Platelet-rich plasma (PRP) is a preparation for therapeutic purposes that is increasingly accepted for various musculoskeletal disorders, due to its theoretical potential to repair tissues with poor healing capacity. Several randomized clinical trials have investigated the capacity of PRP to repair tendons, ligaments, muscles and cartilage, and to date there is level 1a evidence to support its use for lateral epicondylitis, osteoarthritis of the knee, plantar fasciitis and rotator cuff tendinopathy; and level 1b for patellar tendinopathy and osteoarthritis of the hip. Retrospective cohort studies and case series describe promising results with PRP for treating other musculoskeletal disorders.
Since its side effects are fewer than those of the control groups, the treatment is considered practically harmless and is being increasingly used. Further randomized clinical trials are necessary to establish future indications, and to confirm effectiveness and safety.
El plasma rico en plaquetas (PRP) es un preparado con fines terapéuticos cada vez más aceptado en diversas patologías musculoesqueléticas, debido a su teórico potencial para reparar tejidos con baja capacidad curativa. Se han realizado diversos ensayos clínicos aleatorizados que investigan la capacidad del PRP para la reparación de tendones, ligamentos, músculos y cartílago. Hasta la fecha existe evidencia 1A que apoya su uso para la epicondilitis lateral, la osteoartritis de rodilla, la fascitis plantar y tendinopatías del manguito rotador, y evidencia 1B en la tendinopatía del tendón rotuliano y la osteoartritis de cadera. Estudios retrospectivos, de cohortes y series de casos describen resultados prometedores del PRP para el tratamiento de otras patologías musculoesqueléticas.
Al ser sus efectos secundarios menores que los de los grupos controles hacen que sea un tratamiento considerado como prácticamente inocuo y cada vez más usado. Son necesarios nuevos ensayos clínicos aleatorizados para establecer futuras indicaciones y confirmar su efectividad y seguridad.
Platelet-rich plasma (PRP) is a concentrate with therapeutic goals that has become more and more popular for the treatment of several musculoskeletal conditions due to its theoretical potential to repair tissues with low regenerative capacity1 and apparent innocuousness.
Since its inception back in the decades of 1980 and 1990 when it was first used in cardiac and maxillofacial surgeries2 it has reached new heights during the last few years in the world of high-performance sports for the management of lesions in tendons, muscles, ligaments and cartilages.1–3
The PRP is defined as a plasma preparation with higher platelet concentrations than the ones found in the blood flow of a healthy individual (200,000 per microliter)1,4,5 – usually 5 times higher.6 The role of platelets is to trigger the inflammatory response that will in turn trigger the presence of tissue growth factors that will eventually be involved in the process of tissue repair.
In this study we will be reviewing the biological foundation of PRP, its applications in musculoskeletal conditions and the actual evidence available.
A review on this issueThe science behind platelet-rich plasmaIn general, the process of tissue repair has three (3) phases: inflammation phase, proliferation phase, and remodeling. Progression inside these phases is mediated by cytokines and growth factors. In certain tissues such as ligaments or tendons, the repair process is very slow due to decreased blood flow and low cellular exchange of these tissues.6
The role of PRP is due to the presence of growth factors and cytokines found inside the alpha granules of platelets that play a role in the process of tissue repair and can be administered locally in tissues with decreased blood flow.1–3
Also, PRP contains other components such as leukocytes. And yet despite the fact that it plays a role in tissue repair and minimizes the risk of local infection, it also shows inflammatory and immune agents that can be counterproductive. In vitro studies confirm the existence of an inflammatory setting with the use of high concentrations of elements of the white series which works to the detriment of healing.1,2,7–10
With the right spin cycles the PRP should not contain any red blood cells. During the phase of oxidative stress, the iron contained in the hemo group can produce free radicals that will eventually damage the cells. This premise suggests that we should avoid or reduce the presence of erythrocytes in PRP preparations.2,8,11–13
Preparation of platelet-rich plasmaTraditionally, PRP is obtained after one single tube centrifugation (and less commonly after plasmapheresis) of autologous blood obtained from the patient's blood flow after venous blood extraction in variable volumes and with the presence of anticoagulants in the syringe or the extraction system. The preparation can be conducted in the lab, OR, office or radiology room with the right centrifuge kit.14,15
The different existing protocols for the preparation of PRP is one of the reasons that can explain the variability of the outcomes obtained in the different clinical trials conducted. One recent systematic review conducted by Chahla et al.14 aimed at standardizing the preparation of plasma confirmed a mean of 51ml of blood extracted from the patient in all cases with anticoagulants (mostly a citrate dextrose solution). Up to 24 centrifuge or plasmapheresis machines available today were used being the most widely used the following centrifuge kits: GPS (Biomet®) and Magellan Autologous Platelet Separator System (Arteriocyte®) both considered high-perfomance machines due to their higher concentration of platelets.16 Most studies refer to one single spin cycle at 3200rpm for 15min. In those cases where there was a second spin, the mean was 3300rpm for 10min.
Another recent study15 compared different spin methods at room temperature and established that even through there are no statistically significant differences in the number of plasma components, the samples were centrifuged in 2 consecutive spins at 160×g (g or centrigufal force) for 10min and 250×g for 15min with leukocyte layer extraction showing platelets and growth factors whose functions and conditions were better preserved.
The following ones seem to be determinant factors in the better quality of plasma: the number of spins (the actual tendency seems to be 2 consecutive spins), high revolutions per minute (at least 3200rpm) and high g forces (rpm by the radius rotor in millimeters).
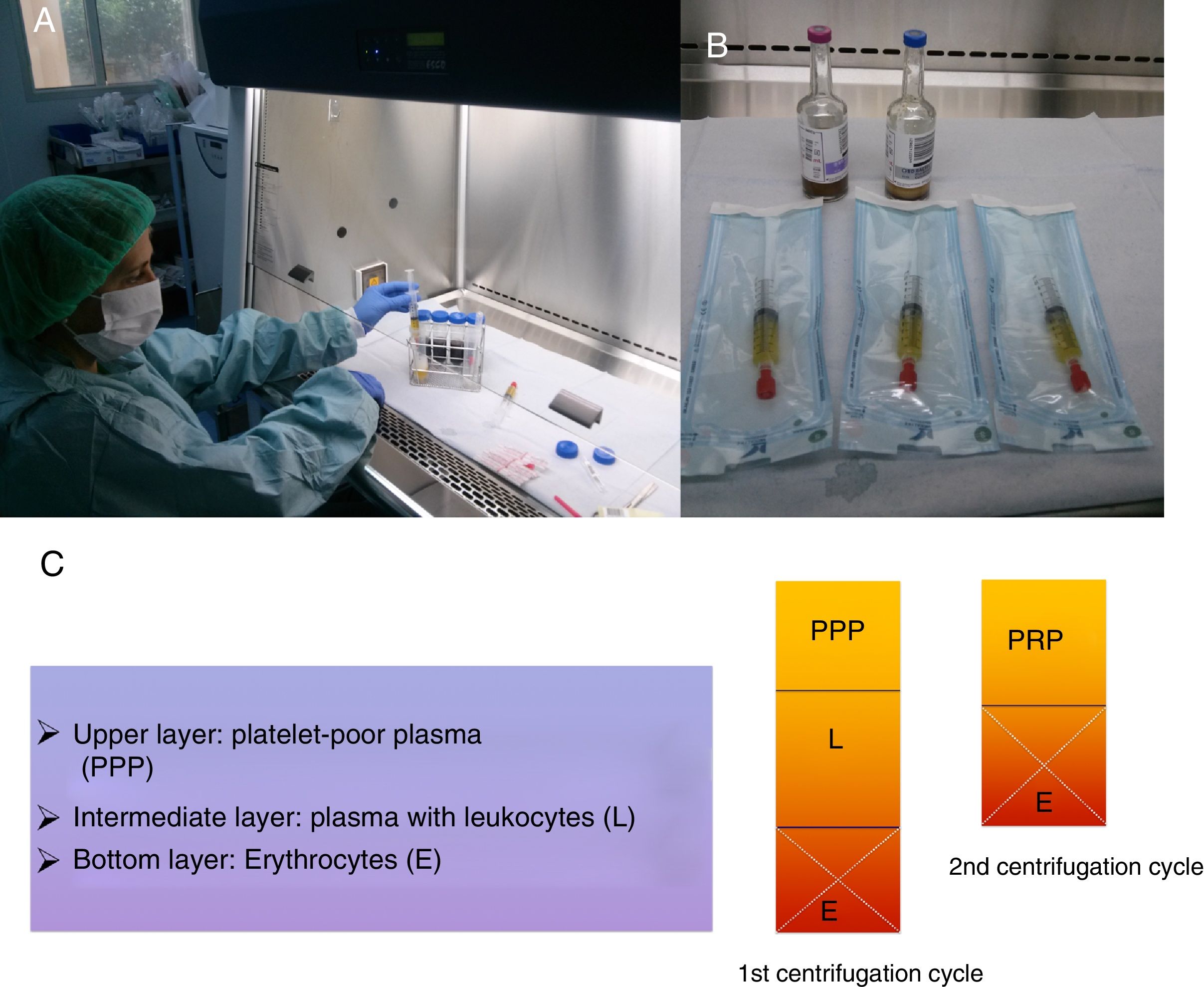
After the spin at around 3200rpm for 15min, the blood extracted is separated into 3 layers.2,3 After discarding the bottom level, a second spin will obtain more concentrated plasma in concentrations of around 10% of initial extraction (2–3ml) (Fig. 1).
(A) After the second spin cycle, the platelet-rich plasma (PRP) is obtained under sterile conditions. (B) Then it is introduced into 2 syringes for later administration and the PRP is ready for injection in sterile bags. (C) Scheme showing how to obtain the PRP from platelet-poor plasma (PPP) after 2 spin cycles.
Once the plasma has been obtained, it can be activated by substances such as calcium chloride or thrombine depending on what will the use be. When plasma is to be used to treat soft tissue injuries, most authors do not care for activation because it will occur in situ when in contact with the tendon collagen (especially after the tendon fenestration with the needle) or with the blood clot from the fiber tear. The activated version is preferred when plasma is administered through an intra-articular injection or for osseointegration purposes. In the latter case, it makes application easier since activation provides more consistency.2,3
If not used after its preparation, the PRP can be frozen and kept in a sterile environment until it is used in the future.
Although consensus is low, scientific literature suggests the following basic requirements2,17:
- •
Platelet concentration factor 4–6 times higher than the normal concentration of blood.
- •
0 leukocyte count or <1000/ml.
- •
Erythrocyte count <1000/ml.
During the last few years the use of PRP and hemoderivatives for the management of sports lesions in muscles, tendons and ligaments has reached new heights.
A Cochrane's review from 201417 that included controlled randomized studies revealed low evidence of pain relief and functionality. In general, the findings only showed better short-term pain relief but no benefits when it comes to functional capacity. These studies were not very comparable due to their heterogeneity and how difficult it was to draw clear conclusions.
Other non-randomized or controlled studies have revealed different findings.
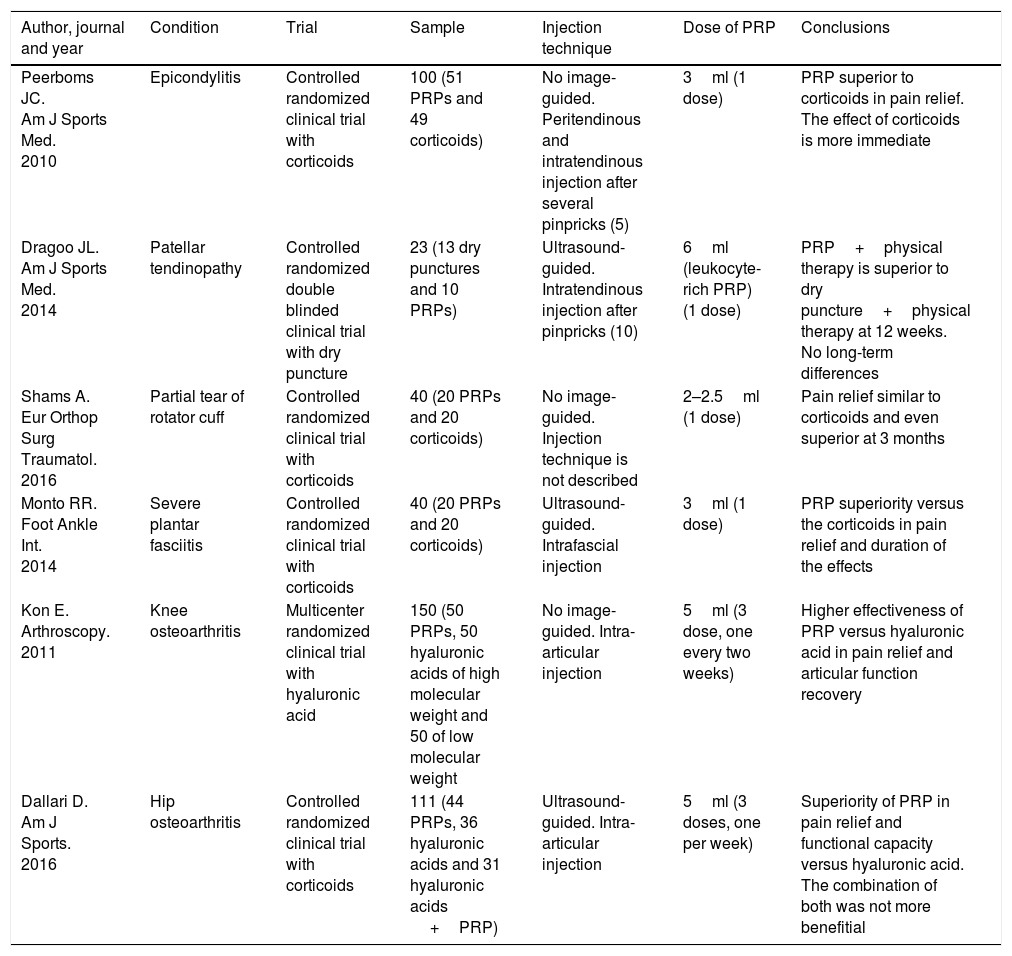
The oldest evidence-based indications in randomized clinical trials are for the management of epicondylitis and knee osteoarthritis. Recently, new evidence-supported indications have been added such as for the management of plantar fasciitis, rotator cuff tendinopathy, patellar tendinopathy and hip osteoarthritis (Table 1).18–23
Relevant evidences of the actual indications.
| Author, journal and year | Condition | Trial | Sample | Injection technique | Dose of PRP | Conclusions |
|---|---|---|---|---|---|---|
| Peerboms JC. Am J Sports Med. 2010 | Epicondylitis | Controlled randomized clinical trial with corticoids | 100 (51 PRPs and 49 corticoids) | No image-guided. Peritendinous and intratendinous injection after several pinpricks (5) | 3ml (1 dose) | PRP superior to corticoids in pain relief. The effect of corticoids is more immediate |
| Dragoo JL. Am J Sports Med. 2014 | Patellar tendinopathy | Controlled randomized double blinded clinical trial with dry puncture | 23 (13 dry punctures and 10 PRPs) | Ultrasound-guided. Intratendinous injection after pinpricks (10) | 6ml (leukocyte-rich PRP) (1 dose) | PRP+physical therapy is superior to dry puncture+physical therapy at 12 weeks. No long-term differences |
| Shams A. Eur Orthop Surg Traumatol. 2016 | Partial tear of rotator cuff | Controlled randomized clinical trial with corticoids | 40 (20 PRPs and 20 corticoids) | No image-guided. Injection technique is not described | 2–2.5ml (1 dose) | Pain relief similar to corticoids and even superior at 3 months |
| Monto RR. Foot Ankle Int. 2014 | Severe plantar fasciitis | Controlled randomized clinical trial with corticoids | 40 (20 PRPs and 20 corticoids) | Ultrasound-guided. Intrafascial injection | 3ml (1 dose) | PRP superiority versus the corticoids in pain relief and duration of the effects |
| Kon E. Arthroscopy. 2011 | Knee osteoarthritis | Multicenter randomized clinical trial with hyaluronic acid | 150 (50 PRPs, 50 hyaluronic acids of high molecular weight and 50 of low molecular weight | No image-guided. Intra-articular injection | 5ml (3 dose, one every two weeks) | Higher effectiveness of PRP versus hyaluronic acid in pain relief and articular function recovery |
| Dallari D. Am J Sports. 2016 | Hip osteoarthritis | Controlled randomized clinical trial with corticoids | 111 (44 PRPs, 36 hyaluronic acids and 31 hyaluronic acids +PRP) | Ultrasound-guided. Intra-articular injection | 5ml (3 doses, one per week) | Superiority of PRP in pain relief and functional capacity versus hyaluronic acid. The combination of both was not more benefitial |
We will be looking into such evidence below under the section Conditions.
ContraindicationsYet despite its theoretical innocuousness, there are contraindications that should be taken into consideration before administering PRP. It is contraindicated in patients who1–5,17:
- •
Suffer from active infections.
- •
Use anticoagulants and platelet antiaggregants.
- •
Have blood dyscrasias.
- •
Use systemic immunosuppressive drugs.
Imaging modalities should be conducted before the procedure to confirm the diagnosis and it is highly advisable to use them, especially the ultrasound scan, to guide the puncture. Conducting a prior test may useful here too with infiltration of anesthetics or anesthetics with corticoids in the symptomatic area to establish the indication in the most accurate way.1,2
In sports medicine, certain indications promote the use of PRP for the management of tendinopathies and osteoarthritis24:
- •
Pain of more than 3–6-month duration with intensity >4 in the visual analog scale (VAS) of 0–10.
- •
Clinical data, images and diagnostic procedures confirming the presence of tendinopathy or osteoarthritis.
- •
Symptoms resistant to conservative therapies such as non-steroid anti-inflammatory drugs [NSAID] and physical therapy.
- •
When the patient's goal is to avoid surgery.
- •
To shorten the comeback time to physical activity.
- •
When the patient is willing to receive several injections for at least 6 weeks.
The following ones:
- •
Informed consent: providing information on the actual risks (infection, hemorrhage, tissue lesions), benefits, alternative therapies, etc.
- •
Asepsis measures: those common to ultrasound-guided percutaneous interventional procedures.
- •
Inform on the possibility of more pain during the first few days after the procedure, especially when dealing with tendons and fascias, since they will be pinpricked.
- •
Physical therapy before and after the puncture to improve prognosis.
- •
Avoid as much as possible the use of NSAID for 2–6 weeks prior to the procedure so they do not interfere with the inflammatory process (paracetamol can be used as an alternative here).
- •
The optimal time to administer PRP is between 3 and 6 months after pain kicks in with repeated injections in 2–8 week-intervals.
- •
The studies conducted so far reveal contradictory findings when it comes to using single or multiple injections.25,26 There are landmark studies that recommend using 1 single injection for the management of tendinopathies and 3 injections for the management of osteoarthritis (Table 1).
Other than the already mentioned knee osteoarthritis, there are other very common entities in sports practice that can also benefit from the use of PRP – some with stronger scientific evidence than others.
EpicondylitisPRP has been more popularly used for the condition called tennis elbow – and today there is evidence for its use.1,2,17,18,24 Randomized controlled trials have shown its effectiveness improving functional capacity, alleviating pain, and the tendon general appearance in imaging modalities compared to steroid injections. Other studies show greater short-term pain relief with the use of corticoids.18,27–31 Lastly, other studies find no significant differences between the use of PRP and saline solutions.32
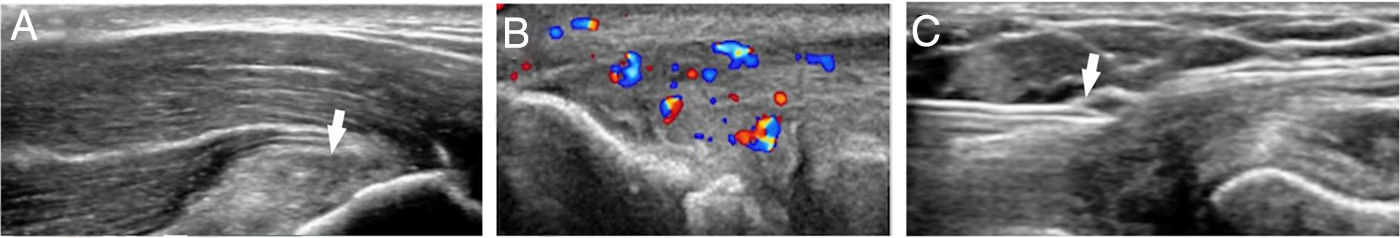
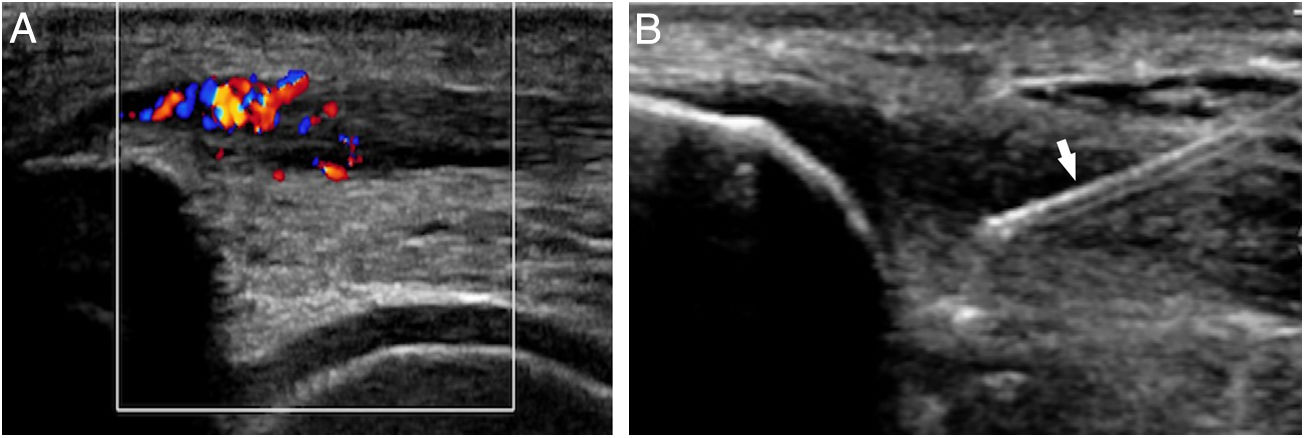
The imaging modalities show thickening of the extensor common tendon with hypoechogenicity on the ultrasound scan and signal hyperintensity on the MRI, fiber disruption and neovascularity on the color Doppler ultrasound scan. The images prior to therapy will confirm the clinical diagnosis and establish the baseline findings for therapy monitoring purposes. Ultrasound guidance will allow us to achieve the therapeutic target more accurately1 (Fig. 2).
(A) Ultrasound image of epicondylitis with thickening of the extensor common tendon and altered fiber pattern (arrow). (B) Epicondylitis with tendinous neovascularity. (C) Ultrasound-guided injection of platelet-rich plasma in the extensor common tendon using one 21G caliber needle (arrow).
The basis for its application is its tendency to become a chronic condition after the use of non-surgical therapies. The outcomes after management with PRP are variable:
- •
Several case series studies, pilot studies and retrospective studies show promising outcomes with an improved functionality after 4 years.33–37
- •
Other controlled randomized studies such as the ones conducted by De Vos et al. and Krogh et al. that compared the PRP to saline solutions do not show a statistically significant superiority of plasma.38,39
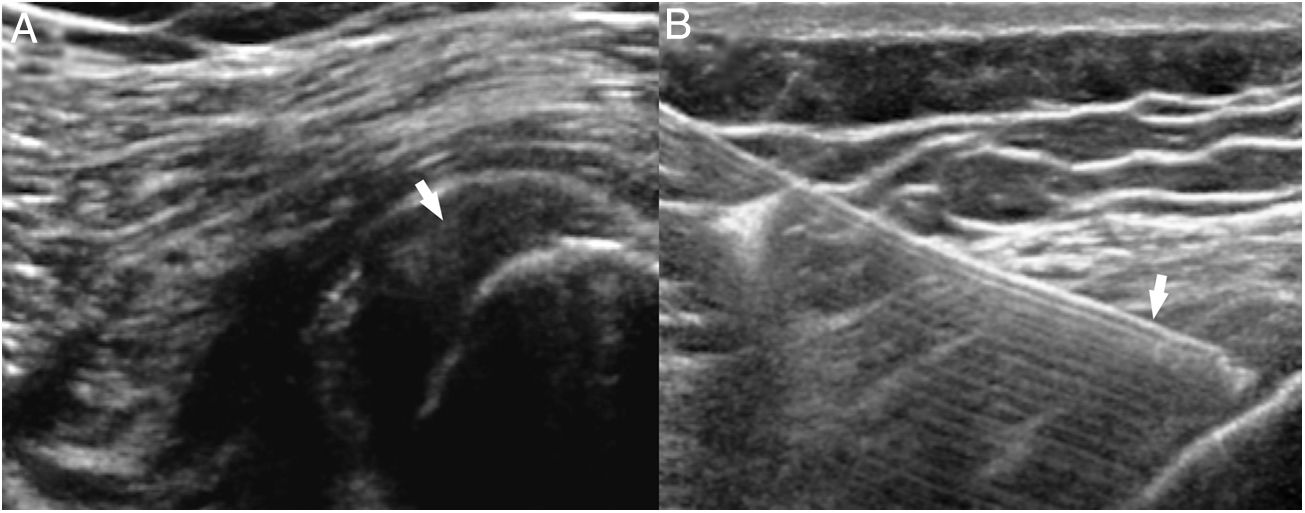
Thus, the PRP can be considered the second-line therapy for the management of Achilles tendinopathy when conservative therapies fail40,41 (Fig. 3).
Patellar tendinopathyUntil recently, there were not randomized controlled quality studies that would recommend the use of PRP over conservative therapies although it may be used in therapy-resistant cases.42,43
One trial19 and one recent meta-analysis44 of randomized trials determine that, even with limited evidence, the PRP is superior to other non-surgical therapies for the management of patellar tendinopathy (Fig. 4).
Rotator cuff tendinopathyAlthough studies provide disparate findings there is growing evidence on its use. A recent meta-analysis conducted in 201845 concludes that the PRP improves the healing rate, levels of pain, and functional capacity of the shoulder. Other very recent studies as well that include systematic reviews and meta-analyses of clinical trials46,47 show favorable outcomes with the use of PRP, but also the need for future studies to evaluate the remaining hemoderivatives.
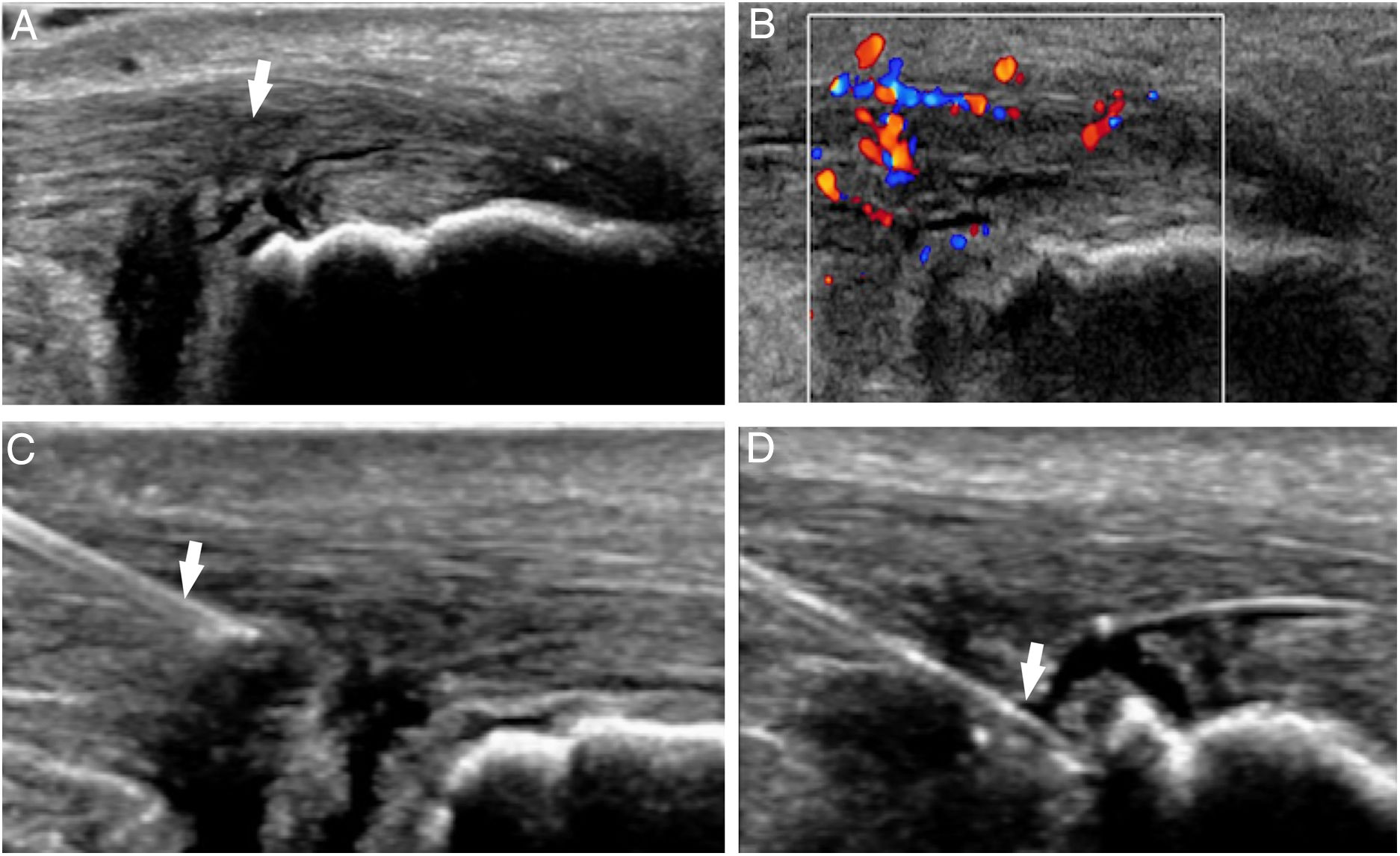
Other tendinopathiesThere is no evidence for the management of other tendinopathies, although the PRP can be used in cases that are resistant to other therapies such as for the management of proximal hamstring tendinopathy48 (Fig. 5) or distal biceps tendinopathy.49
(A) Femoral biceps tendinopathy/semitendinous (arrow) resistant to all conservative therapies used. (B) Three sets of intratendinous platelet-rich plasma were administered using one spinal needle (arrow) with scarce, though more significant pain relief, in the analog visual scale compared to other therapies.
To date there is no such as thing as a uniform therapy for the management of plantar fasciitis which is why so many studies evaluate the use of PRP for the management of plantar fascittis. The first references on the use of PRP for the management of plantar fasciitis go back to 2004 – the first study to ever apply PRP to treat of some type of tendinopathy.50
Future studies have shown its benefits in pain relief, in the homogeneity of the fascia, and in its functional capacity:
- •
Two (2) studies from 2012, one conducted by Aksahin et al. (that made a comparison between the use of PRP and corticoids) and the other conducted by Ragab et al. (without a control group), showed the utility of the PRP (not superior to corticoids) and recommended its use given the lack of adverse events.51,52
- •
Controlled randomized clinical trials conducted by Say et al.53 and Monto et al.21 back in 2014 showed greater effectiveness of PRP compared to corticoids in pain relief and functional capacity that was also statistically significant.
- •
The study conducted by Mahindra et al. back in 2016 showed that the PRP is as useful as corticoids, sometimes even more, compared to physiological serum in the assessment of pain level and functional capacity at 3 months.54
- •
The controlled randomized study conducted by Acosta-Olivo et al. in 2016 did not show any differences between the use of PRP and corticoids.55
- •
The clinical trial conducted by Vahdatpour et al. back in 2016 that compared PRP and corticoids showed the statistically significant superiority of the group of patients treated with PRP with better pain relief and functional capacity, yet no differences were seen in the control ultrasound.56
- •
The systematic review conducted by Chiew et al. in 2016 concludes that the use or PRP may be an effective alternative to conservative therapy without evidence of complications or side effects.57
- •
The 2 latest meta-analyses and systematic reviews conducted in 2017 conclude that the PRP is superior to corticoids, especially in long-term pain relief.58,59
Given the existence of several confirmatory clinical trials, we hereby conclude that the PRP is superior to corticoids for the management of plantar fasciitis with scientific evidence level 1A. Thus, when conservative therapy fails, we can go directly to PRP even ahead of corticoids.
MusclesIn a statistically significant way, Bubnov et al. showed the utility of ultrasound-guided PRP for the management of muscular lesions of the hamstring muscles with pain relief and recovery compared to the control group.60 Other more complete studies on hamstring muscles such as the ones conducted by Hamilton et al. and Guillodo et al. showed no benefits at all.61,62 The latest study conducted on this regard63 was one randomized clinical trial that compared the effectiveness of PRP plus rehabilitation versus rehabilitation for the management of muscle fiber tears in athletes with statistically significant favorable outcomes in the PRP group in terms of convalescence time and comeback to sports activity.
CartilageSeveral clinical trials have confirmed the effectiveness of PRP improving the functional capacity of knee osteoarthritis.22 One meta-analysis conducted back in 2017 by Dai et al., shows favorable outcomes following the knee intra-articular administration of PRP compared to hyaluronic acid and saline solution (level of evidence 1A).64 A different meta-analysis conducted in 2017 by Shen et al. claims that “according to patients, the intra-articular administration of PRP is probably more effective than placebo (saline solution), ozone, hyaluronic acid and corticoids when it comes to pain relief and functional capacity at 3, 6 and 12 months”.65
In other joints such as the hip, evidence is more recent, and there are clinical trials, not meta-analyses, that show that compared to hyaluronic acid23 the intra-articular injection of PRP works better at pain relief and functional capacity. In addition to intra-articular injections, recent studies such as the ones conducted by Sánchez et al.66 and Fiz et al.67 that studied the intraosseous infiltration of PRP in cases of severe knee and hip osteoarthritis did not establish its indication as the standard therapy but described new intra-articular and intraosseous applications of PRP. Nevertheless, future studies still need to be conducted before confirming the benefits of such routes of administration.
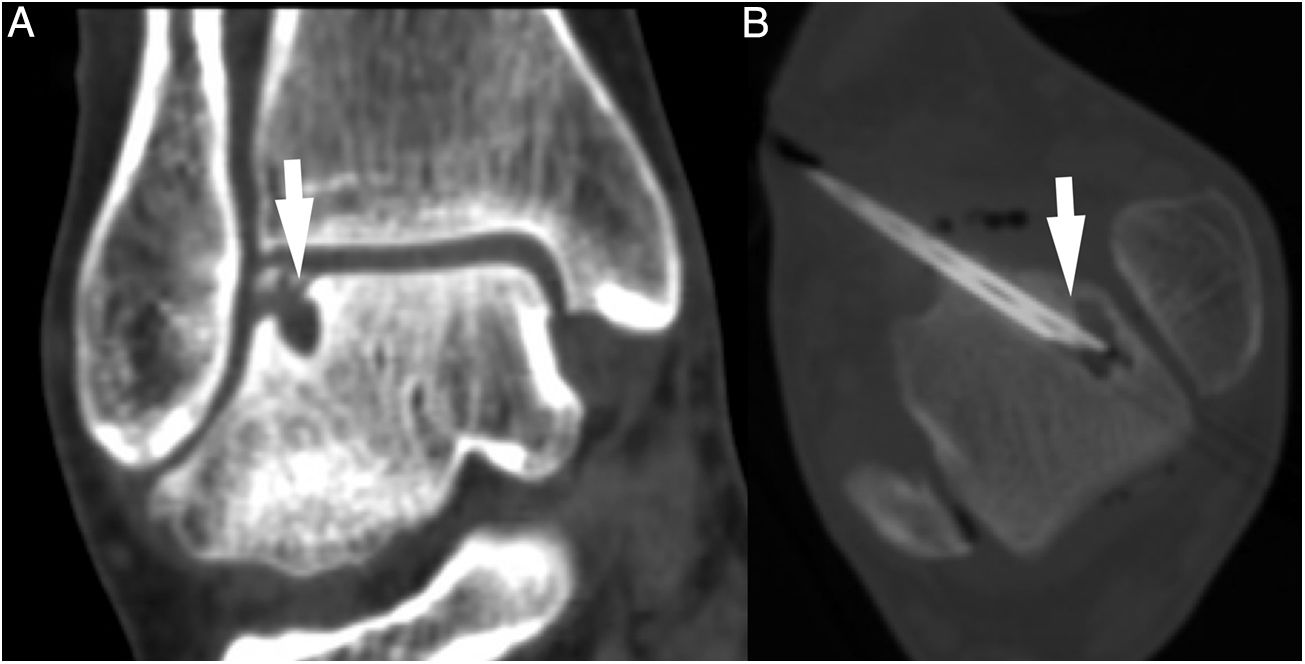
Osteochondral lesionsOne review conducted by Elghawy et al. back in 201868 speaks about 7 studies that assessed the utility of PRP in this type of lesion. Only one of these69 compared the percutaneous approach to hyaluronic acid. There is still no evidence for its percutaneous application meaning that it should always be administered in its activated form (denser) so that the PRP remains in the focus of application (Fig. 6).
Other applicationsAlthough evidence is scarce on this regard there are few clinical trials that deal with the percutaneous application of PRP for the management of meniscal tears or the lumbar facet syndrome with intra-articular administrations of PRP.70,71
The administration of PRP for the management of the anterior cruciate ligament (ACL) plasty is not new and there are clinical trials on this regard that date back to 200972; however, recent systematic reviews73 claim that it can help in the maturation of the graft tissue.
Injection techniqueThe actual technique is the image-guided application of the PRP, especially the ultrasound scan. In joints such as the knee or the hip, the administration of PRP should happen in its activated form being the injection technique similar to that of any intra-articular puncture. According to the most relevant studies conducted so far, the amounts of PRP to be administrated should be 3 doses of 5ml each at 1–2 week-intervals.22,23 In other structures such as tendons and fascias, the PRP should be administered in one single dose of 3ml.18,19,20,21
At our center we administer non-articular plasma following the appropriate requirements of asepsis of all ultrasound-guided procedures and with local anesthetic infiltration with mepivacaine at 2% at the peritendinous or perifascial level (4–5ml). In cases like injections to the plantar fascia, it is possible to perform the anesthetic blockade of the posterior tibial nerve. Then, using 21–23G caliber needles we introduce around 1ml of peritendinous PRP to later perform 4–5 punctures to the tendon to stimulate the healing process and activate the PRP with tendinous collagen or with the blood clot resulting from the fenestration of the tendon. Lastly, we introduce the remaining plasma intratendinously and deep into the tendon.
Post-injection careClinical guidelines published by the University of Wisconsin teaching hospital establish a series of recommendations after the injection of intratendinous PRP from the first day and up to 6–8 weeks after the application of the PRP.74 Here is what we should remember:
- •
Immobilization of the tendon damaged using a bandage or a sling the days 0–3 plus analgesia for around 2–6 weeks – avoiding the use of NSAID.
- •
Increase physical activity progressively.
The PRP is a concentrate with therapeutic goals for the repair of tissues with poor blood supply. Today, there is level of evidence 1A (systematic reviews of controlled clinical trials) for the use of PRP for the management of knee osteoarthritis, epicondylitis, rotator cuff tendinopathy and plantar fasciitis; and level of evidence 1B for the management of patellar tendinopathy and hip osteoarthritis. Imaging modalities are essential as the first-line therapy to establish the diagnosis, assess the patient's baseline status, guide the puncture and conduct the follow-up.
More quality studies are still needed before establishing new indications.
Authors- 1.
Manager of the integrity of the study: AMM.
- 2.
Study Idea: AMM, FRS and JGE.
- 3.
Study Design: AMM, FRS and JGE.
- 4.
Data Mining: AMM, FRS and JGE.
- 5.
Reference: AMM, FRS and JGE.
- 6.
Writing: AMM, FRS and JGE.
- 7.
Critical review of the manuscript with intellectually relevant remarks: AMM, FRS and JGE.
- 8.
Approval of final version: AMM, FRS and JGE.
The authors declare no conflicts of interests associated with this article whatsoever.
Please cite this article as: Martínez-Martínez A, Ruiz-Santiago F, García-Espinosa J. Plasma rico en plaquetas: ¿mito o realidad? Radiología. 2018;60:465–475.