurate presurgical diagnoses and collaborate effectively in clinical decision making, thus impacting decisions on treatment, prognosis, and overall patient care.
La Clasificación 2021 de tumores del SNC por la Organización Mundial de la Salud (OMS) fue acogida con entusiasmo, aunque al principio pudo parecer abrumadora. Con el tiempo, hemos comprendido los puntos clave, y basándonos en nuestra experiencia en unidades de neurooncología de hospitales internacionales, hemos elaborado una guía práctica para radiólogos. Esta guía esclarece los criterios de clasificación de los Gliomas Difusos en los adultos y presenta un formato claro para su aplicación diaria. El manuscrito repasa la evolución histórica de las clasificaciones y destaca las novedades más relevantes. Ofrece un análisis detallado de las entidades principales, centrándose en manifestaciones radiológicas. Además, discute los temas más controvertidos de los últimos años. Con este documento, los radiólogos estarán preparados para realizar diagnósticos prequirúrgicos, y sobre todo colaborar eficazmente en la toma de decisiones clínicas, con impacto directo sobre el tratamiento, el pronóstico o la atención personalizada.
Following the launch of the World Health Organization (WHO) 2021 Classification for Central Nervous System (CNS) tumors, there was a sudden rise of related radiological papers.1–3 Initially, the wealth of these publications could seem daunting to many radiologists. But with time and practice, our grasp of the new key aspects has notably improved. By sharing our expertise, gathered in neuro-oncology units at international hospitals, we believe we can now provide a practical guide. This guide, aimed at radiologists (and other related clinicians), highlights the crucial components of the classification’s 5th edition in a clear, comprehensible manner that can be promptly incorporated into daily practice.
The main aim of this paper is to offer a practical review based on the authors' firsthand experience in characterizing adult diffuse gliomas in multidisciplinary units. Ultimately it is done through visual assessments of radiological images,4 but it is essential not to overlook critical clinical concepts that are foundational for optimal imaging-guided knowledge implementation. While elucidating the fundamental concepts and foundations of the classification from a critical-applied perspective, the content is designed to be comprehensible to a wide audience. The more imaging-centric guide can be adapted to standard MRI protocols.5–7 without requiring complex processing or data analysis, thereby ensuring its applicability, practicality and relevance.
Historical backgroundBack in 2007, the classification relied on the histological examination of surgical samples. Diffuse gliomas were classified as astrocytic, oligodendrocytic, or mixed oligo-astrocytic.8 Thus, each tumor classification depended on pathologists' evaluations with a degree of subjectivity.9 Especially, the consensus among pathologists did not achieve ideal precision when handling tumors with any oligodendroglial elements.10
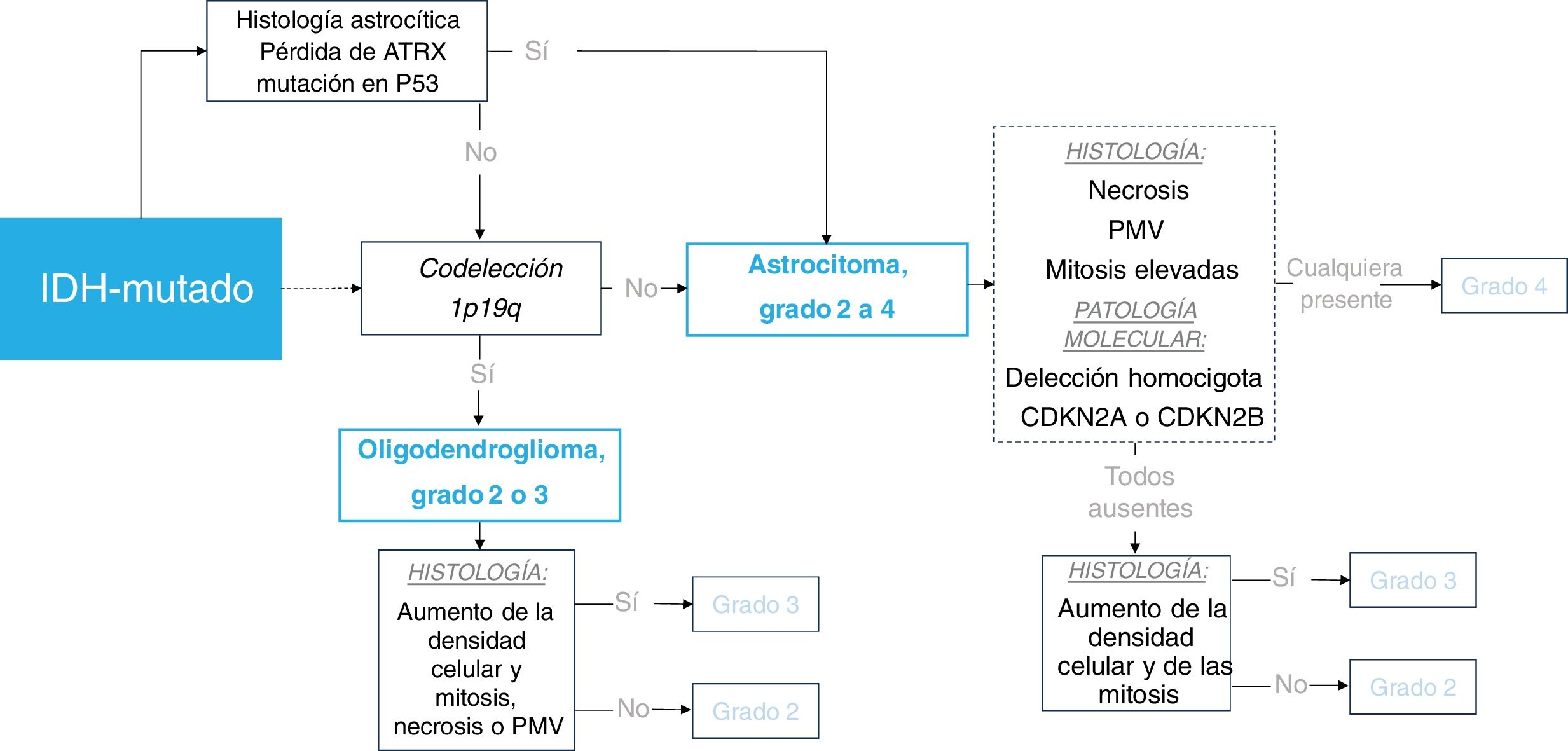
Substantial progress in molecular pathology led to the integration of molecular criteria with histological evaluation in the WHO 2016 classification. A key update involved differentiating oligodendrogliomas from astrocytomas by mandating the presence of IDH mutation and 1p/19q codeletion in oligodendrogliomas, thereby reducing subjectivity in histological classification.10,11 Another significant update involved categorizing astrocytomas based not only on histological grade but also on their IDH mutation status. This change was prompted by the recognition of substantial biological and prognostic distinctions between IDH-mutant and IDH-wildtype tumors, which transcend histological grading.11
After some years of growing influence from molecular pathology,12 the paradigm shift completed in 2021, when the classification focused primarily on genetic entities.
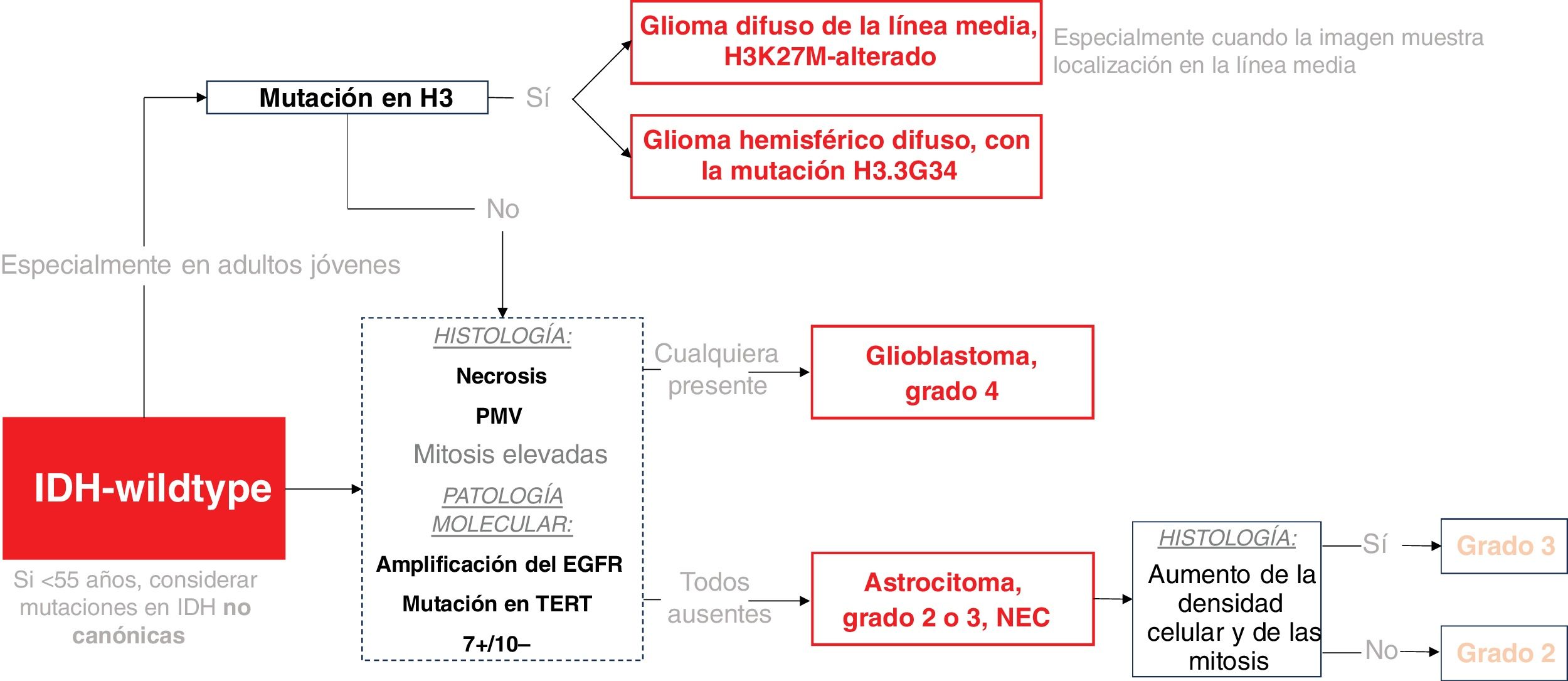
WHO 2021 classification of diffuse gliomas in adults, key summaryThe new classification system now centers around three primary genetically defined categories: IDH-wildtype Glioblastoma, IDH-mutant astrocytoma, and IDH-mutant 1p/19q-codeleted oligodendroglioma. Astrocytomas had grades 2 to 4, while oligodendrogliomas had grades 2 and 3. The term anaplastic was dropped and replaced by grade 3, and grade 4 IDH-mutant astrocytoma was no longer called Glioblastoma (reserved exclusively for IDH-wildtype). The grading system for each tumor still fundamentally relies on histology, with high mitosis and necrosis or microvascular proliferation indicating grade 4. However, new genetic criteria could upgrade a (solely) histological grade 2 or 3 to a WHO grade 4 (molecular grades 4): 1. A homozygous codeletion of CDKN2A or CDKN2B in IDH-mutant astrocytoma carries a grade 4 IDH-mutant astrocytoma, or 2. EGFR-amplification, TERT promoter-mutation, or 7+/10− concurrent gain of chromosome 7 and loss of chromosome 10 in IDH-wildtype carries a glioblastoma (WHO grade 4), regardless of histological traits.13 Therefore, in summary, the major key updates were: 1. Simplification of the classification to three genetically defined categories (IDH-wildtype glioblastoma, IDH-mutant astrocytoma, IDH-mutant 1p/19q-codeleted oligodendroglioma); and 2. Tumor grading incorporation of molecular grades 4 alongside traditional histological assessments.2,13
Moreover, the latest classification notably differentiates between adult- and pediatric-type gliomas, acknowledging that certain pediatric types can occur in young adults. This adds another crucial molecular marker for classifying IDH-wildtype tumors in young adults, especially if midline-located: the histone H3-mutations. H3K27M mutations are frequently observed in diffusely infiltrating gliomas situated in midline structures. H3.3G34R/V mutations are found in a smaller group of high-grade gliomas in cerebral hemispheres, with a more favorable prognosis. Therefore, in young individuals, H3-mutations must be ruled out before confirming a glioblastoma.13–15
Lastly, it is crucial to comprehend two terms: NOS (not otherwise specified) and NEC (not elsewhere classified). NOS indicates that the necessary tests for definitive classification are unavailable. NEC signifies that after performing all necessary tests, the tumor cannot be classified into any of the established WHO categories.13,16 The primary focal entities in the NEC category are grade 2 or 3 IDH-wildtype astrocytic tumors. These tumors do not meet the histological or molecular criteria for grade 4 and thus cannot be classified as glioblastoma.2,17 These somewhat enigmatic entities have attracted significant attention in academic discussions and will be debated here in a subsequent dedicated subsection.
A simplified framework of the classification is shown in Fig. 1 for IDH-wildtype and Fig. 2 for IDH-mutant.
Classification Scheme for IDH-wildtype Gliomas in Adults. H3-mutations are considered pediatric-type gliomas, but they are included here due to their potential relevance in young adults. The dashed box highlights the stage at which molecular pathology can determine a Grade 4 tumor regardless histology. MVP stands for microvascular proliferation.
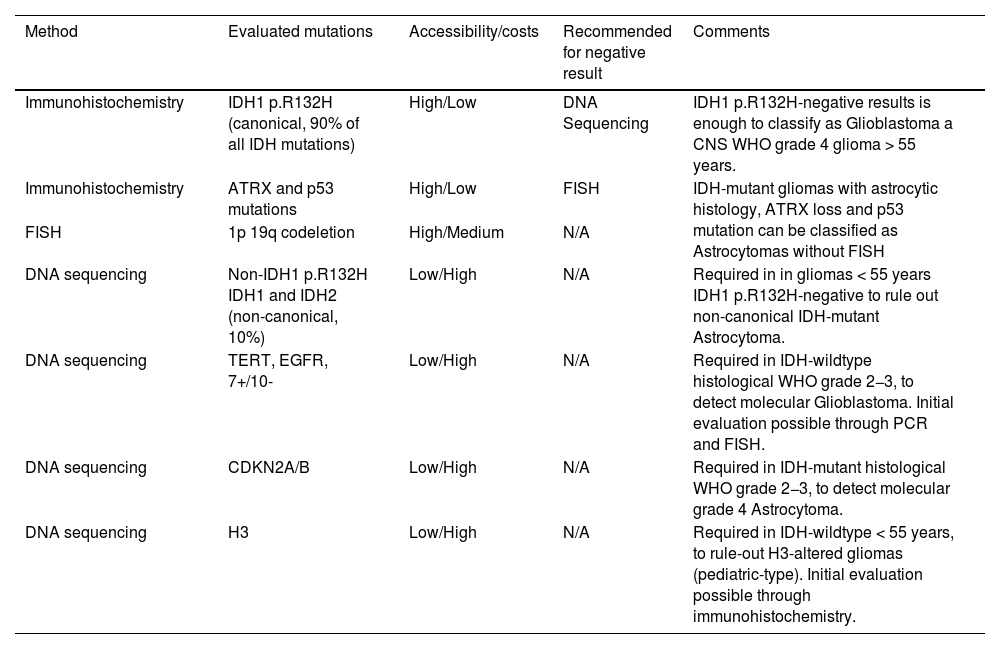
Understanding the available molecular tests, along with their strengths and limitations, is crucial. Immunohistochemistry is the most accessible to evaluate IDH-mutation status. However, it exclusively evaluates IDH1 p.R132H mutations, accounting for more than 90% of all IDH-mutations.
Gene sequencing, less accessible and more costly, extends the detection to other loci of IDH1 and IDH2, known as non-canonical IDH-mutations. Therefore, if the immunohistochemistry result is negative, DNA-sequencing is recommended. However, it is not required for grade 4 gliomas in patients aged ≥55, where a negative immunohistochemistry result is sufficient to classify the tumor as IDH-wildtype13,18,19
Also, regarding oligodendrogliomas and 1p/19q-codeletion; if the histology, IDH-mutation, and p53 and ATRX status are consistent with IDH-mutant astrocytoma, there is no additional need for FISH for 1p/19q-codeletion13,20,21 (Table1).
Molecular pathology tests summary. PCR=polymerase chain reaction.
| Method | Evaluated mutations | Accessibility/costs | Recommended for negative result | Comments |
|---|---|---|---|---|
| Immunohistochemistry | IDH1 p.R132H (canonical, 90% of all IDH mutations) | High/Low | DNA Sequencing | IDH1 p.R132H-negative results is enough to classify as Glioblastoma a CNS WHO grade 4 glioma > 55 years. |
| Immunohistochemistry | ATRX and p53 mutations | High/Low | FISH | IDH-mutant gliomas with astrocytic histology, ATRX loss and p53 mutation can be classified as Astrocytomas without FISH |
| FISH | 1p 19q codeletion | High/Medium | N/A | |
| DNA sequencing | Non-IDH1 p.R132H IDH1 and IDH2 (non-canonical, 10%) | Low/High | N/A | Required in in gliomas < 55 years IDH1 p.R132H-negative to rule out non-canonical IDH-mutant Astrocytoma. |
| DNA sequencing | TERT, EGFR, 7+/10- | Low/High | N/A | Required in IDH-wildtype histological WHO grade 2−3, to detect molecular Glioblastoma. Initial evaluation possible through PCR and FISH. |
| DNA sequencing | CDKN2A/B | Low/High | N/A | Required in IDH-mutant histological WHO grade 2−3, to detect molecular grade 4 Astrocytoma. |
| DNA sequencing | H3 | Low/High | N/A | Required in IDH-wildtype < 55 years, to rule-out H3-altered gliomas (pediatric-type). Initial evaluation possible through immunohistochemistry. |
Understanding this, empowers radiologists to contribute effectively in reaching the most accurate diagnosis and recommending specific tests in unique scenarios. As an example, we might insist on non-canonical IDH-testing when a tumor presents with a T2-FLAIR mismatch, highly specific for IDH-mutants astrocytoma, if immunohistochemistry results are negative.
WHO 2021 diffuse gliomas imaging differential diagnosis in adultsRadiology in the era of genetic classification, current trends and critical perspectiveGiven the recent advances in the genetic characterization of gliomas, there is an increasing interest in radiogenomics: categorizing genetically defined entities based on imaging phenotypes.22 Typically, it is quantitative and deals with big-data, and is widely represented in scientific literature. However, visual/qualitative radiogenomics is also feasible and useful; and, in fact, visual assessment continues to dominate in clinical practice.23–28
When reviewing radiological literature on the 2021 classification, a critical perspective is essential. Many studies claim to distinguish between different genetic entities, but these claims often require careful interpretation because there is a strong correlation between genetic markers and histological grades. For instance, IDH wildtype tumors are mostly glioblastomas with histological grade 4, while IDH mutant astrocytomas are usually grade 2−3. Therefore, some studies' claims of differentiation of IDH-mutation status might actually be a more familiar distinction between grades 2−3 and 4. For example, is the presence of necrosis a marker of IDH-wildtype or is it just a grade 4 marker as we already knew it? All this invites debate. For example, in younger patients, where IDH-mutant and IDH-wildtype coexists more evenly than in the elderly,13,19,29 necrosis may be an unreliable marker of the IDH-status beyond the histological grade 4. Or conversely, an IDH-wildtype grade 2−3 astrocytoma NEC should not present necrosis,13 another situation in which the absence of this radiological marker would fail in determining IDH-status. Therefore, there is a delicate interplay between clinical-epidemiological data, histological grade, and genetic profile which the radiologist should skillfully manage when suggesting a specific diagnosis in clinical reports or neuro-oncology boards.
Preliminary key conceptsThe first factor to consider when approaching the appropriate differential diagnosis may be the patient's age. IDH-mutations are much more common in patients under 55 years of age.13,17,19,29 Therefore, in patients over 55, there exists a substantial likelihood of identifying an IDH-wildtype tumor, which is almost always a glioblastoma.
Imaging accuracy in detecting histological grade 4 in astrocytic gliomas, regardless of the IDH-mutation status, is crucial to remember for radiologists. Aligned with the WHO's histological criteria for grade 4, there are two primary methods. The first is by identifying necrosis on post-contrast T1-weighted (T1w) sequences.30,31 The second involves assessing indirect signs of microvascular proliferation using DSC-PWI.32,33 To simplify: unremarkable (without significant increases nor decreases) CBV maps are typically associated with grade 2 astrocytomas. In grade 3, one might observe a range from unremarkable to some foci of slightly elevated CBV. In contrast, clear nodular or diffuse frank elevations in CBV are more indicative of a grade 4.34 Additionally, Diffusion-Weighted-Imaging (DWI) can provide information on cellular density and therefore mitotic activity, important for histological grading.35,36 All these imaging-based grading criteria are more controversial for oligodendrogliomas.24,37
Finally, it is important to mention a well-known and extremely specific radiological sign for IDH-mutant astrocytomas: the T2-FLAIR mismatch. This consists of a high T2w and low FLAIR signal within solid tumor, often accompanied by a peripheral rim of FLAIR hyperintensity.38 This sign serves as a specific marker for IDH-mutation and is absent in 1p19q-codeleted gliomas, facilitating the differentiation between astrocytomas (IDH-mutant, 1p19q- non codeleted) and oligodendrogliomas (IDH-mutant, 1p19q- codeleted). Additionally, it may function as a favorable prognostic indicator for astrocytomas.23
IDH-wildtype, glioblastomaGlioblastoma is the most common malignant primary tumor in adults, particularly affecting individuals over 55 years.13,17,19,29,39 The clinical symptoms are acute to subacute.40 It is an astrocytic glioma, IDH-wildtype and H3-wildtype, with: microvascular proliferation or necrosis; or altered TERT, EGFR, or 7+/10−.13,15 It arises in the subcortical white matter and infiltrates cortex and deep grey matter. Extension through the corpus callosum is a well-known growth pattern.41 Survival prognosis is poor, around 15–18 months after therapy.13 MGMTpromoter-methylation, is an independent predictor of longer survival beyond age and performance status.42,43
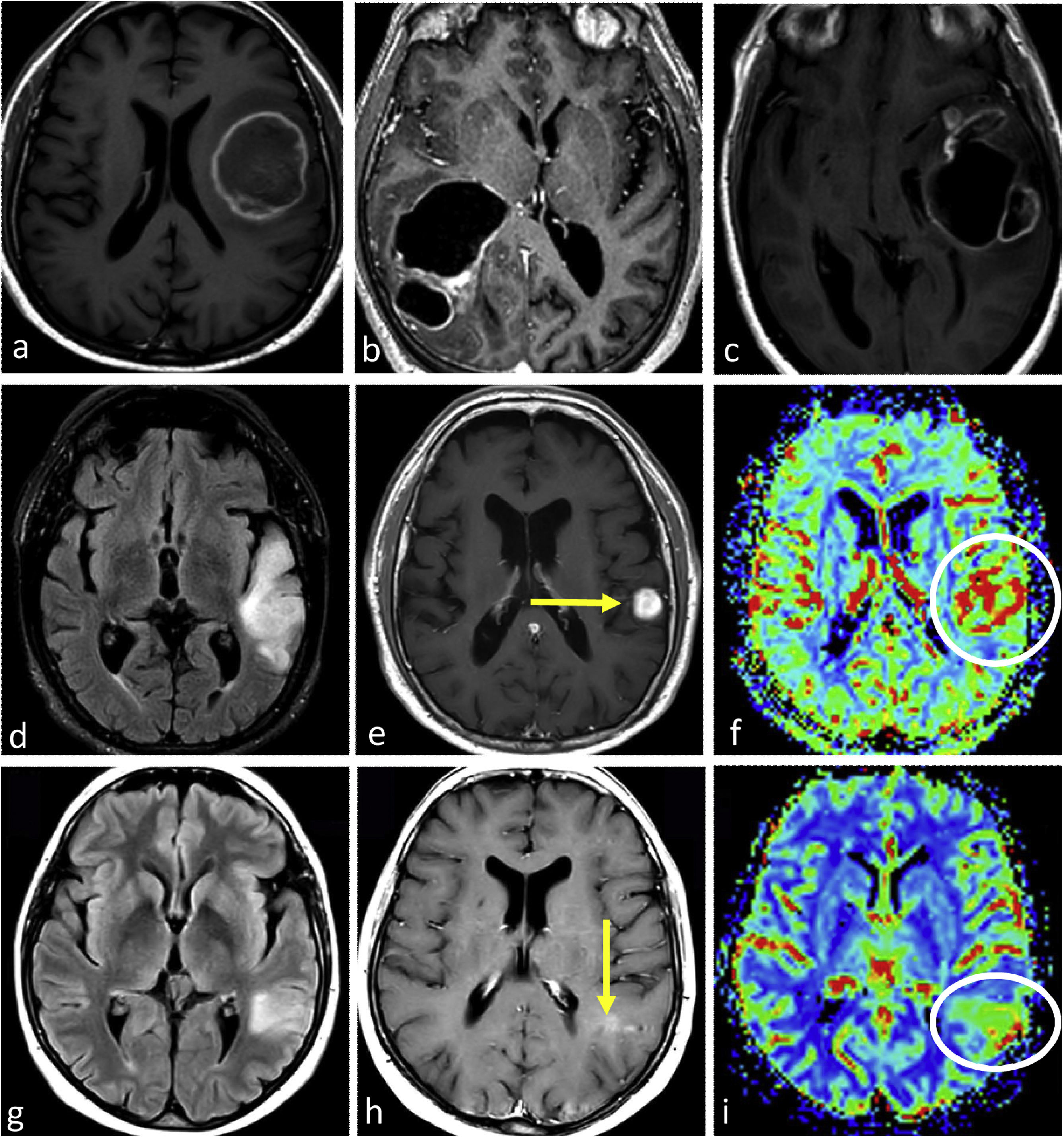
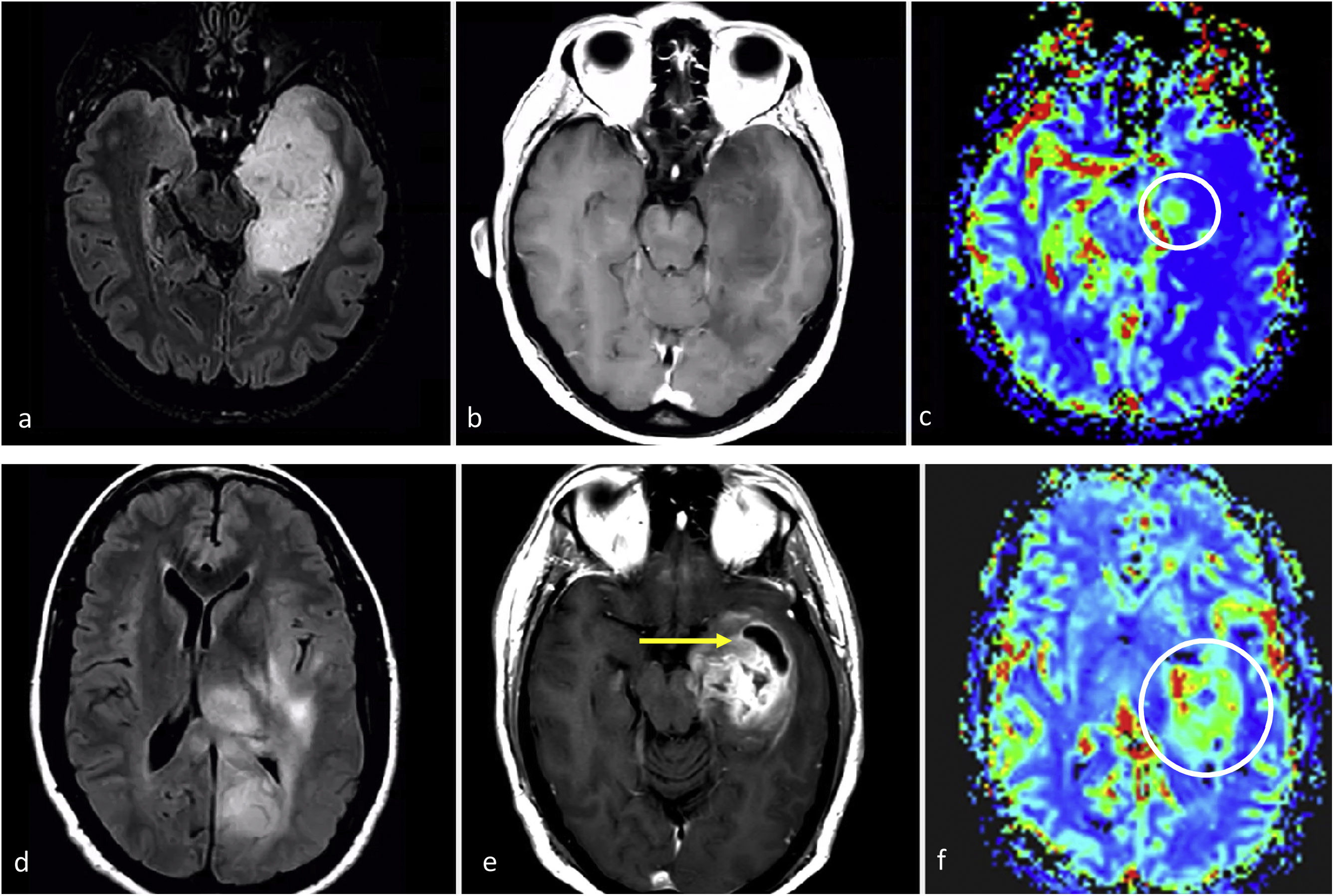
ImagingGlioblastoma characteristic imaging consists of an expansive-infiltrative lesion with prominent irregular enhancement, extensive central necrosis and ill-defined margins. Different degrees of hemorrhage are frequently observed. Moderate to large areas of edema and mass effect are usual.1–3,23 Signs of pial and ependymal invasion are frequent.44 Non-enhancing T2w and FLAIR tumor components may be radiologically visible, although usually not the dominant part of the tumor.45,46 Areas of clearly elevated CBV and restricted diffusion, within enhancing tumor, are very frequent.47,48 Furthermore, the detection of these hyperperfused/diffusion restricted areas beyond the enhancing tumor is helpful in differentiating glioblastoma from solitary metastases49–51 (Fig. 3).
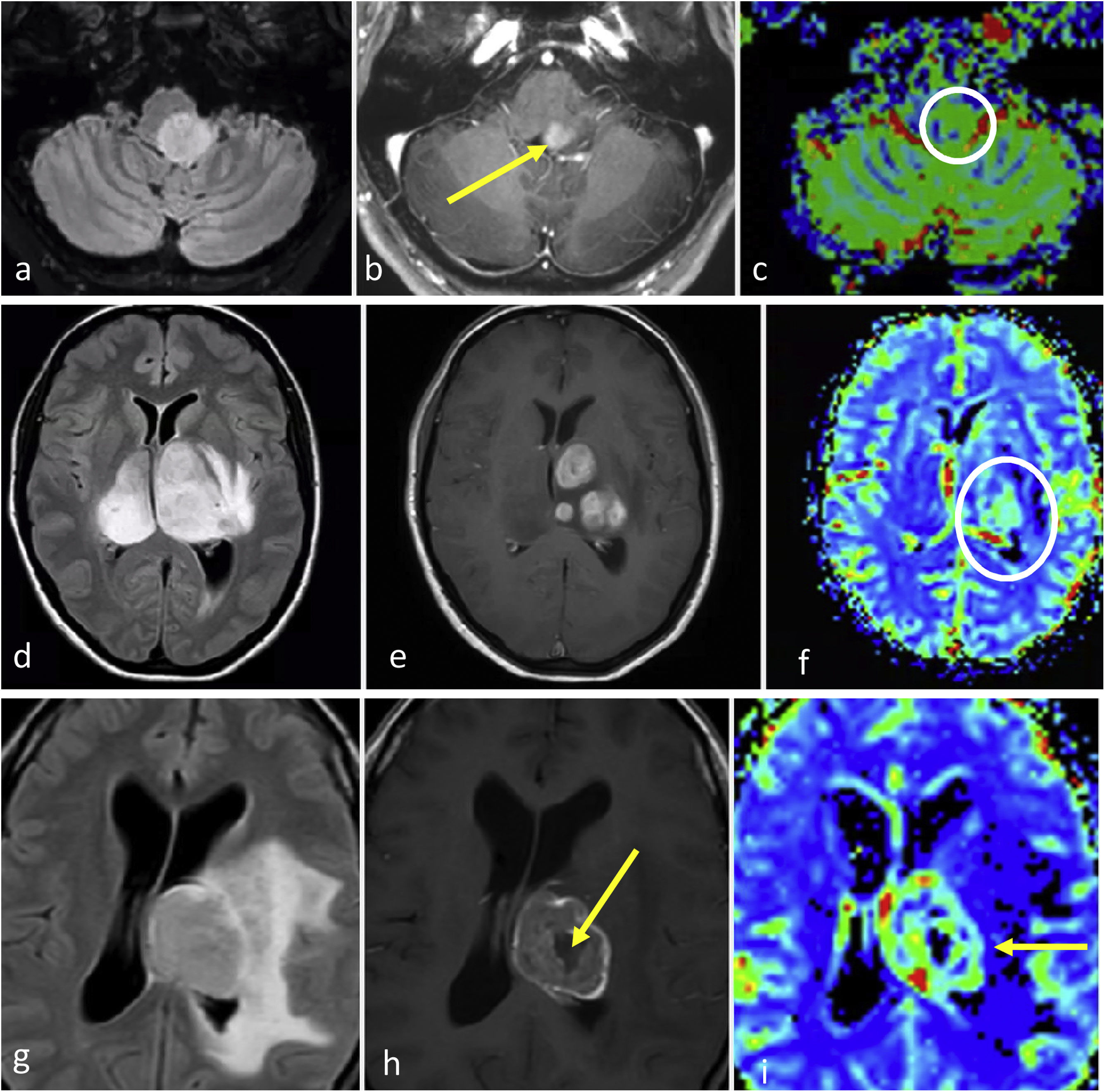
Three different typical presentations for IDH-wildtype glioblastoma in patients between 60- and 70 years old. In a–c, the most paradigmatic imaging features are seen in axial T1w-post contrast images of three different patients, with ring-enhancing irregular margins and extensive necrosis. In d–f: axial FLAIR, T1w-postcontrast, and CBV color maps from DSC-PWI. Extensive FLAIR left temporal infiltrative lesion (d) with associated solid nodular enhancement without necrosis (arrow in e) but with clearly elevated CBV (circle in f). In g–i: axial FLAIR, T1w-postcontrast, and CBV color maps from DSC-PWI. Focal FLAIR left temporal infiltrative lesion (g) with minimal enhancement and absent necrosis (arrow in h) but clearly elevated CBV (circle in i).
In certain instances, glioblastoma can manifest without clear necrosis and predominantly display an infiltrative pattern. These tumors are characterized by large and poorly defined non-enhancing components, with absent or different degrees of enhancement.13 In these non-enhancing and non-necrotic tumors the presence of clear restricted diffusion or increased CBV are particularly relevant, as they strongly support the diagnosis of glioblastoma23,52 (Fig. 3).
In summary, when faced with an expansive-infiltrative lesion in an adult over 55, the neuroradiologist should thoroughly search for signs of histological grade 4 which should strongly suggest a diagnosis of glioblastoma. Specifically, necrosis in T1w post-contrast, hypercellularity on DWI and hypervascularization on DSC-PWI.
As an aside, no sound qualitative imaging markers for MGMTpromoter-methylation status have been elucidated. Nevertheless, according to one study, MGMTpromoter-methylated glioblastoma is more likely to show less edema, higher ADC, and lower CBV than unmethylated.53
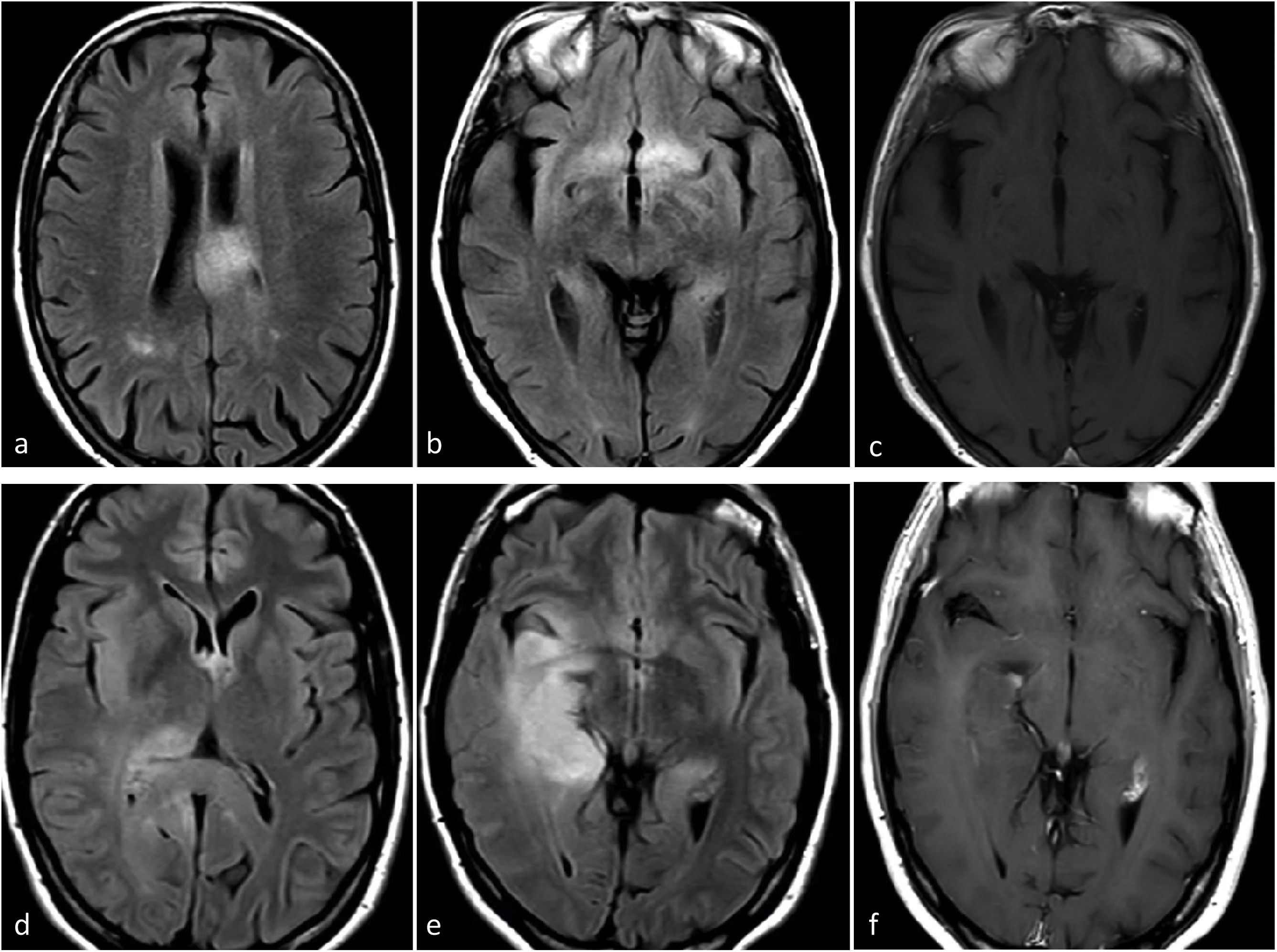
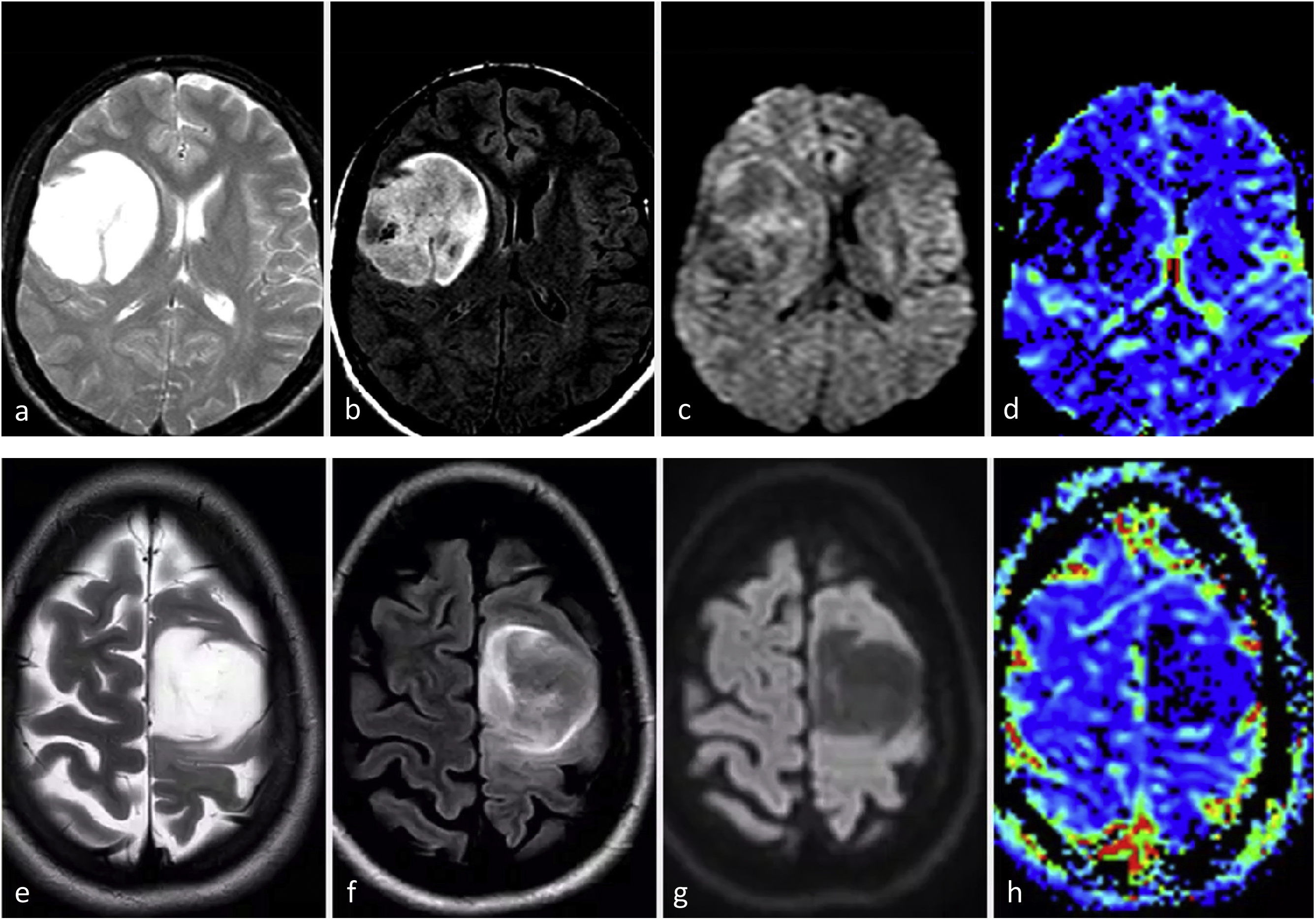
Molecular glioblastomaThe typical radiology for glioblastoma may not work for those that are not histologically but only molecularly defined (TERT, EGFR, 7+/10−), for which imaging remains relatively unknown. They can present as ill-defined infiltrative lesions on T2w and FLAIR sequences, with minimal or no-enhancement, absent necrosis, and unremarkable findings on DWI and DSC-PWI. In fact, they may present as a classic histological grade 2−3 appearance. However, some studies have indicated a tendency towards multifocality and multicentricity, as well as a gyriform pattern of cortical infiltration in tumors with EGFR or TERT mutations.54–56 From a practical perspective, the presence of a gliomatosis cerebri pattern (with some gyriform cortical infiltration) in an adult over 55 may raise suspicion of a molecular glioblastoma, particularly TERT mutated54–56 (Fig. 4). No qualitative imaging description have been found for 7+/10−.
Possible Presentation of TERT or EGFR Altered Molecular IDH-wildtype glioblastoma. Two patients, 80-year-old (a-c) and 55-year-old (d–f). In axial FLAIR sequences (a–b and d–e), diffuse and extensive infiltrative hyperintensities reminiscent of a gliomatosis cerebri pattern in TERT promoter mutated glioblastomas. T1w post-contrast images (c and f) show no enhancement or signs of necrosis.
These tumors, not included as a distinct WHO entity, are the primary focal entities in the NEC category and have been subject of debate. They are diffuse, astrocytic gliomas IDH-wildtype and H3-wildtype, lacking histological and molecular markers of grade 4.
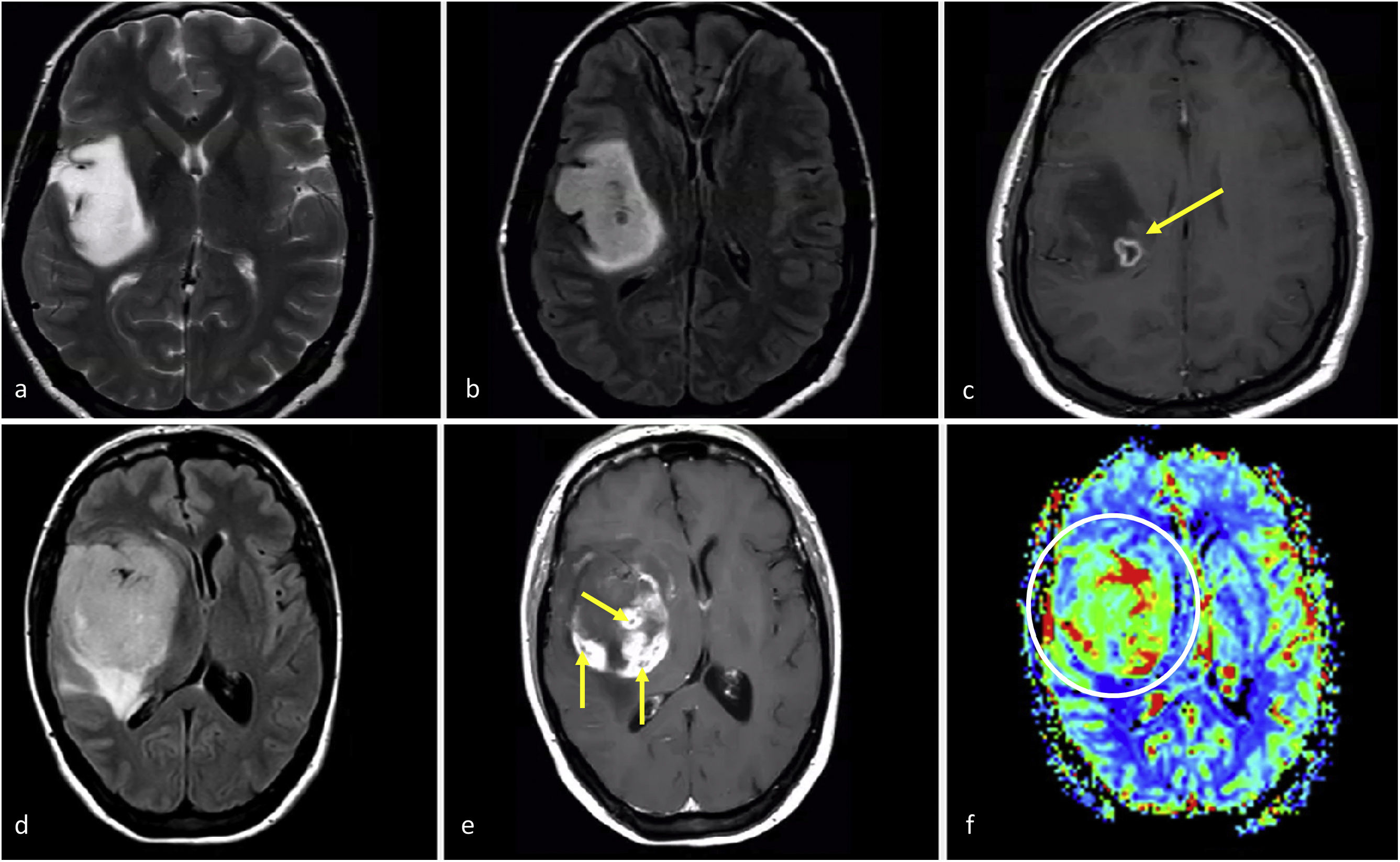
These somewhat enigmatic entities have attracted significant attention in the most recent academic discussions. In theory, such tumors should be exceptional, and any potential biases in their characterization need to be ruled out. For instance, there could be a biopsy bias: gliomas are heterogeneous, and different histological grades may coexist, so a focal sample from a biopsy may not reflect the highest histological grade within a tumor that might otherwise be undergraded. This bias can potentially be addressed with imaging: initially guiding the biopsy; and subsequently detecting necrosis or clear high vascularization possibly missed in the biopsied tissue, both of which would suggest a grade 4.31 Although this is not explicitly specified in the WHO 2021 classification, potential biopsy biases are not exceptional in neuro-oncology units. Therefore, radiologists need to be aware of this possibility and understand their crucial role in its prevention.
On imaging, based on assumptions from publications prior to the 5th edition, these tumors could present as ill-defined infiltrative lesions, with minimal or no-enhancement, absent necrosis, and unremarkable findings on DWI or DSC-PWI. They may present more ill-defined margins, and possible propensity for temporal location, as well as absent T2-FLAIR mismatch compared to IDH-mutant counterparts57 (Fig. 5).
Two exemplifying cases in which biopsy results could render histological grade 2–3 results for an actual grade 4 tumor. A-c, 57-year-old patient. Axial FLAIR (a), T1w post-contrast (b) and DSC-PWI derived CBV color maps (c). Extensive FLAIR hyperintense medial left temporal infiltrative lesion (a), without enhancement or necrosis (b), and a subtle nodular focus of clearly elevated CBV (circle in c). i.e., a biopsy not capturing the high CBV foci would be at risk of undergrading this pathology proven IDH-wildtype astrocytic tumor as grade 2-3. (d–f), 58-year-old patient. Axial FLAIR (d), T1w post-contrast (e) and DSC-PWI derived CBV color maps (f). Extensive FLAIR hyperintense medial left temporal infiltrative lesion in (d), with a more focal area of prominent enhancement and necrosis (arrow in e) as well as high CBV (circle in f). i.e., a biopsy not capturing the necrosis and/or high CBV component would be at risk of undergrading this pathology proven IDH-wildtype astrocytic tumor as grade 2–3. Both patients were treated as IDH-wildtype glioblastomas under a tumor board's consensus decision.
From a radiological management perspective, the authors recommend to keep in mind the radiologists’ role in: 1. guiding biopsies to target the higher-grade regions of tumors, and 2. carefully analyzing the images to identify markers of histological grade 4 which may not have been captured in the biopsy. These practices should minimize potential biopsy biases and resultant undergrading.31
IDH-mutant, astrocytomaAstrocytoma IDH-mutant is 1p/19q-non codeleted and frequently associated with ATRX loss and TP53 mutation.58 Overall they mostly occur in patients under 55 years13,17,19,29: grade 2−3 between 30–40 years, and grade 4 in slightly older patients.59 They have a predilection for the frontal lobes57,60 and symptoms are rarely abrupt unless they present with seizures.60
Focal oligodendroglioma-like components are possible and were a source of misclassifications prior to the establishment of molecular criteria.9,10 Recent years have seen debate regarding the prognostic value of tumor grading among grade 2−3 IDH-mutant astrocytomas, with several scientific publications discussing these grades collectively.18,59,61,62 According to the last WHO,13 median overall survival is >5 and >10 years respectively. Conversely, grade 4 IDH-mutant astrocytomas show shorter survival rates (about 3 years13); yet longer than IDH-wildtype, despite sharing histological characteristics.63 Molecular grade 4 are determined by homozygous deletion of CDKN2A or CDKN2B, even in the absence of necrosis and microvascular proliferation.13,17 Finally, contrary to classical belief, recent work indicates that most grade 4 IDH-mutant astrocytomas occur de novo, rather than with a history of a lower-grade glioma.13,64
ImagingGrade 2−3 IDH-mutant, astrocytomaThese two different grades are usually treated together due to similar behaviours.18,59,61,62 The most frequent imaging presentation is an infiltrative lesion, non- or scarcely-enhancing, with well-defined margins and nodular/oval morphology. Despite relatively low sensitivity, T2–FLAIR mismatch sign shows an almost perfect specificity in adults (when it represents at least >25–50% of tumor extent). The mass effect is relatively limited as well as the perilesional edema. They should lack imaging signs of necrosis.4,23–27,38 DWI may vary between facilitated and homogeneous in grade 2 to slightly heterogeneous in grade 3. No frank CBV elevations should be seen with DSC-PWI34–36 (Fig. 6). Lack of calcification and cysts as well as the nodular/oval morphology instead of a gyriform pattern following the cortex may help in the main differential with oligodendrogliomas.4,23–27
Two different patients with typical imaging features of IDH-mutant grade 2–3 astrocytoma. 31 (a–d) and 34 (e–h) years-old patients. Axial T2w (a and e), FLAIR (b and f), DWI (c and g), and DSC-PWI derived CBV color maps (d and h). Rounded, well-defined T2w hyperintense masses with corresponding FLAIR hypointensity, with a thin peripheral rim of hyperintensity: T2-FLAIR mismatch sign. Without significant diffusion restriction in DWI and low CBV in CBV color maps.
In summary, the neuroradiologist should consider this entity when encountering a young adult with a frontal lobe tumor that appears nodular or oval, exhibits well-defined borders, lacks necrosis, shows minimal to no contrast enhancement, and displays unremarkable DWI and DSC-PWI. If a T2-FLAIR mismatch is observed, it indicates a strong diagnostic possibility; and in the event of negative immunohistochemistry results, DNA-sequencing should be considered for additional assessment of IDH-mutations from a radiologist’s perspective.
Grade 4 IDH-mutant, astrocytomaThis subgroup of IDH-mutant astrocytomas has been less extensively studied compared to grades 2−3, remaining a major radiological challenge. Unfortunately, these tumors often get grouped together (and consequently under-represented) with grades 2−3 under the broader category of IDH-mutant astrocytomas. However, this generalized grouping may be counterproductive because: 1. The differences between IDH-mutant grades 2−3 and 4 are vital for patient management, and 2. The application of the same imaging indicators across grades 2−3 and 4 may not yield accurate results for grade 4, which share imaging characteristics with glioblastomas (also grade 4). Indeed, the imaging of grade 4 IDH-mutant astrocytomas is hypothesized to fall somewhere between that of grade 2−3 IDH-mutant astrocytomas (with which they share IDH-mutation status) and IDH-wildtype glioblastomas (with which they share grade). Therefore, based on histological grade this tumor should present necrosis, restricted diffusion and elevated CBV often; while based on its IDH-mutation status should appear in patients below 55 years, being well-defined and nodular/oval in morphology. Furthermore, the presence of a T2-FLAIR mismatch has been recently described specific also for grade 4 IDH-mutant astrocytoma. Added to prior knowledge, this suggests that if a tumor with a T2-FLAIR mismatch exhibits necrosis, prominent enhancement, restricted diffusion, or elevated CBV, it should be highly indicative of an IDH-mutant astrocytoma grade 465,66 (Fig. 7).
Imaging features in two patients with IDH-mutant astrocytoma grade 4. a–c, 37 years-old. Axial T2w, FLAIR, and T1w post-contrast. Extensive well-defined lesion hyperintense on T2w (a) with corresponding FLAIR hypointensity and thin peripheral hyperintense rim in (b), consistent with T2-FLAIR mismatch sign suggesting IDH-mutation. A small focus of enhancement and necrosis is seen within the deep margin of the tumor (c), suggesting grade 4. Also note that in this case, a biopsy not capturing the necrosis could undergrade the tumor as grade 2–3. Detection of grade 4 imaging features within a tumor with T2-FLAIR mismatch could be a specific presentation of IDH-mutant astrocytoma grade 4. d–f, 49 years-old. Axial FLAIR (d), T1w post-contrast (e), and DSC-PWI derived CBV color map (f). Well-defined, rounded tumor mass on FLAIR (d) with internal areas of solid enhancement (e) within non-enhancing tumor. Small foci of necrosis (arrows in e) and clearly elevated CBV (circle in f). Of note, more extensive necrosis existed in other parts of the tumor not captured in this figure. A grade 4 looking glioma in a patient under 55 with some atypical features for glioblastoma such as clear rounded morphology and well-defined margins could suggest an IDH-mutant astrocytoma grade 4.
Regarding molecular grade 4 IDH-mutant astrocytomas (CDKN2A/B homozygous deletion), to the authors’ knowledge there are not described imaging features that allow presurgical detection.2,25
Oligodendroglioma, IDH-mutant 1P/19Q-codeletedThese tumors are uncommon in patients >55 years.13,17,19,29 Approximately two-thirds of patients present with seizures as initial symptom.13,67 Survival data for genetically defined oligodendrogliomas (only since WHO 2016) are lacking, because prior registries are confounded by the inclusion of gliomas without IDH-mutation or 1p/19q-codeletion. However, overall, they are associated with favorable response to therapy and possibly the highest median survival, above 10 years.68,69 There is also a tendency in radiological literature to treat grade 2−3 together.
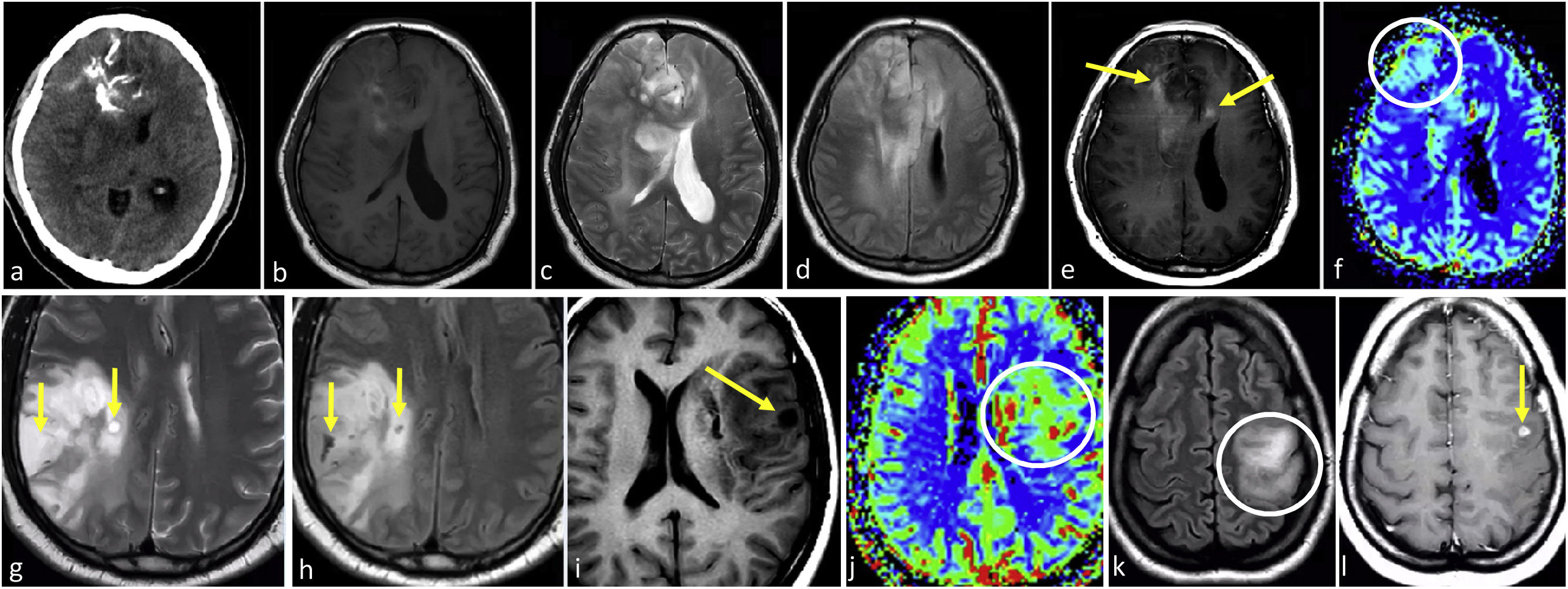
ImagingThese tumors often demonstrate a predilection for the frontal lobes. They typically present as mass lesions centered in the cortex and subcortical white matter. Their cortical epicenter is characteristic and often marked by a typical continuous-cortex sign. This term refers to the involvement of the cortex in more than 50% of the tumor extension. Calcifications (best detected on CT) and cysts are commonly seen and can be distinctive. Hemorrhage is possible. These three features are infrequent for IDH-mutant astrocytomas. Also, they typically exhibit prominent heterogeneity on T1w and T2w images, with indistinct tumor margins. They most often appear non-enhancing, but some do enhance, possibly more common in grade 3. Their morphology often follows a gyriform pattern along the cortex, instead of the nodular/rounded appearance of IDH-mutant astrocytomas.4,23–27 Additionally, oligodendrogliomas exhibit more heterogeneous DWI and DSC-PWI patterns, possibly presenting areas of higher CBV and restricted diffusion,34–36 and imaging-based grading is not reliable24,37 (Fig. 8).
IDH-mutant 1p/19q-codeleted oligodendrogliomas grade 2–3. a–f, 53-year-old patient. Axial non-enhanced CT (a), T1w (b), T2w (c), FLAIR (d), T1w post-contrast (e), and CBV color map (f). Ill-defined diffuse bifrontal mass with extensive cortical infiltration (c and d) and heterogeneous signal on T1w and T2w (b and c). Prominent calcifications on CT (a). Very subtle irregular enhancement (arrows in e) and areas of elevated CBV (circle in f). (g and h) 52-year-old patient. Axial T2w (g) and FLAIR (h). Ill-defined infiltrative parietal mass with heterogeneous signal and extensive cortical infiltration (g–h). Presence of small characteristic intratumoral non-enhancing fluid signal intensity-like areas consistent with cystic foci (arrows in g and h). (I and j), 38-year-old patient. Axial T1w (i) and CBV color map (j). An example of a small intratumoral cyst on T1w (arrow in i) and clearly elevated CBV (j). K-l, 35-year-old patient. Axial FLAIR (k) and T1w post-contrast (l). Infiltrative ill-defined FLAIR hyperintense mass clearly centered on the cortex and following its gyriform morphology: continuous cortex sign (circle in k). Associated small foci of solid enhancement (arrow in l). Also note absent T2-FLAIR mismatch sign in c-d and g-h.
In summary, radiologists should suspect an oligodendroglioma in young adults presenting with a near-frontal mass that exhibits heterogeneity on T1w and T2w calcifications or cysts, ill-defined borders, gyriform morphology and continuous cortex sign. Radiologists should also be cognizant of possible heterogeneity on DWI and DSC-PWI.
Some authors postulate that additional molecular testing should be considered for IDH-mutant gliomas with discordant neuroimaging and FISH results. For instance, if imaging is highly suggestive of oligodendroglioma and FISH is negative for 1p19q co-deletion, other techniques, such as chromosomal microarray analysis can act as a tiebreaker.70
Finally, leptomeningeal spread is occasionally seen in patients with oligodendroglioma, particularly at recurrence.71
Diffuse midline glioma H3K27-altered and diffuse hemispheric glioma, H3 G34-mutantThese pediatric-type gliomas, which lack IDH-mutations, can occasionally present in younger adults. Consequently, in this age group, without IDH-mutations in immunohistochemistry and DNA-sequencing, radiologists must be attentive. They should promptly inform clinical colleagues when a tumor is midline located, because it becomes crucial to rule out a diffuse midline glioma H3K27-altered before classifying the tumor as a glioblastoma. Moreover, in cases of hemispheric tumors in the same patient subset, it is also important to consider H3.3G34R/V-mutant diffuse hemispheric gliomas, as recommended in the latest classification.13,15 In the authors’ experience, H3K27-altered midline diffuse gliomas in adults may manifest as classic brainstem gliomas, occasionally with certain atypical features, that serve as red flags, such as clear enhancement and DWI or DSC-PWI anomalies. Alternatively, these tumors can present in brainstem or other midline structures (thalamus) with clear aggressive features, including avid heterogenous enhancement, restricted diffusion, high CBV, necrosis and hemorrhage (Fig. 9). This latter presentation has been described to be more frequent when they have extended outside the strict midline.72
Diffuse mid-line gliomas H3K27-altered. a–c, 52-year-old patient. Axial FLAIR (a), T1w post-contrast (b), and CBV color map (c). Brain-stem glioma, slightly paramedian, nodular, and well-defined in FLAIR (a), with foci of enhancement (arrow in b) and slightly elevated CBV (circle in c). d–f, 48-year-old patient. Axial FLAIR (d), T1w post-contrast (e), and CBV color map (f). Bithalamic FLAIR hyperintense infiltrative mass (d) with multifocal solid nodular enhancements (e) and high CBV. G-i, 33-year-old patient. Axial FLAIR (g), T1w post-contrast (h), and CBV color map (i). Thalamic FLAIR hyperintense mass with extensive edema (g), irregular thick ring enhancement with central necrosis (arrow in h) and elevated CBV in the enhancing ring (arrow in i).
Imaging descriptions and key-features of all documented tumors can be found in Table2.
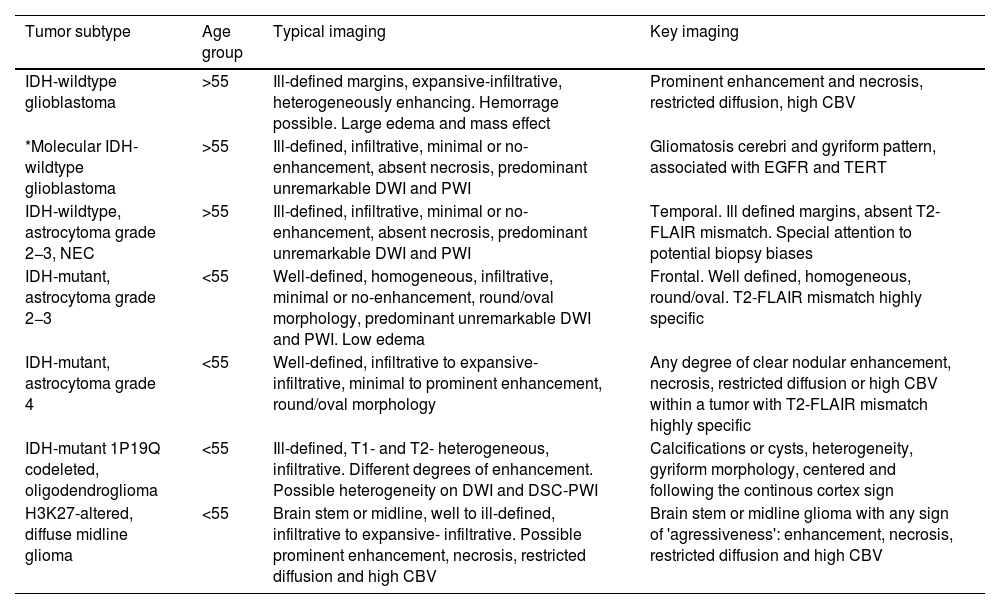
Summary of imaging features of diffuse gliomas in adults. The “*” in “Molecular IDH-wildtype glioblastoma” indicates this is not a specific WHO category, but a subgroup within IDH-wildtype glioblastomas that can appear with different imaging patterns.
| Tumor subtype | Age group | Typical imaging | Key imaging |
|---|---|---|---|
| IDH-wildtype glioblastoma | >55 | Ill-defined margins, expansive-infiltrative, heterogeneously enhancing. Hemorrage possible. Large edema and mass effect | Prominent enhancement and necrosis, restricted diffusion, high CBV |
| *Molecular IDH-wildtype glioblastoma | >55 | Ill-defined, infiltrative, minimal or no-enhancement, absent necrosis, predominant unremarkable DWI and PWI | Gliomatosis cerebri and gyriform pattern, associated with EGFR and TERT |
| IDH-wildtype, astrocytoma grade 2−3, NEC | >55 | Ill-defined, infiltrative, minimal or no-enhancement, absent necrosis, predominant unremarkable DWI and PWI | Temporal. Ill defined margins, absent T2-FLAIR mismatch. Special attention to potential biopsy biases |
| IDH-mutant, astrocytoma grade 2−3 | <55 | Well-defined, homogeneous, infiltrative, minimal or no-enhancement, round/oval morphology, predominant unremarkable DWI and PWI. Low edema | Frontal. Well defined, homogeneous, round/oval. T2-FLAIR mismatch highly specific |
| IDH-mutant, astrocytoma grade 4 | <55 | Well-defined, infiltrative to expansive- infiltrative, minimal to prominent enhancement, round/oval morphology | Any degree of clear nodular enhancement, necrosis, restricted diffusion or high CBV within a tumor with T2-FLAIR mismatch highly specific |
| IDH-mutant 1P19Q codeleted, oligodendroglioma | <55 | Ill-defined, T1- and T2- heterogeneous, infiltrative. Different degrees of enhancement. Possible heterogeneity on DWI and DSC-PWI | Calcifications or cysts, heterogeneity, gyriform morphology, centered and following the continous cortex sign |
| H3K27-altered, diffuse midline glioma | <55 | Brain stem or midline, well to ill-defined, infiltrative to expansive- infiltrative. Possible prominent enhancement, necrosis, restricted diffusion and high CBV | Brain stem or midline glioma with any sign of 'agressiveness': enhancement, necrosis, restricted diffusion and high CBV |
A subset of gliomas occurs with multiple lesions, termed multifocal or multicentric. Multifocal gliomas demonstrate contiguous pathways of spread between foci, whereas multicentric are widely separated. Also, widespread intracerebral dissemination may be referred to as gliomatosis cerebri. These pattern can be seen in IDH-wildtype glioblastomas and IDH-mutant 1p/19q-codeleted oligodendrogliomas.13,73
The terms primary and secondary gliomas should be used cautiously in radiological context, given current knowledge. It is now understood that most grade 4 IDH-mutant astrocytomas are de novo,13,64 and the presence of non-enhancing tumor components may not necessarily indicate subjacent lower aggressiveness.74 Therefore, radiologists should categorize a glioma as secondary only when a prior lower-grade tumor is demonstrable. Simply put, radiologists should avoid labeling a glioma as secondary just because they observe high-grade-looking foci accompanied by low-grade-looking areas, unless there is further evidence to support it.
Clinical relevance of non-invasive presurgical diagnosisAs radiologists, we could encounter the question: “which is the importance of attempting to diagnose specific entities non-invasively if the definitive diagnosis is ultimately based on pathology?” This perspective minimizes the radiologist's role and misrepresents the reality in neuro-oncology units. Although at face value this statement might seem valid, it overlooks the vital role radiologists play in securing a definitive diagnosis efficiently and safely.
The first-line molecular biology testing typically involves immunohistochemistry and FISH due to their wide availability. However, DNA-sequencing is the preferred method for detecting non-canonical IDH-mutations and other genetic alterations involved in classification. Unfortunately, in many centers worldwide, DNA-sequencing is either costly or unavailable. This lack of access to DNA-sequencing in many centers underscores the pivotal role of the radiologist in guiding testing procedures.13,18–21,70
Early identification of specific molecular subtypes is becoming increasingly important. This aids in selecting optimal candidates for specific treatments or clinical trials that evaluate novel targeted therapies. For example, a recent study showcased the significant efficacy of IDH-targeted therapy in managing IDH-mutant astrocytomas.75
Accurate non-invasive imaging diagnosis, especially in certain locations like the brain stem, corpus callosum, or basal ganglia, can help avoid aggressive diagnostic or treatment interventions. It is essential to identify early those tumors that may benefit from aggressive and rapid treatment, and those that might not. Ultimately, in exceptional situations, treatment could be initiated without a definitive diagnosis, an option considered in European guidelines.15
Also, detecting and correcting possible biopsy biases can significantly impact treatment strategies. For example, if a patient is diagnosed with a grade 2−3 tumor, but the radiologist clearly identifies necrosis suggesting a grade 4, the treatment approach must be more aggressive than if we assume that pathology is the only truth. It is important for radiologists to be aware and speak up in such situations.31
Finally, although often also inherently linked to specific tumor presurgical classifications, radiological descriptions encompass relevant prognostic information. For example, a present T2-FLAIR mismatch can indicate a more favorable prognosis, while the presence of necrosis, enhancement, restricted diffusion, or elevated CBV usually implies less favorable outcomes. Radiologists should remain mindful of these prognostic indicators, recognizing that their significance goes beyond merely achieving an accurate non-invasive presurgical diagnosis aligned with the final pathology results.23
ConclusionsRadiologists must have an in-depth understanding of the WHO classification, its strengths, and limitations, and recognize how radiology can contribute to ensuring optimal patient care.
This work highlights areas where further research is needed to optimize the role of radiology in the application of the WHO classification and its potential to improve patient outcomes.
Finally, the authors wish to emphasize the pivotal role of radiology in optimally applying the WHO classification, and to express confidence in that future editions will incorporate radiology to a greater extent. Nonetheless, radiologists must be prepared to rise to such a responsibility.
Authorship- 1
Responsible for study integrity: AP-E
- 2
Study conception: AP-E
- 3
Study design: AP-E
- 4
Data acquisition: AP-E
- 5
Data analysis and interpretation: AP-E
- 6
Statistical processing: NA
- 7
Literature search: AP-E
- 8
Drafting of the manuscript: AP-E
- 9
Critical review of the manuscript with intellectually significant contributions: AP-E, CM, MS, LO
- 10
Approval of the final version: AP-E, CM, MS, LO
This study did not receive any funding.
Conflicts of interestMarion Smits declares consultancy fees from Bracco and Speaker fees from GE Healthcare, AuntMinnie and Fondazione Internazionale Menarini (all paid to institution). Albert Pons-Escoda, Carles Majos and Laura Oleaga confirm that they have no conflicts of interest to declare pertaining to this manuscript.
Albert Pons-Escoda and Carles Majos acknowledge support from the Instituto de Salud Carlos III (Proyectos de Investigación en Salud, PI20/00360).