Acute mesenteric ischaemia is an abdominal emergency because reduced blood flow to bowel loops rapidly leads to irreversible necrosis and death.
This paper reviews the different conditions (arterial, venous, low-flow states) that can result in reduced blood flow to bowel loops.
Since the clinical and laboratory findings are nonspecific, imaging tests play an important role in the diagnosis of mesenteric ischaemia. Multidetector computed tomography is the first-choice technique for the initial workup in cases of suspected acute mesenteric ischaemia because it can rule out other causes of acute abdominal pain. It is important to know the characteristic radiological signs of this entity, because early diagnosis is essential to prevent progression to life-threatening intestinal necrosis.
La isquemia mesentérica aguda constituye una urgencia abdominal con elevada mortalidad, debido al escaso tiempo que transcurre desde la disminución del flujo vascular a las asas intestinales hasta la instauración de una necrosis intestinal irreversible.
Esta disminución del flujo puede deberse a diferentes causas, objeto de revisión de este estudio (arteriales, venosas y estados de bajo gasto).
Las pruebas de imagen tienen un importante papel en su diagnóstico, ya que ni los síntomas ni las pruebas de laboratorio son específicos. La tomografía computarizada multidetector (TCMD) es la técnica de imagen inicial de elección para el diagnóstico de sospecha de la isquemia mesentérica aguda y permite excluir otras causas de dolor abdominal agudo. Es importante conocer los signos radiológicos típicos de esta enfermedad, ya que resulta imprescindible su reconocimiento precoz para evitar la progresión de la enfermedad a necrosis intestinal, que puede poner en riesgo la vida del paciente.
Acute mesenteric ischaemia (AMI) is an uncommon condition that constitutes one of the abdominal emergencies with the worst prognosis. Its incidence increases with age and seems to be equal in men and women.1 It represents approximately one in every 1000 patients admitted to hospital for acute care.2–5 Its mortality rate is around 40–80%,6–8 due to the difficulty of early detection and the limited time that elapses between the decrease in vascular flow to the intestinal loops and the development of irreversible intestinal necrosis.3,4,9 The prognosis for these patients depends on the time to diagnosis and initiation of treatment. A delay in diagnosis of 24h decreases survival rates by up to 20%.10 Therefore, early diagnosis and rapid management are essential.11–13 In this regard, imaging tests play an important role, since, as shall be seen later on, neither symptoms nor laboratory tests are specific. At present, multidetector computed tomography (MDCT) is the initial imaging technique of choice for the diagnosis of suspected AMI and, in addition, enables other causes of acute abdominal pain to be ruled out.
AetiologyThe most common cause of AMI is arterial, such as mesenteric artery embolism (MAE) (Fig. 1A) and mesenteric artery thrombosis (MAT) (Fig. 1B); other less common causes are venous, such as mesenteric vein thrombosis (MVT) (Figs. 2 and 3), and low-output states such as non-occlusive mesenteric ischaemia (NOMI) (Fig. 4). There are other causes of AMI that should also be borne in mind, such as vasculitis, arterial dissection, internal hernias (Fig. 5), adhesions (Fig. 6), volvulus and mesenteric trauma.14
Mesenteric ischaemia of arterial origin. (A) Embolism of the superior mesenteric artery. Multiplanar reconstruction (MPR) on a sagittal plane in an abdominal and pelvic computed tomography (CT) scan with iodinated intravenous contrast in an arterial phase showing an embolus inside the superior mesenteric artery (white arrow), distal to the origin of the artery. (B) Thrombosis of the superior mesenteric artery. MPR on a sagittal plane in an abdominal and pelvic computed tomography (CT) scan with iodinated intravenous contrast in an arterial phase showing the presence of a thrombus in the main trunk of the superior mesenteric artery (white arrow) involving the first 2–3cm from the origin of the artery. In addition, an atheromatous load greater than that seen in an embolic condition is visualised and striking calcifications due to atherosclerotic changes at the origins of the superior mesenteric artery (black arrow) and the abdominal aortic artery (white arrow tips) are observed.
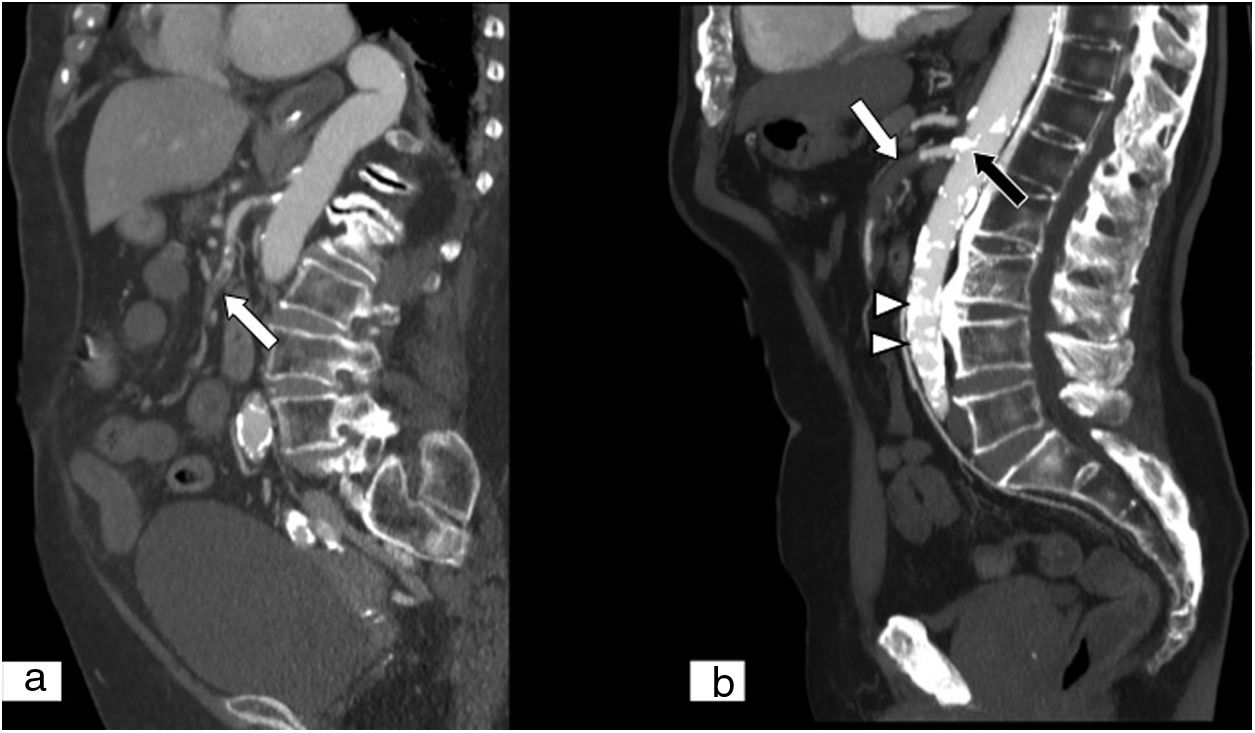
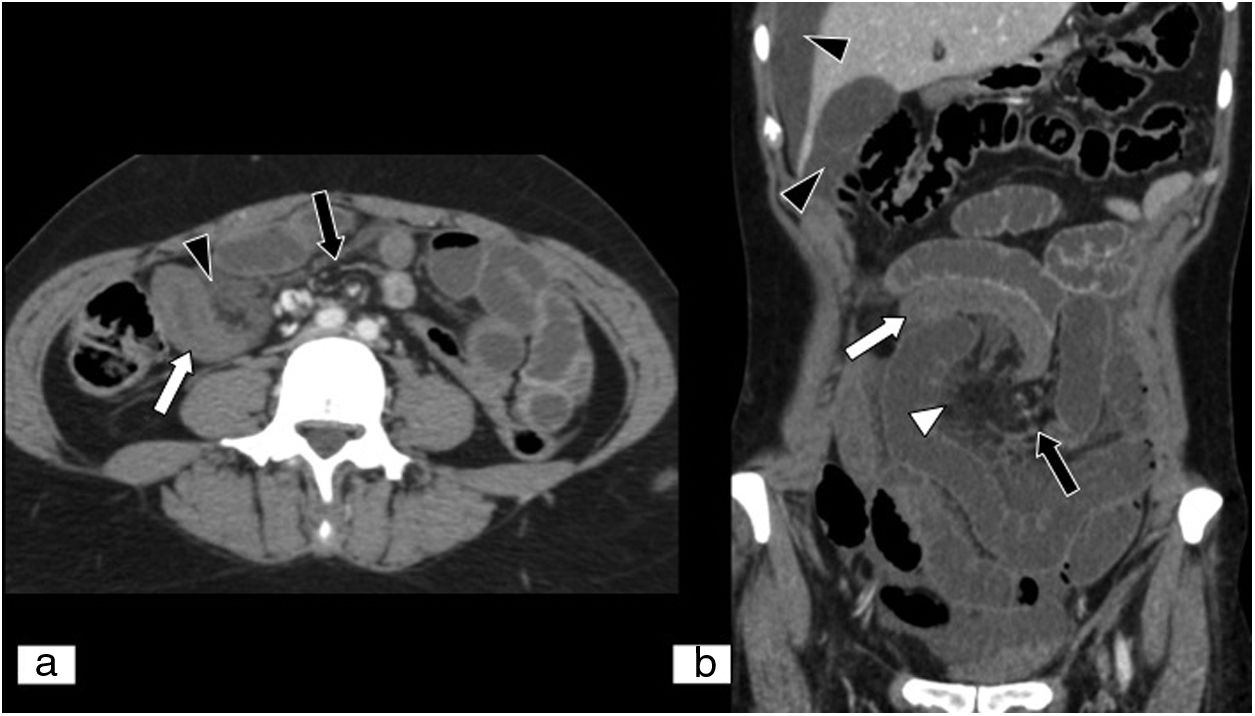
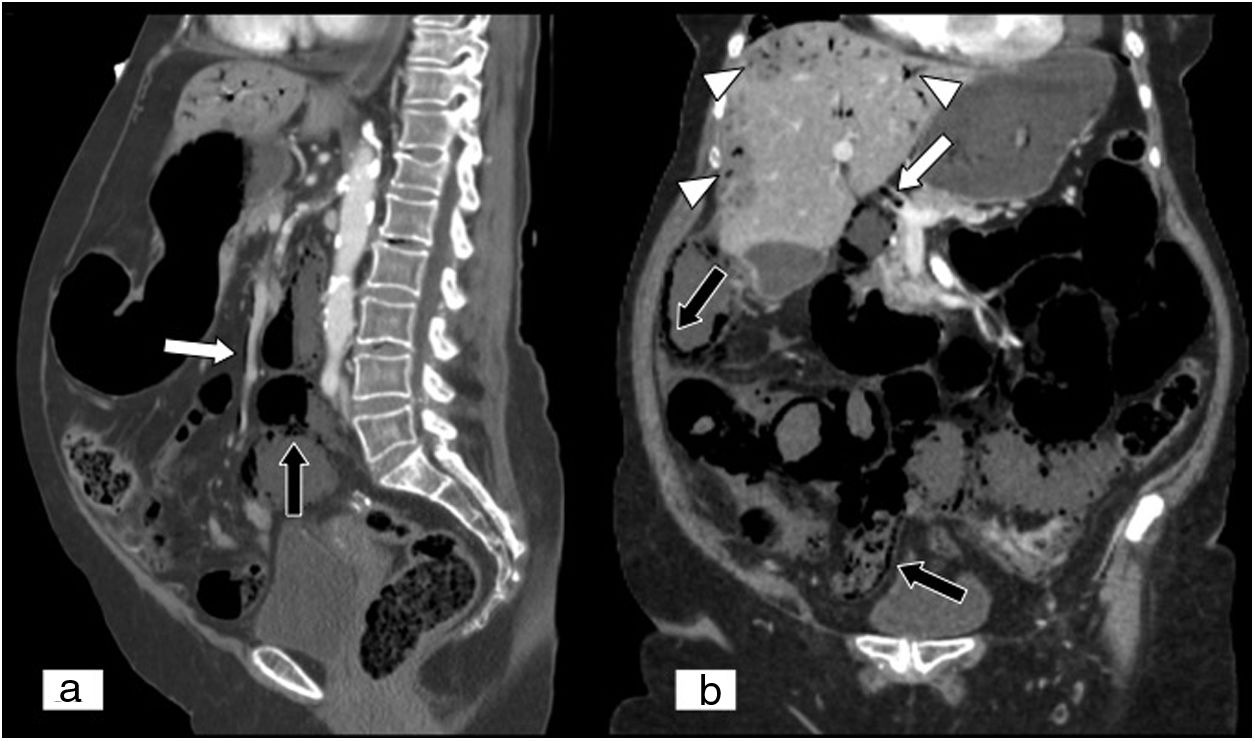
Mesenteric ischaemia of venous origin. (A) MPR on a sagittal plane in an abdominal and pelvic computed tomography (CT) scan. (B) MPR on an oblique coronal plane in an abdominal and pelvic CT scan, both with iodinated intravenous contrast in a portal phase. Filling defect of the superior mesenteric vein extending towards the proximal end of the portal vein (black arrows). Loops of small intestine with decreased parietal uptake (white arrow), engorgement of mesenteric vessels and increased density or striation of fat due to oedema are seen (white arrow tip). Free fluid is also seen in the right paracolic gutter, perihepatic space and pelvis (curved white arrow).
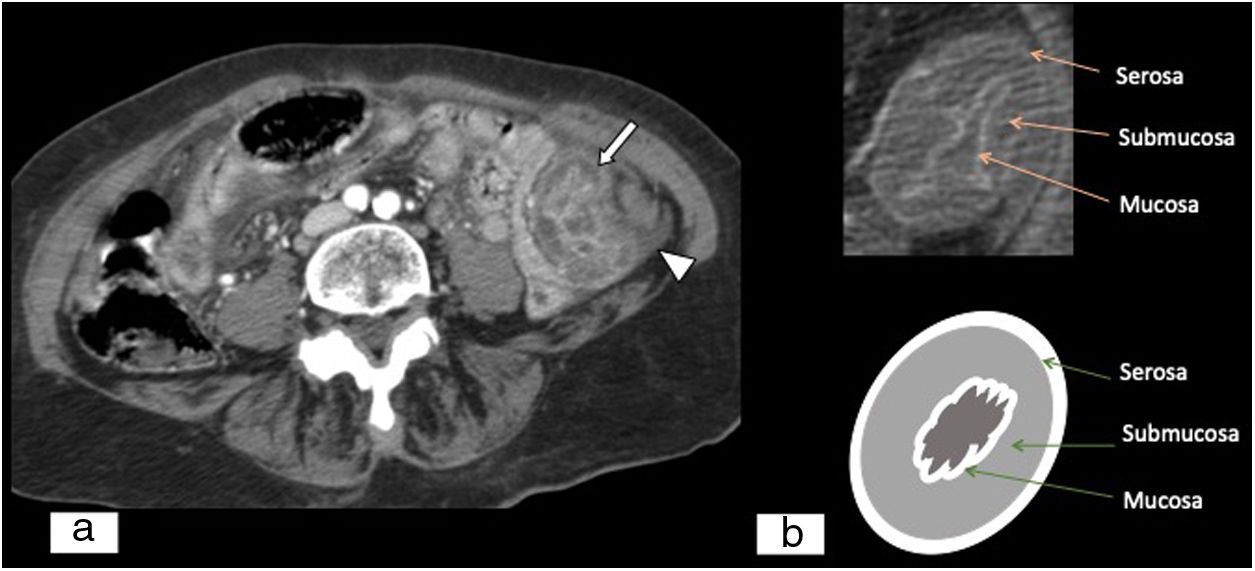
Oedema of the intestinal wall due to venous thrombosis. (A) Abdominal and pelvic CT scan, axial plane, with iodinated intravenous contrast in a late arterial/early portal phase. Concentric thickening of the intestinal wall at the expense of the submucosa due to oedema thereof. Hyperuptake by the mucosa and the serosa with hypodensity of the submucosa (bull's-eye sign) (white arrow) and associated free fluid (white arrow tip). (B) Schematic image of the bull's-eye sign.
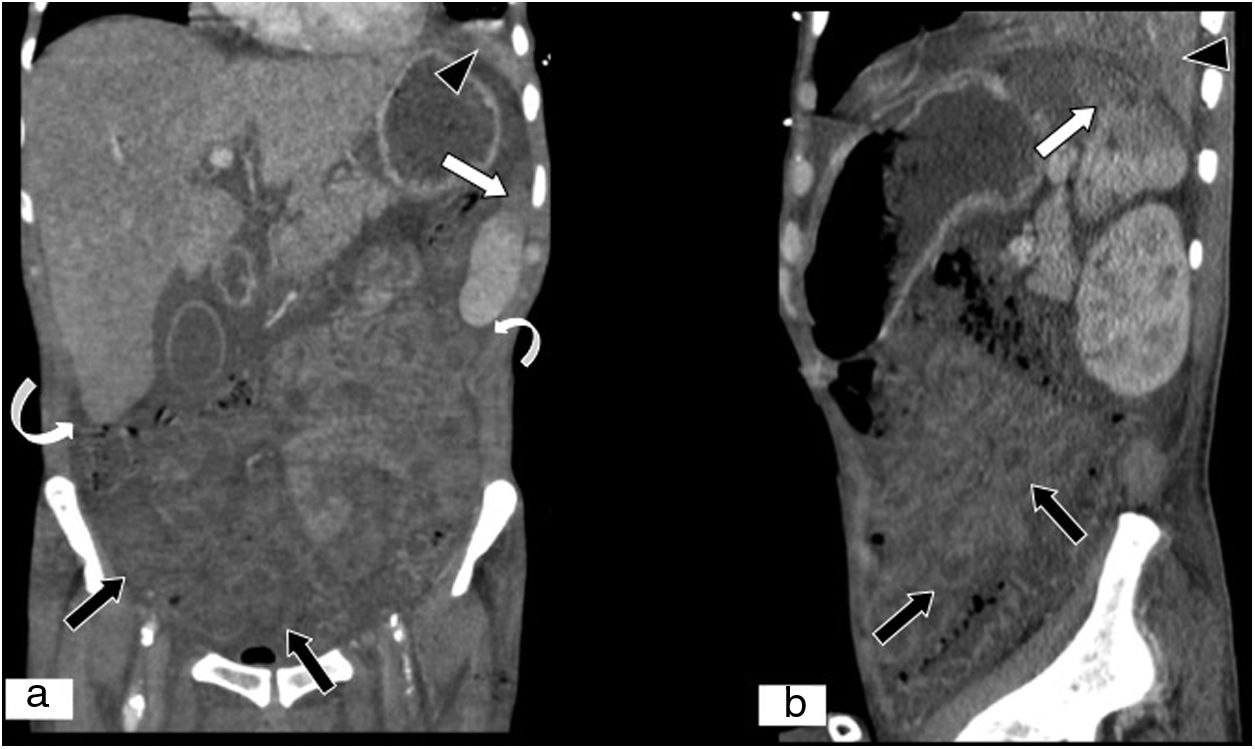
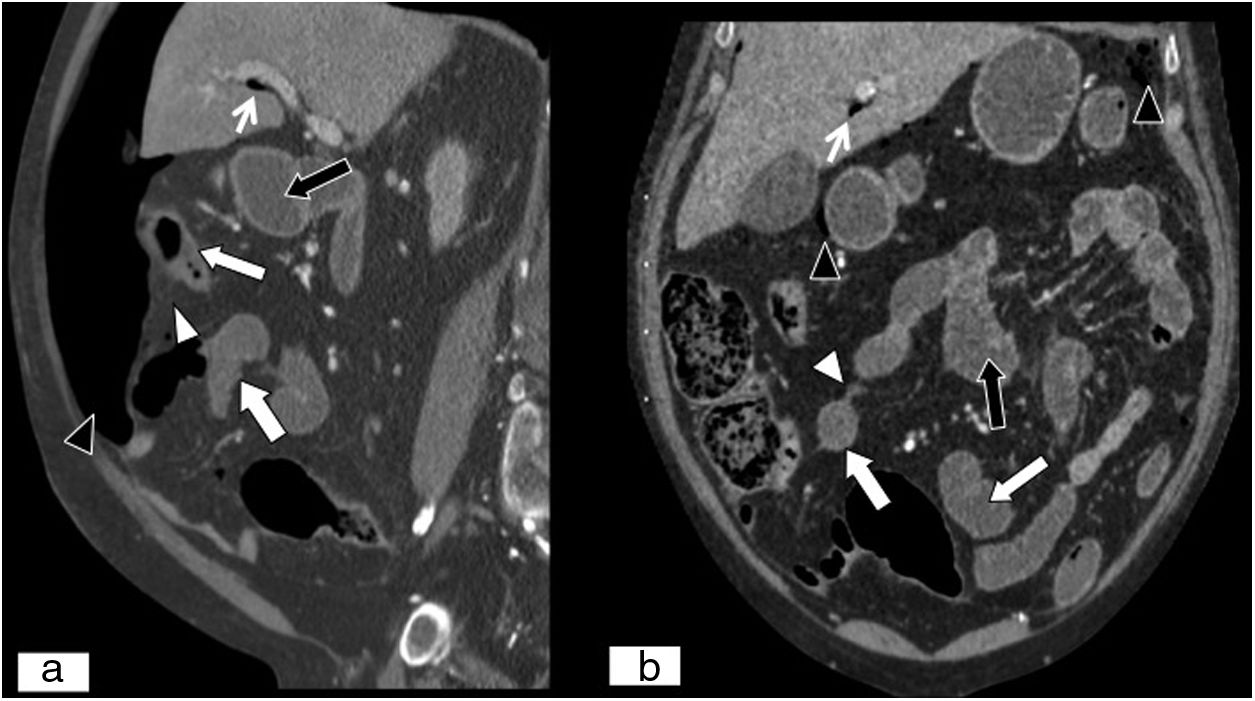
Non-occlusive mesenteric ischaemia in a patient with septic shock. (A) MPR on a coronal plane. (B) MPR on a sagittal plane in an abdominal and pelvic CT scan. Decreased attenuation at the superior–lateral end of the spleen due to infarction in a context of septic shock (white arrows). Decreased parietal uptake by loops of small intestine and colon due to hypoperfusion secondary to low output due to septic shock (black arrows). Presence of free fluid (curved white arrows). Alveolar condensation in left lower lobe (black arrow tips).
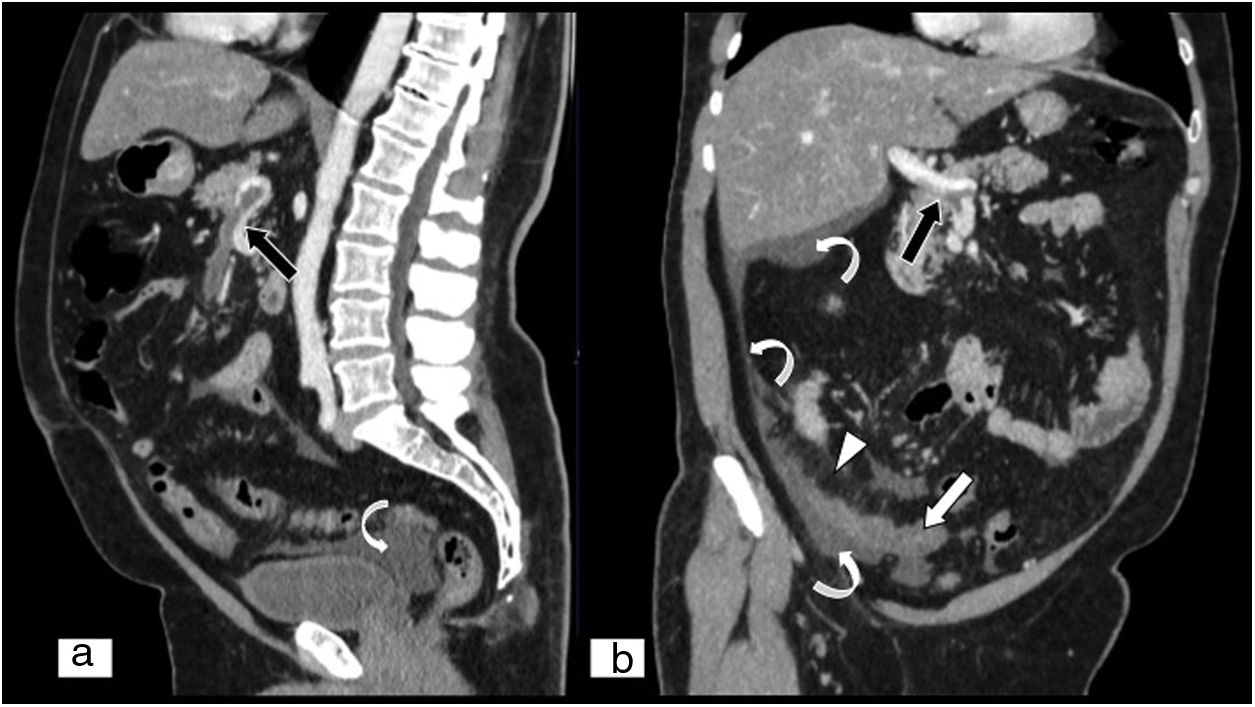
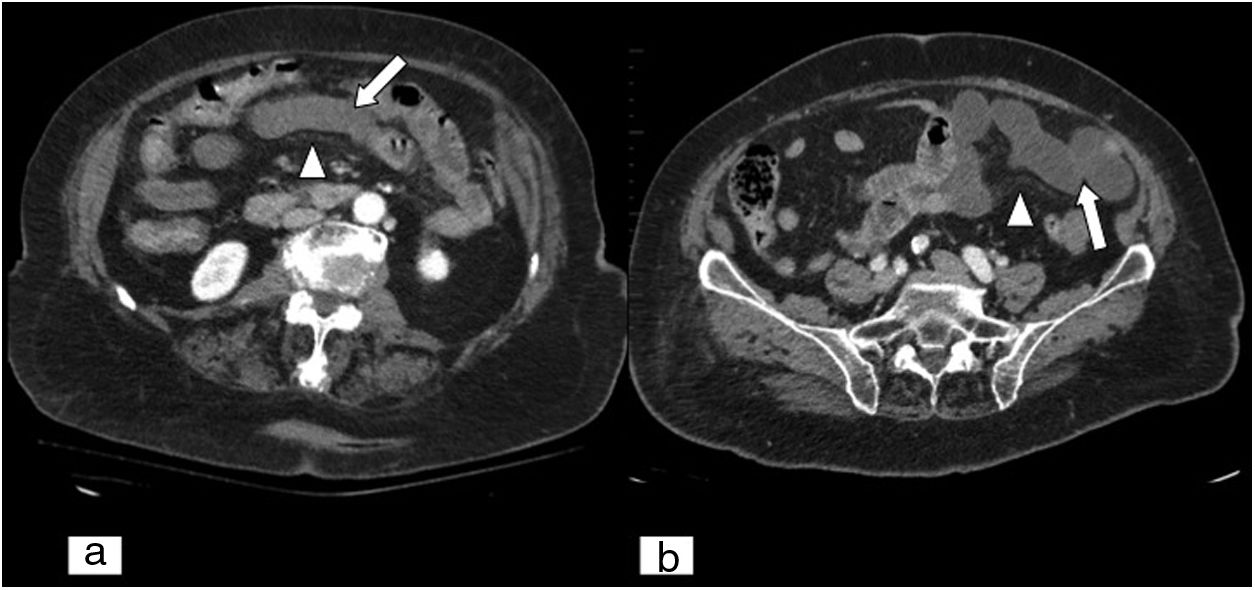
Acute mesenteric ischaemia secondary to internal hernia. Abdominal and pelvic CT scan on an axial plane (A) and MPR reconstruction on a coronal plane of an abdominal and pelvic CT scan (B), both with iodinated intravenous contrast in a portal phase. Loops of small intestine showing parietal thickening and lesser contrast uptake (white arrows), as well as free fluid (black arrow tips) and engorgement of mesenteric vessels, and increased density or striation of fat due to oedema (white arrow tip). Mesenteric vessels with disturbance (black arrows) are seen, due to the presence of an internal hernia.
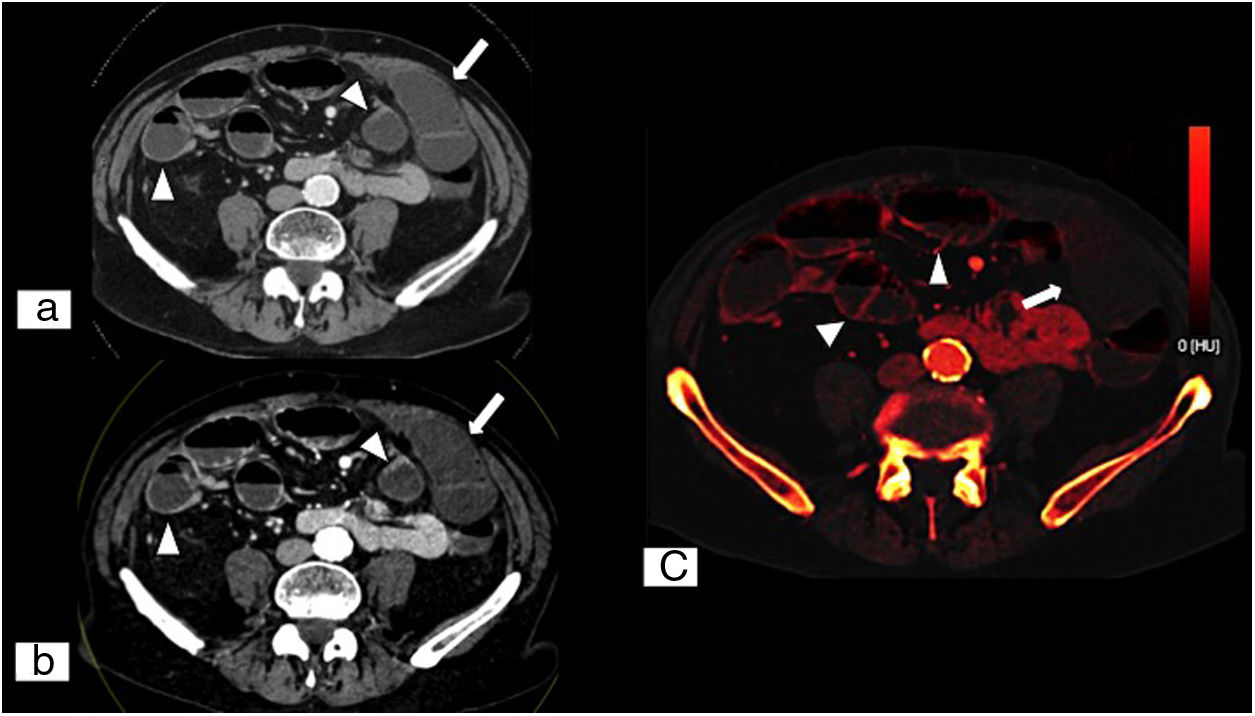
Acute mesenteric ischaemia secondary to adhesions. Abdominal and pelvic dual energy CT on an axial plane with intravenous contrast in a portal phase (A), low-kilovoltage (40KeV) single energy image on an axial plane (B) and iodine map on an axial plane (C). These show lesser contrast uptake in the ischaemic loops (white arrows) compared to other loops with normal perfusion (tips of white arrows), with no obvious parietal thickening. Compared to conventional CT, low-kilovoltage single energy imaging enables tissues that exhibit iodine uptake to stand out more, which facilitates detection of areas of poorly perfused tissues. (Image courtesy of Marta Calvo Imirizaldu and Isabel Vivas Pérez, Radiology Department, Clínica Universidad de Navarra).
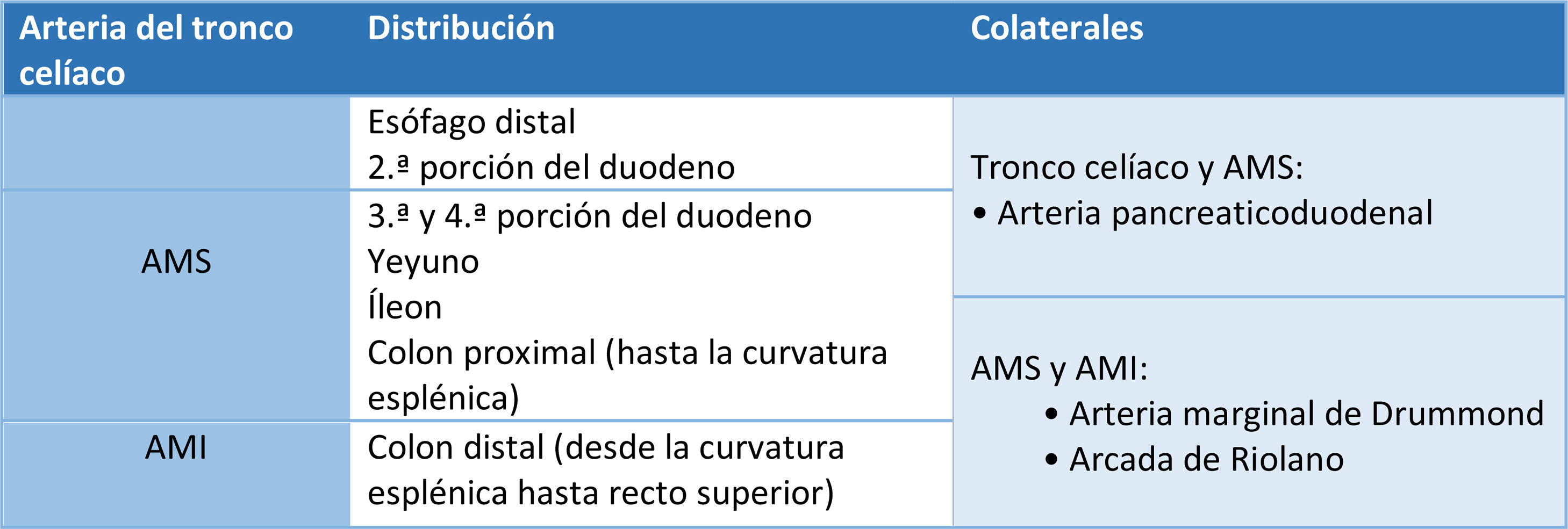
Knowledge of intestinal vascular anatomy is essential for proper diagnosis of AMI. The gastrointestinal system is supplied by three main arteries that are branches of the abdominal aorta: the coeliac trunk, the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA).15–17 The coeliac trunk supplies blood to the distal oesophagus and the second segment of the duodenum; the SMA supplies the third and fourth segments of the duodenum, the jejunum, the ileum, and the proximal colon up to the splenic flexure; and the IMA supplies the distal colon from the splenic flexure to the superior rectum. The branches of the internal iliac arteries and the medial and inferior rectal arteries supply the distal rectum16 (Table 1).
There are a number of collateral branches between these three vessels; they are the pancreaticoduodenal artery, a branch of the common hepatic artery that provides collateral circulation between the coeliac trunk and the SMA; the marginal artery of Drummond; and the arc of Riolan. The latter two are collateral routes between the SMA and the IMA.18 The greatest risk of ischaemia is in the border areas between the two routes as they are poorly vascularised areas. These are located at the splenic flexure (Griffith's point), the ileocaecal junction and the rectosigmoid junction (Sudeck's point).19,20
The superior mesenteric vein (SMV) and the inferior mesenteric vein (IMV) are responsible for venous return. The SMV provides venous drainage of the small intestine and the proximal colon, whereas the IMV provides venous return from the descending colon and the rectum.21,22 The latter drains into the splenic vein, which joins the SMV, and the three together form the portal vein.23 In contrast with arterial collateral circulation, there is venous collateral circulation between the mesenteric veins and the systemic circulation, but it cannot offset acute thrombosis of the SMV or portal vein.24
Stages of ischaemiaAcute intestinal ischaemia is divided into three stages according to degree of intestinal wall impairment.25 Stage 1 (reversible disease) is characterised by necrosis, erosions, ulcerations, oedema and bleeding in the mucosa.26,27 It can resolve spontaneously without sequelae. Stage 2 represents the spread of the necrosis towards the submucosal and muscularis propia layers. Resolution in this stage may give rise to fibrotic stenosis.28,29 Stage 3 involves all three layers of the intestinal wall (transmural necrosis) and is associated with a high mortality rate.30
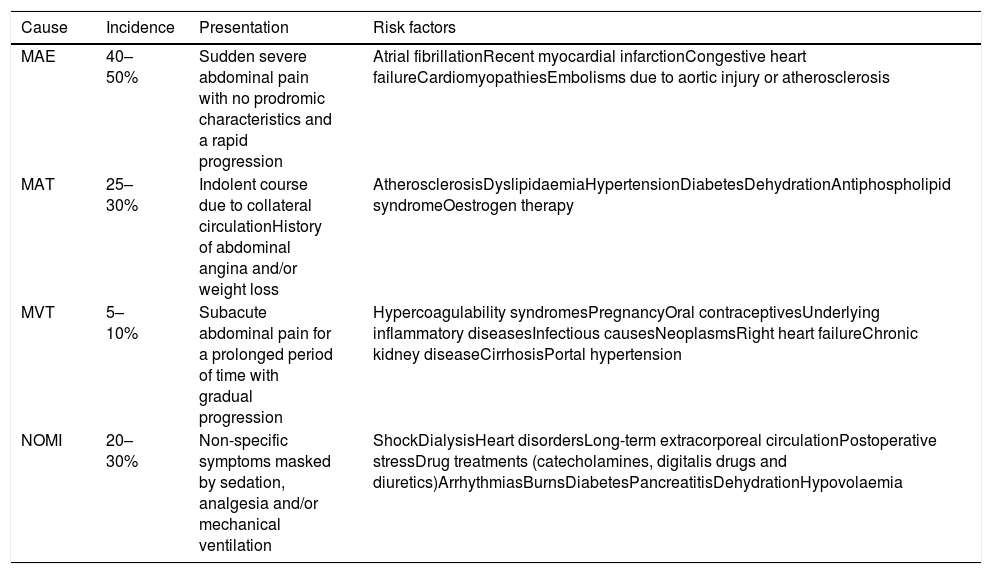
Epidemiology and risk factorsThe epidemiological characteristics and risk factors associated with the different aetiologies of AMI are very important in guiding the suspected diagnosis (Table 2).
Incidence, symptoms and risk factors by aetiology of acute mesenteric ischaemia.
| Cause | Incidence | Presentation | Risk factors |
|---|---|---|---|
| MAE | 40–50% | Sudden severe abdominal pain with no prodromic characteristics and a rapid progression | Atrial fibrillationRecent myocardial infarctionCongestive heart failureCardiomyopathiesEmbolisms due to aortic injury or atherosclerosis |
| MAT | 25–30% | Indolent course due to collateral circulationHistory of abdominal angina and/or weight loss | AtherosclerosisDyslipidaemiaHypertensionDiabetesDehydrationAntiphospholipid syndromeOestrogen therapy |
| MVT | 5–10% | Subacute abdominal pain for a prolonged period of time with gradual progression | Hypercoagulability syndromesPregnancyOral contraceptivesUnderlying inflammatory diseasesInfectious causesNeoplasmsRight heart failureChronic kidney diseaseCirrhosisPortal hypertension |
| NOMI | 20–30% | Non-specific symptoms masked by sedation, analgesia and/or mechanical ventilation | ShockDialysisHeart disordersLong-term extracorporeal circulationPostoperative stressDrug treatments (catecholamines, digitalis drugs and diuretics)ArrhythmiasBurnsDiabetesPancreatitisDehydrationHypovolaemia |
MAE: mesenteric artery embolism, NOMI: non-occlusive mesenteric ischaemia; MAT: mesenteric artery thrombosis; MVT: mesenteric vein thrombosis.
Arterial embolism is the most common cause of AMI and accounts for 40–50% of all cases.31–33 The main risk factors include atrial fibrillation, recent myocardial infarction, congestive heart failure, cardiomyopathies and embolisms due to aortic lesion or atherosclerosis.15,34 The SMA is the artery most commonly seen to be affected due to its minimal angulation where it branches from the aorta compared to the angulation of the coeliac trunk and the IMA. Embolisms often lodge distal to the origin of the first jejunal branches and of the medial colic artery.10,35 This presents a classic pattern of ischaemia that spares both the proximal small intestine and the proximal colon.10,13
Arterial thrombosis accounts for approximately 25–30% of cases of AMI.7,36 The main risk factors are atherosclerotic disease and dyslipidaemia, followed by hypertension, diabetes, dehydration, antiphospholipid syndrome and oestrogen therapy. The pattern of intestinal infarction is more extensive than in MAE, and neither the proximal small intestine nor the proximal colon is spared. This widespread impairment is due to the involvement of the origin of the SMA,15 proximal to the medial colic artery and to the jejunal arteries.
MVT accounts for 5–10% of all cases of AMI.8,25 It may occur in younger populations compared to other causes.23 Obstruction of venous blood flow secondary to thrombosis causes intestinal wall oedema and luminal distension; this increases pressure, which decreases arterial blood flow and consequently leads to intestinal ischaemia.37 It is often associated with hypercoagulability syndromes, such as antiphospholipid antibody syndrome, protein C and protein S deficiency, polycythemia vera, and factor V Leiden mutation, and with hypercoagulable states, such as pregnancy and use of oral contraceptives. Other less common causes are underlying inflammatory diseases such as vasculitis, infectious causes such as enterocolic lymphocytic phlebitis,38–40 neoplasms, chronic kidney failure, cirrhosis and portal hypertension.15 It is deemed idiopathic in 21–49% of cases.41
NOMI is often seen in patients of advanced age, and is responsible for approximately 20–30% of cases of AMI.1,42 Unlike the above-mentioned disorders, it is an acute disorder of the mesenteric circulation not caused by organic occlusion of the blood vessels43 that often persists even after the precipitating event is corrected.42 In terms of pathogenesis, NOMI is believed to arise from a combination of low cardiac output and vasoconstriction. Underlying diseases and risk factors include shock, dialysis, heart disorders, long-term extracorporeal circulation, postoperative stress, use of certain drug treatments (catecholamines, digitalis drugs and diuretics), arrhythmias, burns, diabetes, pancreatitis, dehydration and hypovolaemia.37 This condition is characterised by high rates of morbidity and mortality, due to patients’ advanced age and diagnostic delay.42 This disease may affect the entire gastrointestinal tract (from the oesophagus to the rectum); therefore, impairment of the entire colon should be considered a distinctive element in the diagnosis of this condition compared to occlusive forms of ischaemia.43
Signs and symptomsClinical diagnosis is difficult, since often symptoms are non-specific and the initial presentation imitates that of other abdominal conditions.44 The main clinical symptom is severe abdominal pain, which may appear along with nausea, vomiting, diarrhoea, abdominal distension and blood in faeces.45,46 The early phase may be characterised by an initial discrepancy between the severity of the abdominal pain and the minimal findings in the physical examination.3,8,15,21 The classic triad consists of abdominal pain, haematochezia and fever, and is only observed in a third of patients.47
Patients with AMI have an abrupt onset of symptoms, without prodromes and with rapid progression.34 This is because SMA occlusion is sudden, with no time for collateral circulation to develop.8,48,49
MAT has a relatively indolent course due to development of collateral circulation,8 and is often associated with a history of abdominal angina and/or weight loss, which suggests chronic mesenteric ischaemia.15,37 As the occlusion occurs close to the origin of the SMA, depending on the collateral circulation, often a wide range of intestinal segments is affected, and the course is rapid once complete artery occlusion occurs.37
In MVT, the onset of signs and symptoms is characterised by acute or subacute abdominal pain, which may manifest over a prolonged period with gradual progression.39
Patients with NOMI may have an insidious onset, symptoms that are non-specific and often concealed as they are usually already very seriously ill.15
Laboratory testsThere are no specific laboratory tests for early detection of AMI.50 Patients present elevated leukocytosis, metabolic acidosis, D-dimer and serum lactate, but these markers are not sensitive enough to establish or rule out the diagnosis.8 According to a recent review by Evennett et al.,51 the most promising plasma markers are: intestinal fatty acid binding proteins (I-FABPs) and α-glutathione S-transferase (GST), which are produced in the small intestine and may be released into the bloodstream after a tissue injury;51,52 and D-lactate, which is produced by intestinal bacterial organisms such as Escherichia coli and has been defended as a marker of bacterial translocation.53 These markers may have a potential use as tools for early diagnosis in AMI, but larger-scale studies are needed to validate them and incorporate them into regular clinical practice.
Main radiological techniquesPlain X-rayPlain X-ray of the abdomen is capable of detecting dilatation of the loops of the small intestine and colon, as well as, in some cases, intestinal wall oedema, intraperitoneal free gas, portal vein gas and intestinal pneumatosis.49,54 However, these findings are generally identified late, often when ischaemia or intestinal infarction has already developed.55 In addition, it must be borne in mind that a normal X-ray does not rule out the diagnosis.8,56
UltrasoundThe use of ultrasound enables the coeliac trunk and the SMA to be visualised. The Doppler mode is highly specific (92–100%), but has lower sensitivity (70–89%) for identifying vascular occlusions, mainly non-occlusive thrombi and distal occlusion.54 In advanced cases, intramural gas and portal vein gas may be evident. This technique is useful for narrowing down differential causes of abdominal pain,54,56 but given the time needed to perform the study and its likelihood of failure, it is not recommended in the diagnosis of AMI.54
Magnetic resonance imagingAngiography by magnetic resonance imaging with gadolinium has also demonstrated high specificity and sensitivity for visualising stenosis or obstruction of the SMA or coeliac trunk.57 This technique's main advantage over MDCT is that it does not use ionising radiation. Its limitations are its ineffectiveness in both non-occlusive forms and occlusion of distal branches,56,58,59 lengthier examination time, lower spatial resolution and inability to visualise vascular calcium (atherosclerosis).34
Mesenteric angiographyMesenteric angiography is indicated in patients in whom AMI is strongly suspected with no clear indication for emergency laparatomy.60 Its advantages include its near-100% sensitivity and specificity, its capacity for distinguishing between occlusive and non-occlusive forms, and the fact that it enables thrombolytic drugs and arterial vasodilators to be administered.58 However, it also has disadvantages, such as its invasive nature, nephrotoxic potential and high dose of radiation.60
Computed tomographyMDCT is the initial imaging technique of choice for the diagnosis of suspected AMI, as it has high sensitivity and specificity50 and, furthermore, enables other causes of acute abdominal pain to be ruled out.50 MDCT has a number of advantages including a high speed of image acquisition, which minimises the artefact of intestinal movement, a large exposure field and region of coverage, and excellent patient tolerance. Its potential risks are its use of ionising radiation and nephrotoxicity as well as reactions to iodinated intravenous contrast material.23 MDCT has managed to replace catheter angiography; the latter is now reserved for cases of suspected NOMI and for endovascular management.15
MDCT images are obtained from the dome of the diaphragm to the pelvis.50 Different studies have proposed image acquisition with biphasic intravenous contrast (arterial and venous phase). This acquisition with contrast enables identification of thrombi in the mesenteric arteries and veins, uptake abnormalities in the intestinal wall and embolism or infarction in other organs. Additional acquisition in a phase without contrast is not essential, although it may contribute certain information, such as detection of vascular calcifications, hyperdense intravascular thrombi and intramural bleeding.15,23
The use of oral contrast material, whether positive or neutral, is not indicated in patients with AMI, since intraluminal positive contrast material impedes evaluation of intestinal wall uptake, and the frequent presence of vomiting and adynamic ileus limit the passage of the contrast material through the intestine, which delays definitive diagnosis and treatment.61
There are multiple MDCT study acquisition protocols in the specialised literature, but the most widely accepted protocol and the one that we use at our hospital (Philips Brillance 64 system) consists of acquisition of two series following administration of iodinated intravenous contrast. The clinician administers a volume of 1.5ml per kilogram of weight of low-osmolarity water-soluble contrast at a concentration of 370mg/ml and a speed of 4–5ml/s, followed by a bolus of 20ml of normal saline. An initial arterial phase (thickness 1mm with interval 0.75mm) with an automated bolus tracking technique situating the measurement region of interest (ROI) in the infradiaphragmatic abdominal aorta (with a measurement threshold of 150HU) and a second portal phase after 80 seconds have elapsed since the start of the injection.23 Another option, which is used with increasing frequency (due to limited reliability depending on the patient's haemodynamic status) consists of using post-contrast injection lag times of 25 seconds for the arterial phase and 60–70s for the portal phase.50,62
Thin slices should be used so that multiplanar reconstructions may be prepared.23 Sagittal reconstructions are very useful for evaluating permeability, the origin in the aorta of the mesenteric arteries and variations therein. Acquisition of coronal reconstructions with maximum intensity projection (MIP) and volumetric representation (VR) is useful for evaluating the vascular anatomy and detecting emboli.62–64 In addition, it is recommended that MDCT images be visualised with different window settings, essentially the soft-tissue window and the lung parenchyma window, which aid in recognising extraluminal gas.65
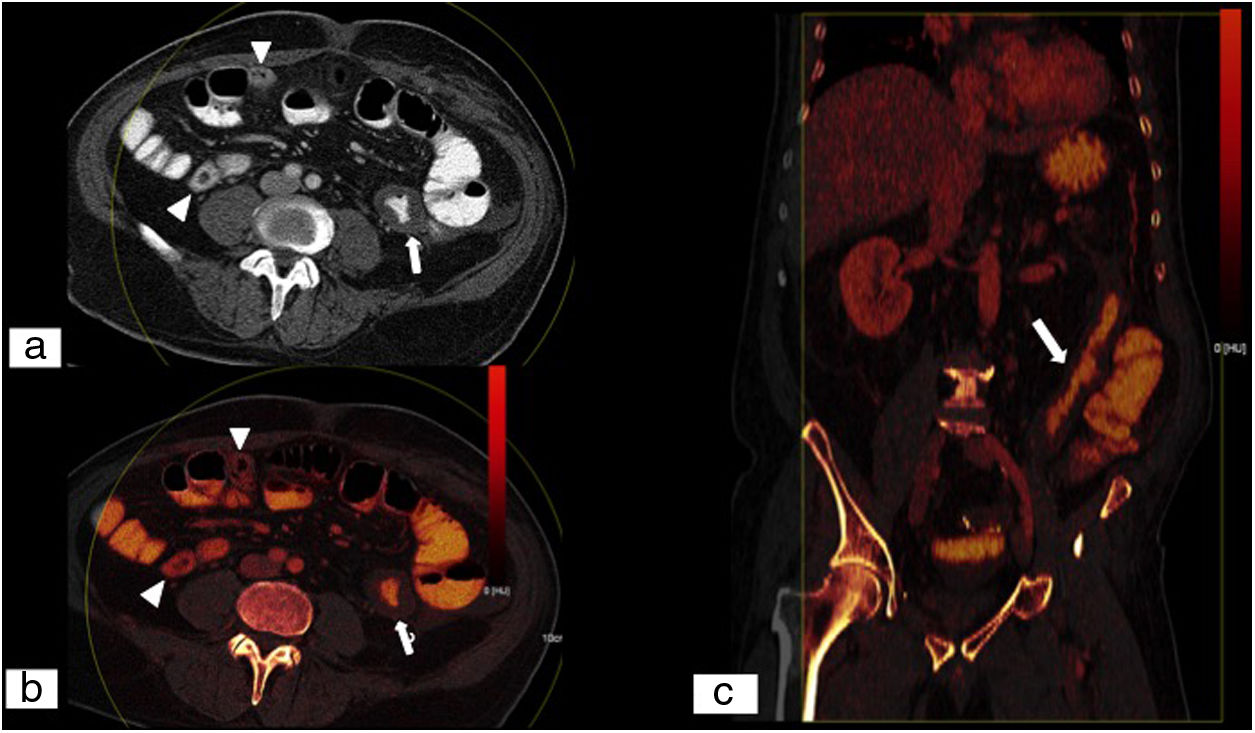
Dual energy CT (DECT), also called spectral CT, is a technique based on CT data acquisition in two different energy configurations, generally high energy (140–150kVp) and low energy (80–100kVp).66,67 Using it to evaluate AMI increases sensitivity for detecting intestinal ischaemia through the use of low-kilovoltage virtual single energy images which increase the difference in attenuation between ischaemic and non-ischaemic segments compared to conventional CT, and also by assessing iodine maps and iodine quantification (Figs. 6 and 7).67–69 Furthermore, recent studies have shown that the radiation dose of MDCT may be similar to single energy CT, or even lower.66,70–72
Ischaemic colitis. Abdominal and pelvic CT scan with intravenous contrast in a portal phase following ingestion of oral contrast. Single energy image obtained with low kilovoltage (70KeV) on an axial plane (A), iodine map on an axial plane (B) and MPR on a coronal plane of an iodine map (C). Despite the difficulty of assessing the intestinal wall following ingestion of oral contrast, lesser contrast uptake is seen in the thickened wall of the descending colon (white arrow) compared to the normoperfused wall of the loops in the right flank (white arrow tips), related to ischaemic colitis. Low-kilovoltage virtual single energy images increase the difference in attenuation between ischaemic segments and non-ischaemic segments compared to conventional CT. (Image courtesy of Marta Calvo Imirizaldu and Isabel Vivas Pérez, Radiology Department, Clínica Universidad de Navarra).
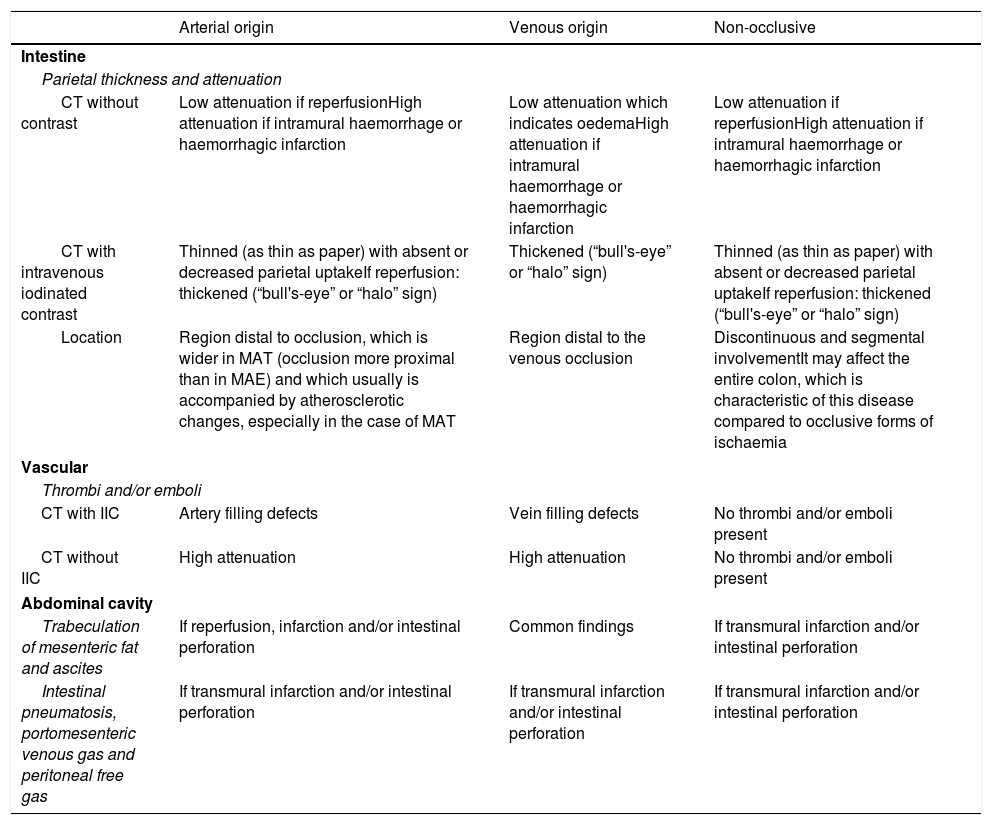
The main findings that appear on MDCT, for the diagnosis of the different aetiologies of AMI are described below. Attenuation and intestinal wall thickness will be assessed, bearing in mind that the latter may vary not only by aetiology, but also by the course of a single process, vascular findings and the presence of abnormalities in the abdominal cavity. These findings are summarised in Table 3.
Findings on multidetector CT by aetiology of acute mesenteric ischaemia.
| Arterial origin | Venous origin | Non-occlusive | |
|---|---|---|---|
| Intestine | |||
| Parietal thickness and attenuation | |||
| CT without contrast | Low attenuation if reperfusionHigh attenuation if intramural haemorrhage or haemorrhagic infarction | Low attenuation which indicates oedemaHigh attenuation if intramural haemorrhage or haemorrhagic infarction | Low attenuation if reperfusionHigh attenuation if intramural haemorrhage or haemorrhagic infarction |
| CT with intravenous iodinated contrast | Thinned (as thin as paper) with absent or decreased parietal uptakeIf reperfusion: thickened (“bull's-eye” or “halo” sign) | Thickened (“bull's-eye” or “halo” sign) | Thinned (as thin as paper) with absent or decreased parietal uptakeIf reperfusion: thickened (“bull's-eye” or “halo” sign) |
| Location | Region distal to occlusion, which is wider in MAT (occlusion more proximal than in MAE) and which usually is accompanied by atherosclerotic changes, especially in the case of MAT | Region distal to the venous occlusion | Discontinuous and segmental involvementIt may affect the entire colon, which is characteristic of this disease compared to occlusive forms of ischaemia |
| Vascular | |||
| Thrombi and/or emboli | |||
| CT with IIC | Artery filling defects | Vein filling defects | No thrombi and/or emboli present |
| CT without IIC | High attenuation | High attenuation | No thrombi and/or emboli present |
| Abdominal cavity | |||
| Trabeculation of mesenteric fat and ascites | If reperfusion, infarction and/or intestinal perforation | Common findings | If transmural infarction and/or intestinal perforation |
| Intestinal pneumatosis, portomesenteric venous gas and peritoneal free gas | If transmural infarction and/or intestinal perforation | If transmural infarction and/or intestinal perforation | If transmural infarction and/or intestinal perforation |
IIC: iodinated intravenous contrast; CT: computed tomography.
In acute artery occlusion, the intestinal wall involved looks thinner and may have the thickness of paper when ischaemia is advancing towards a transmural infarction. Only if reperfusion occurs will a thickening thereof be observed.37,43,64
In the first stage of venous occlusion, when there is still arterial flow and the intestinal wall is viable, a thickening thereof is seen. In a subsequent stage, when ischaemia progresses towards transmural infarction, the intestinal wall appears thinner. This condition usually spares the colon, unlike arterial causes.73
In NOMI, findings are similar to those observed in acute arterial ischaemia.43,61
Attenuation of the intestinal wallIn acute ischaemia of arterial origin, arterial blood supply decreases or ceases, resulting in reduced or absent intestinal wall uptake (Fig. 8).25,63 In cases in which reperfusion occurs, the intestinal wall appears thickened and may exhibit a “halo” or “bull's-eye” appearance on MDCT images with contrast, due to mucosal and serosal uptake, and to submucosal oedema.37,43,64
Abnormality in intestinal parietal uptake. (A) Abdominal and pelvic CT scan, axial plane, with iodinated intravenous contrast in an arterial phase (A) and in a portal phase (B). Loops of small intestine with decreased parietal uptake suggestive of ischaemia of arterial origin (white arrows) and mild striation of fat due to oedema are observed (white arrow tips).
In the first stage of MVT, the intestinal wall affected may show a “halo” or “bull's-eye” uptake pattern, due to uptake by the mucosa and the serosa and the absence of uptake by the submucosa and the muscularis propia (Fig. 3).23,74 When transmural infarction occurs, intestinal wall uptake is absent or decreased in MDCT with contrast.73
In NOMI, MDCT with contrast shows decreased or absent uptake by the intestinal wall in the ischaemic phase (Fig. 4) or during ineffective reperfusion, whereas in the effective reperfusion phase uptake may be increased.
MDCT without contrast may show low attenuation of the intestinal wall which indicates oedema in cases in which there is reperfusion, or high attenuation which generally represents the presence of intramural haemorrhage or haemorrhagic infarction due to ineffective reperfusion.43
Vascular findingsIn MAE, emboli may have high attenuation on MDCT without contrast and may cause defects in filling close to the origin of the medial colic artery on MDCT with contrast (Fig. 1A).64
In MAT, MDCT without contrast may show calcifications due to atherosclerotic changes, and stenosis or luminal occlusion often occurs in the first 2cm from the origin of the SMA (Fig. 1B).64 Caution should be exercised in distinguishing between MAT and chronic mesenteric ischaemia, since symptoms and appearance of vascular findings on MDCT are similar, especially in cases of critical artery stenosis. The presence of abnormal findings in the intestinal wall and the surrounding mesentery are necessary for their differentiation.37
In MVT, thrombi in the mesenteric veins appear as luminal filling defects (Figs. 2 and 3),47,73,74 and the vessels show an increase in normal calibre.
In an early phase of NOMI, vascular findings on MDCT overlap with those on angiography. In particular, original axial images and multiplanar reconstruction (MPR) images show an irregular narrowing of the SMA and the IMA, spasm of the arches of the mesenteric arteries and deficient intramural vessel filling.75
Abnormalities in the abdominal cavityIn acute arterial ischaemia, trabeculation of mesenteric fat and ascites are rare early in the course of the disease; they appear when reperfusion, transmural infarction and/or intestinal perforation occur.25,63 In these situations intramural gas (pneumatosis) may be observed in the mesenteric veins and portal vein, in addition to extraintestinal gas if there is perforation. (Figs. 9 and 10)37 However, it must be borne in mind that pneumatosis is not a specific finding of ischaemia, and it may be seen in various non-ischaemic conditions.76 Therefore, these findings should be interpreted with caution, taking into consideration that isolated intestinal pneumatosis with no other findings of ischaemia should not trigger a definitive diagnosis of intestinal ischaemia.61
Intestinal pneumatosis and portomesenteric venous gas. MPR on a sagittal plane (A) and MPR on a coronal plane (B) of an abdominal and pelvic CT scan with iodinated intravenous contrast in a portal phase. An 85-year-old patient who sought care due to abdominal distension, tympanism, diffuse abdominal pain and signs and symptoms suggestive of bowel obstruction for the past 24h. Gas in superior and portal mesenteric veins (white arrows) extending to intrahepatic portal branches and arranged peripherally (white arrow tips), unlike the presence of gas in the bile ducts (aerobilia). Absence of uptake in the intestinal wall and pneumatosis (black arrows). All this in a context of an intestinal ischaemic process.
Ischaemic intestinal perforation. MPR on a sagittal plane (A) and MPR on a coronal plane (B), from an abdominal and pelvic CT scan with iodinated intravenous contrast in a portal phase. Loops of small intestine with abundant fluid content in relation to paralytic ileus (black arrows). Loops of small intestine with limited parietal uptake (white arrows) and a thin sheet of adjacent free liquid (white arrow tips). Pneumoperitoneum (black arrow tips) and periportal gas (thin white arrows).
In MVT, trabeculation of mesenteric fat and ascites (Figs. 2 and 3) are present in almost all patients; therefore, they are not necessarily suggestive of disease severity.61 In cases with irreversible infarction pneumatosis, portomesenteric venous gas and extraintestinal gas may also be seen; the latter appears when there is perforation of the affected loop.37
In NOMI, trabeculation of mesenteric fat and ascites may be observed; as in arterial occlusive mesenteric ischaemia, it appears only when there is reperfusion, transmural infarction and/or intestinal perforation.43
Use of imaging tests in therapeutic managementImaging studies play a very important role in AMI diagnosis and treatment planning. They enable visualisation of the cause of ischaemia and assessment of the extent of intestinal impairment.49 They are essential to taking a proper therapeutic approach, which is different in occlusive and non-occlusive forms. They also aid in evaluating treatment effectiveness and guide surgical excision when radiological signs of irreversible necrosis of the intestinal tract are detected.43 A therapeutic alternative in cases in which this necrosis does not occur is endovascular management. This can be applied in ischaemia caused by embolism or thrombosis, where fibrinolytic drugs and vasodilators such as streptokinase, urokinase and recombinant tissue plasminogen activator are infused, or mechanical devices such as balloon catheters and endoprostheses are used, and to cases of ischaemia due to low output, which seem to benefit from infusion of vasodilators such as papaverine.9
ConclusionAcute mesenteric ischaemia represents an abdominal emergency with a high mortality rate. Early diagnosis and treatment are essential for improving the patient's prognosis. As symptoms and laboratory tests are non-specific, radiologists play a critical role in its diagnosis; MDCT with contrast is the imaging technique of choice. This techniques enables determination of the suspected aetiology (occlusive causes [embolism or arterial or venous thrombosis] or non-occlusive causes [low output]), offers indications of severity (detection of signs of intestinal wall ischaemia, ascites, trabeculation of mesenteric fat, pneumatosis, pneumoperitoneum and/or portomesenteric gas) and helps in treatment planning (surgery or endovascular treatment). Therefore, it is essential for radiologists to be familiar with vascular and intestinal mesenteric anatomy, as well as the pathophysiology, epidemiology, risk factors, signs and symptoms, and imaging findings characteristic of the different aetiologies of AMI.
Authors’ contributions- 1.
Study integrity: RNC, LMC, AEC and DIM.
- 2.
Study concept: RNC, LMC, AEC and DIM.
- 3.
Study design: RNC, LMC, AEC and DIM.
- 4.
Data acquisition:
- 5.
Data analysis and interpretation:
- 6.
Statistical processing:
- 7.
Literature search: RNC, LMC, AEC and DIM.
- 8.
Drafting of the study: RNC, LMC, AEC and DIM.
- 9.
Critical review of the manuscript with intellectually significant contributions:
- 10.
Approval of the final version: RNC, LMC, AEC and DIM.
None.
Conflicts of interestThe study's promoter and first author, as well as all of the study's other authors, declare that there was no economic funding and that there were no conflicts of interest in the conduct of this study.
We would like to express our appreciation for the support and assistance of all our colleagues who contributed to this study, in particular Marta Calvo Imirizaldu and Isabel Vivas Pérez, from the Radiology Department at the Clínica Universidad de Navarra [University Clinic of Navarre], for their iconographic contribution to this study. We would also like to thank Adrià Esplugues Vidal for his help and his capacity for making things easier.
Please cite this article as: Navas-Campo R, Moreno-Caballero L, Ezponda Casajús A, Ibáñez Muñoz D. Isquemia mesentérica aguda. Revisión de las principales técnicas y signos radiológicos. Radiología. 2020;62:336–348.