Reversible Cerebral Vasoconstriction Syndrome (RCVS) is a not very well known clinical-imaging entity; it is characterized by thunderclap headache, which mimics an aneurysmal subarachnoid haemorrhage, and a diffuse and segmental constriction of cerebral arteries, that resolves spontaneously within 3 months. The pathophysiology remains unknown. The female gender is the more affected and more than half of cases occur in the puerperium or after exposure to vasoactive substances. Typically, RCVS is self-limited and has a benign course, although it may have more serious complications with permanent neurologic sequelae and death. Treatment is predominantly supportive and directed to the symptoms.
Reversible Cerebral Vasoconstriction Syndrome (RCVS) is a cerebrovascular disease characterized by severe headache, mimicking aneurysmal subarachnoid haemorrhage,1 with or without other acute neurologic symptoms, and noninflammatory, nonatherosclerotic diffuse segmental constriction of cerebral arteries, which resolves spontaneously within 3 months.2 For nearly 30 years, it has been described in several clinical settings, with other names, such as post-partum angiopathy,3 Call syndrome,4 central nervous system (CNS) pseudovasculitis,5 benign angiopathy of the CNS.6,7
In 2007, Calabrese et al. proposed the term RCVS, unifying all of these angiopathies with similar clinical course and angiographic characteristics, and a set of diagnostic criteria.8 In the following years, the publication of two large prospective cohorts9–14 and one large retrospective series,15 provided a better recognition of this syndrome.
RCVS is clinically important as it occurs in various clinical settings, in young people, especially females, in which the differential diagnosis with other more serious entities is urgent because it implies different attitudes and therapeutic strategies. It typically has a benign course but serious complications arise in more than 10% of patients, particularly ischaemic and haemorrhagic strokes, and even death.
The aim of this study was to perform a narrative review of RCVS in order to alert the medical community about the importance of early diagnosis and proper treatment of the most serious complications of this disease and the need for research to clarify in an objective manner the pathophysiology and therapeutic orientation.
MethodsWe searched PubMed using the term “reversible cerebral vasoconstriction syndrome” and we selected review articles from 01/01/2010 to 31/01/2015; the list of bibliographic references of the review articles considered were also screened, totalling 81 articles.
DefinitionRCVS is characterized by recurrent acute severe headaches which may be associated with seizures and focal neurological deficits. There is no specific laboratory investigation and cerebrospinal fluid (CSF) analysis is normal in most cases. Cerebral angiography shows alternating segmental constrictions and dilations. However, computed tomography (CT), magnetic resonance imaging (MRI) and angiography may be normal until the first week of evolution of the disease. It is a self-limited entity, with headache resolution and subsequent disappearance of arterial changes from 1 to 3 months after the onset of symptoms. Meanwhile, severe complications can occur with permanent neurological damage and death.
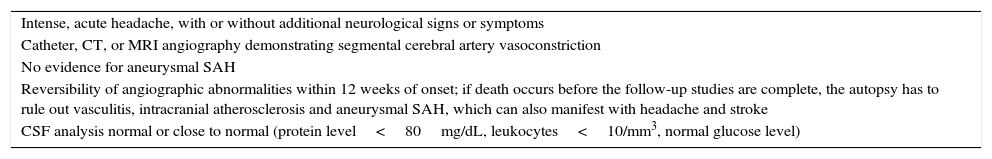
A set of diagnostic criteria is not universally accepted; however those proposed by Calabrese et al. (Table 1)8 are the basis for diagnostic approach.
Diagnostic criteria of the reversible cerebral vasoconstriction syndrome.
| Intense, acute headache, with or without additional neurological signs or symptoms |
| Catheter, CT, or MRI angiography demonstrating segmental cerebral artery vasoconstriction |
| No evidence for aneurysmal SAH |
| Reversibility of angiographic abnormalities within 12 weeks of onset; if death occurs before the follow-up studies are complete, the autopsy has to rule out vasculitis, intracranial atherosclerosis and aneurysmal SAH, which can also manifest with headache and stroke |
| CSF analysis normal or close to normal (protein level<80mg/dL, leukocytes<10/mm3, normal glucose level) |
CT, computed tomography; MRI, magnetic resonance imaging; SAH, subarachnoid haemorrhage; CSF, cerebrospinal fluid.
Despite the aetiological heterogeneity, clinical manifestations are similar.
RCVS has a typical acute and self-limited course without new symptoms after 1 month.2,13
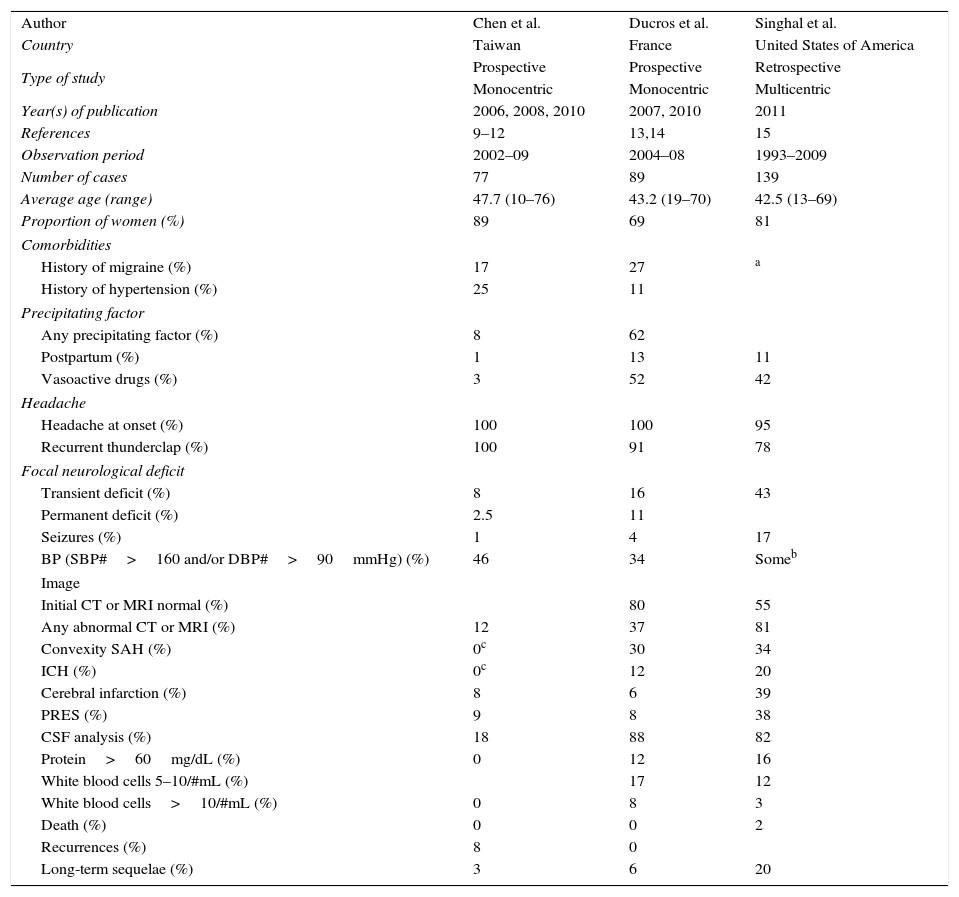
The main symptom in 95–100% of cases is headache2 and it can be the only symptom8,13,16–19 (Table 2). It is typically a thunderclap headache (TH) which reaches maximum intensity in seconds, mimicking the onset of an aneurysm ruptured headache. On average, it lasts 3h,20 and repeats four times over a period of 1–4 weeks,2,9,10,13 often triggered by sexual activity, stress, Valsalva manoeuvre and/or emotions. The typical headache is bilateral, although it may be unilateral, starting in the occipital region, followed by diffuse pain,2 and often accompanied by nausea, vomiting, photophobia and phonophobia. In some cases, the headache does not correspond to the definition of TH, being less intense or more progressive; the presentation of other symptoms without the headache is exceptional.2 If accompanied by neck pain, it is especially necessary to exclude cervical artery dissection.12,13,21 Although patients may have migraine as a comorbidity, they report the TH of RCVS as having location, degree and quality different from their usual headache.20,22 This entity may also be associated with focal neurological deficits, transient or permanent, and seizures, as reported in three major series (Table 2), in 8–43% and 1–17% of cases, respectively.2,11,13–15
Summary of the three main series of reversible cerebral vasoconstriction syndrome.
| Author | Chen et al. | Ducros et al. | Singhal et al. |
| Country | Taiwan | France | United States of America |
| Type of study | Prospective | Prospective | Retrospective |
| Monocentric | Monocentric | Multicentric | |
| Year(s) of publication | 2006, 2008, 2010 | 2007, 2010 | 2011 |
| References | 9–12 | 13,14 | 15 |
| Observation period | 2002–09 | 2004–08 | 1993–2009 |
| Number of cases | 77 | 89 | 139 |
| Average age (range) | 47.7 (10–76) | 43.2 (19–70) | 42.5 (13–69) |
| Proportion of women (%) | 89 | 69 | 81 |
| Comorbidities | |||
| History of migraine (%) | 17 | 27 | a |
| History of hypertension (%) | 25 | 11 | |
| Precipitating factor | |||
| Any precipitating factor (%) | 8 | 62 | |
| Postpartum (%) | 1 | 13 | 11 |
| Vasoactive drugs (%) | 3 | 52 | 42 |
| Headache | |||
| Headache at onset (%) | 100 | 100 | 95 |
| Recurrent thunderclap (%) | 100 | 91 | 78 |
| Focal neurological deficit | |||
| Transient deficit (%) | 8 | 16 | 43 |
| Permanent deficit (%) | 2.5 | 11 | |
| Seizures (%) | 1 | 4 | 17 |
| BP (SBP#>160 and/or DBP#>90mmHg) (%) | 46 | 34 | Someb |
| Image | |||
| Initial CT or MRI normal (%) | 80 | 55 | |
| Any abnormal CT or MRI (%) | 12 | 37 | 81 |
| Convexity SAH (%) | 0c | 30 | 34 |
| ICH (%) | 0c | 12 | 20 |
| Cerebral infarction (%) | 8 | 6 | 39 |
| PRES (%) | 9 | 8 | 38 |
| CSF analysis (%) | 18 | 88 | 82 |
| Protein>60mg/dL (%) | 0 | 12 | 16 |
| White blood cells 5–10/#mL (%) | 17 | 12 | |
| White blood cells>10/#mL (%) | 0 | 8 | 3 |
| Death (%) | 0 | 0 | 2 |
| Recurrences (%) | 8 | 0 | |
| Long-term sequelae (%) | 3 | 6 | 20 |
BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SAH, subarachnoid haemorrhage; ICH, intracerebral haemorrhage; PRES, posterior reversible encephalopathy syndrome; CSF, cerebrospinal fluid.
The seizures can be at the onset; their recurrence is rare.2,3,22–24 Transient focal deficits are present in little more than 10% of patients, last 1min to 4h, they are often visual, but can also be motor, sensory or dysphasic.2 The majority have sudden onset and are similar to a transient ischaemic attack (TIA), though they can mimic positive symptoms of a migraine aura.2,13
Neurological deficits exceeding 12h are not likely to improve. Permanent deficits suggest a stroke and include hemiplegia, aphasia, hemianopia or cortical blindness.2,7,14,15,23 Cerebellar strokes may also occur.
In more than a third of patients, hypertensive peaks (systolic blood pressure>160mm Hg) occur during headache crisis.11,13,23 It is not clear whether this is due to the pain, a response to the vasoconstriction of arteries, or integrates the manifestations of the disease.
Risk/susceptibility factors and complicationsFemale gender appears to be a risk factor in the development of RCVS,25 which is also more severe in women than in men.13,14
Physiological changes during pregnancy and the postpartum period appear to increase susceptibility to develop this disease,25 as well as hormonal therapy,26 pharmaceutical vasoconstrictors, including those used in the treatment of migraine,27 which is more common in women, and the use of recreational drugs25; genetic factors appear to be the basis of individual susceptibility or can affect severity and clinical course.12,26
More than 90% of patients have a good prognosis.25 However, 10% of patients have severe complications15 that can lead to death. Complications include cerebral infarction (4–40%), intracerebral haemorrhage (ICH) (6–20%), subarachnoid haemorrhage (SAH) (22–34%) and posterior reversible encephalopathy syndrome (PRES) (less than 10%).9,13,15,25 These values of incidence reported may be the result of bias in recruitment of participants (perhaps with the most symptomatic patients seeking medical help) selection criteria, and the context of each patient.2,16 For example, the cases of ischaemic stroke and ICH in patients who developed postpartum RCVS described by Fugate et al. appear to be greater than those described in Ducros et al.16,28,29
Haemorrhagic complications (parenchymal and SAH), PRES and seizures often occur in the first week of the disease.10,13,16,30,31 In contrast, ischaemic events and resulting focal neurological deficits occur later, with a peak between the first and second week of RCVS onset. Brain ischaemia can occur even later in the course of the syndrome, occasionally after the resolution of symptoms such as headache, reflecting the well documented apparent delay in resolving cerebral vasoconstriction.11,16
The female gender and a history of migraine are independent risk factors for haemorrhagic complications.14 On the contrary, a history of hypertension and peaks of elevation in systolic blood pressure are not associated with the risk of haemorrhage.14 The identification of migraine, mainly with aura, as a risk factor for haemorrhagic complications, is particularly relevant, since previous studies have not highlighted migraine as a risk factor for global RCVS.9,13,14 More studies are needed to re-evaluate migraine as a risk factor for RCVS haemorrhagic complications, for RCVS, or both.14
AetiologyRCVS can occur spontaneously, without apparent cause, or be secondary to endogenous or exogenous precipitating factors (Table 3).
Potential aetiologies and associated conditions of RCVS.
| General categories | Specific entities |
|---|---|
| Primary (idiopathic) | Primary headaches (thunderclap headache, sexual activity, exercise) |
| Secondary | |
| Pregnancy | Postpartum, pre-eclampsia, eclampsia |
| Vasoactive substances | Recreational drugs: cannabis, cocaine, LSD, amphetamines, ecstasy, binge-drinking |
| Ergots: ergotamine tartrate, bromocriptine, methylergometrine, lisuride, methergine | |
| Sympathomimetics: ephedrine, pseudoephedrine, phenylpropanolamine, isometheptene, diet pills | |
| Serotonergic drugs: SSRI, triptans | |
| Immunosuppressants: tacrolimus (FK-506), cyclophosphamide, Interferon alfa | |
| Others: ginseng, liquorice, nicotine patches, indomethacin, oral contraceptive pills, hormonal ovarian stimulation for intrauterine insemination | |
| Catecholamine secreting tumours | Pheochromocytoma, glomus tumours, bronchial carcinoid tumour |
| Extra- or intra-cranial arterial disorders or procedures | Cervical artery dissection, aortic dissection, unruptured intracranial aneurysm, fibromuscular dysplasia, post carotid endarterectomy |
| Blood products | Erythropoietin, intravenous immunoglobulin, massive blood transfusion |
| Intracranial disorders or surgery | CSF hypotension, intracranial haemorrhage, spinal subdural haematoma, neurosurgery, head trauma |
| Miscellaneous | Systemic lupus erythematosus, Hypercalcemia, porphyria, microangiopathic haemolytic anaemia, autonomic dysreflexia, ascent to high altitudes |
LSD, lysergic acid diethylamide; SSRI, selective serotonin reuptake inhibitors.
There are differences in the results presented by the series (Table 2). In Chen et al., 96% of cases of RCVS were idiopathic, in contrast to the 37% in Ducros et al. In the Ducros’ series,13,14 the use of vasoactive drugs contributed to most cases of RCVS (52%), with postpartum being the second most common cause with 13% of cases. With respect to drugs, cannabis was the most recorded, particularly in males. Despite being the most commonly consumed illicit drug,32 it had until now been rarely implicated in this syndrome.20,33 Other vasoactive drugs are pointed to, especially in females, namely selective serotonin reuptake inhibitors and nasal vasoconstrictors.
Ducros et al.13 suggests two new precipitating factors: binge-drinking, at least in combination with vasoactive substances, which is already considered a risk factor for stroke and a precipitating factor previously associated with RCVS,34 and the use of interferon alpha, previously implicated in a case of multiple brain infarcts35 and in another one with PRES,36 though both without documented cerebral vasoconstriction. Ducros et al.13 even underlines the need for a case–control study to assess the strength of the association between these risk factors and RCVS.
The results obtained by Singhal et al.15 corroborate those obtained by Ducros et al.13 Other potential precipitating factors have been mentioned in literature, especially in isolated observations, and some raise doubts about the nosological boundaries between RCVS and PRES.37,38
The epidemiological differences in the Western and Taiwanese series can be due to bias in the recruitment of participants or to the consumption of illicit substances.37
Time latency may vary between exposure to a precipitating factor and the development of RCVS, which can be a few days to several months.17 This finding and the ubiquity of precipitating factors may be a result of chance in part of these associations.8,16,17,28,39–51
In spontaneous cases, there may be a genetic predisposition or there might be some unknown aetiologies that make individuals particularly susceptible.16
PathophysiologyThe pathophysiology of this syndrome remains an enigma, it seems to be a multifactorial process.
The dysfunction of the vascular tone regulation appears to be the central element in the pathophysiology.8,13,17,19,25,52 A rapid change of vascular tone can lead to vasoconstriction and vasodilatation of segmental small vessels and the sudden stretching of arterial walls can cause the TH in the initial phase.14,26 This appears to be induced by sympathetic stimulation, endothelial dysfunction and oxidative stress.34–36,49,50 The association of RCVS with outbreaks of hypertension, vasoactive substance intake and phaeochromocytoma support the role of sympathetic stimulation. On the other hand, the overlap with PRES supports the role of endothelial dysfunction. Some hormones and biochemical factors have been involved in vascular tone dysregulation, namely endothelin-1, oestrogen, serotonin, nitric oxide and prostaglandins. For example, levels of 8-iso-prostaglandin F2alfa, a marker of oxidative stress and a potent vasoconstrictor, seem to be related to the severity of the syndrome, thus supporting the role of oxidative stress. Other factors, including the placental growth factor, soluble placental growth factor receptor and soluble endoglin, play a role in angiogenesis and have been implicated in postpartum RCVS.31,52
Genetic factors may be the basis of individual susceptibility to develop RCVS and in the severity of its clinical course.52 Recently, the Brain-derived neurotrophic factor gene (BDNF) polymorphism (Val66Met), which is important for neuronal survival, neurogenesis and synaptic plasticity, has been associated with severe vasoconstriction in patients with RCVS.12,52
EpidemiologyRCVS occurs in all age groups, including paediatric patients.11,53,54 The peak occurrence is at 42 years of age, though a decade earlier in men13,19,20 and is more common in women.
Incidence is unknown, although the syndrome does not appear to be rare, considering cases reported in retrospective and prospective studies.2 Recent work even suggest a possible increase in incidence; however it remains unclear if this is due to a real increase in new cases or whether it is a consequence of the improvement of imaging techniques and awareness of the disease.52,55 Nevertheless, RCVS probably remains misdiagnosed and should be included in the list of differential diagnoses in younger patients with cryptogenic stroke or intense headache.16,56 Cases have been reported in all continents and the three great series from Asia, Europe and North America have documented various forms of presentation, from the most common, benign, to the rarest, deadly2 (Table 2). Despite the differences observed between sets, it is not possible to say whether this is due to ethnic differences or differences in the recruitment criteria of the participants.2
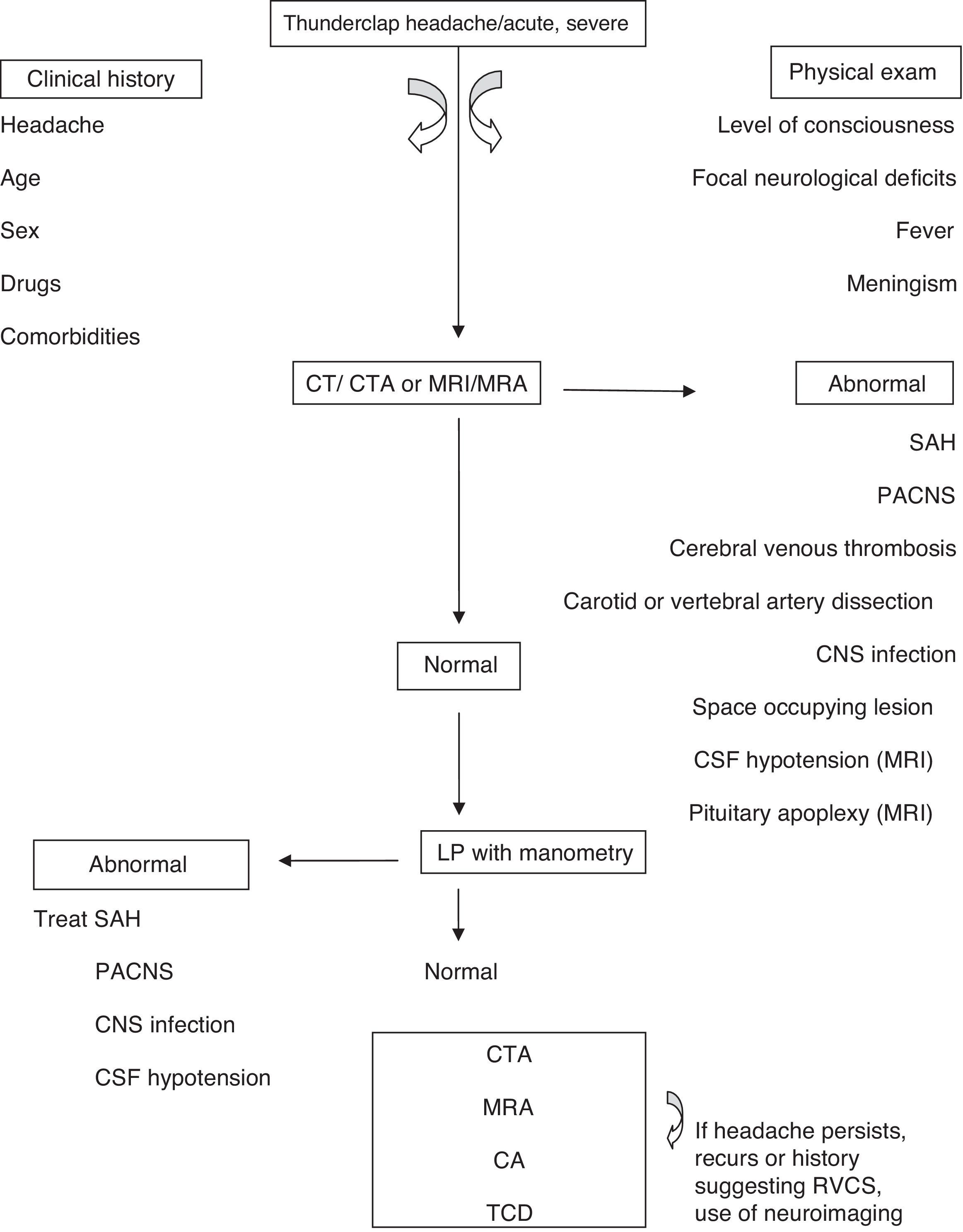
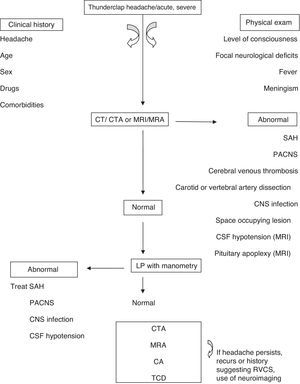
DiagnosisAs TH is the main symptom, it is necessary to explore it thoroughly, since there are other diseases that present with TH as well, although with some particularities as will be discussed.
Considering the algorithm (Fig. 1) and the proposed diagnostic criteria by Calabrese et al., a good clinical history is needed, with characterization of the headache, past medical history, usual medication, comorbidities and a complete physical examination, to discard the presence of meningeal signs and fever, to evaluate the level of awareness and the presence of focal neurological deficits. After exclusion of the most common pathologies by neuroimaging and study of the CSF, additional studies are necessary for the characterization of the cerebral vasculature. The complete blood count and biochemistry are generally normal. Exclusion of vasculitis and vasoactive tumours (carcinoid and pheochromocytoma) is useful. Additionally, it is important to screen serum and urine for vasoactive drugs.
An algorithm for RCVS diagnosis. CTA, computed tomography angiography; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; PACNS, primary angiitis of the central nervous system (CNS); CA, catheter angiography; TCD, transcranial Doppler.
The diagnosis is based on angiographic exams and requires the initial demonstration of diffuse segmental vasoconstriction of cerebral arteries, as well as its reversibility (complete or marked normalization of arteries) in 12 weeks after onset of the disease.8
Initially, 55–80% of patients have a normal CT or MRI (Table 2). However, in subsequent images, 12–81% of patients have changes consistent with SAH, convexity ICH, cerebral infarction and PRES. Haemorrhagic complications are the most frequent.
Particular attention must be payed to the ICH, which seems to be more common in women and people with migraine.14 It occurs early in the course of RCVS, revealing itself in most cases by a TH with a persistent focal deficit. Attention is also important to PRES, due to clinical and imaging overlapping with RCVS and the possibility of simultaneous occurrence.3,38,57,58 More than 85% patients with PRES demonstrate cerebral vasoconstriction similar to RCVS in conventional angiography. Reversible cerebral oedema alike PRES may also occur in 9–38% of patients with RCVS.2,16
Regarding imaging, catheter cerebral angiography is the gold standard diagnostic exam. It allows a real-time assessment of the calibre and flow of vessels, provides a good view of the small and peripheral vessels, with good spatial and temporal resolution, and allows for acting therapeutically with intra-arterial vasodilators. It has the disadvantage of being an invasive method, with potential vascular damage and stroke,59 and it is impractical for follow-up. In one of the series by Ducros et al.13, over 9% of patients suffered transient neurologic deficits after cerebral angiography.13 This type of angiography should be reserved for cases whose diagnoses raise additional questions.26
CT and MRI angiographies (CTA and MRA) allow vascular diagnosis, with the advantage of not being intrusive.
CTA overcomes the limitations of conventional angiography, allowing a good assessment of vasoconstriction in patients with RCVS.9 It has been singled out as a specific and very sensitive tool in the assessment of intracranial vasculature,60 however it is not feasible for sequential follow-up due to exposure to radiation and contrast.
Chen et al. demonstrated that the assessment with MRA is valid.11 Transcranial Doppler (TCD) imaging has long been used and validated in the study of intracranial vessel spasm,59–61 allowing sequential monitoring. It is a non-invasive method, innocuous, with high reproducibility, which makes it possible to evaluate the vessel vasoconstriction and monitor haemodynamic changes in patients with RCVS. According to a study, cerebral vasoconstriction indicators, including high mean flow velocity of the middle cerebral artery (>120cm/s) and a high hemispheric index (>3), were associated with an increased risk of developing PRES or ischaemic stroke.10,16 However, the acceleration of the velocity in arteries at the base of the skull do not often reach these values in RCVS as in aneurysmal SAH and the focal character and the temporal evolution of the flow velocities should be valued in this case. The TCD has the disadvantage of not allowing the evaluation of small vessels.56
It is necessary to keep in mind that the arterial vasoconstriction may not be evident in the first angiographic study; in cases with high clinical suspicion, the repetition of these exams is indicated.62 As suggested by Ducros et al.,13 the segmental vasomotor dysregulation seems to start in the most peripheral arterioles, progressing centripetally to the medium and large calibre cerebral arteries, which are viewed more easily.52,63 This centripetal progression can explain the time lag between headaches and vasoconstriction, given that headaches appear before any detectable vasoconstriction and this extends on average 14 days after headache resolution. Therefore, headache may be due to the involvement of small distal arteries, whose rapid changes of calibre (constriction or dilation) stimulate perivascular pain-sensitive fibres.13
In imagining, the main differential diagnosis, after aneurysmal HSA is ruled out, is CNS vasculitis (primary angiitis of the CNS, PACNS).26,64 This distinction is important, though difficult, because the treatment is quite different.7,8,17,52 The resolution of the cerebral vasoconstriction in the mid-term angiographic control allows for the differentiation between these two entities. In addition, advanced techniques of MRI that evaluate the arterial wall can help the differential diagnosis at an early stage of the disease, because there are abnormal captures of the contrast product on the wall of the involved arteries in PACNS.62 Additionally, the headache of the vasculitis has an insidious onset, progressing gradually, and thus distinct from that typical of RCVS,7,8,17,30,65,66 and it is more common in older men, unlike RCVS that is typical in young/middle aged women.52,65,67 There are also differences in the CSF analysis because, unlike RCVS, in vasculitis it is not normal, with proteins and leucocyte counts increase with values generally >100mg/dL and 5–10cells/mm3, respectively.8,15,17,30,40,52,65 Finally, patients with vasculitis have a fulminant course, with poor prognosis, particularly if treatment with immunosuppressant steroids and cytotoxic agents is delayed; however, lack of these drugs benefits RCVS.17,52,65,67
Another differential diagnosis to be noted is cervical artery dissection, as it evolves with headache/neck pain and can even be associated with RCVS.20
TreatmentThere is no established treatment for RCVS25 and there is no form of treatment which clearly modifies the natural history of vasoconstriction.3 The therapeutic approach is based on observational studies and the experience of the doctor. However, the timely recognition of the syndrome is important. All patients with suggestive clinical history, no evidence of another cause for the symptoms, and initial normal angiograms, should be considered as having possible or likely RCVS and thus receive the same symptomatic treatment as patients with proven vasoconstriction.2
All patients need symptomatic treatment, which includes the discontinuation of any vasoactive drug2,6,15,20 and the suspension of Valsalva manoeuvres or other headache precipitating factors for a few days to weeks, depending on the severity of the headache.
Treatment includes analgesics, antiepileptic drugs for seizures, blood pressure monitoring and admission to the intensive care unit in severe cases.2
Drugs targeting the vessel spasm should be considered when cerebral vasoconstriction is documented. Vasodilators, including calcium channel blockers with systemic action, by reducing systolic blood pressure, can compromise the cerebral perfusion in patients with severe vasoconstriction, and should therefore be used with caution. Nimodipine is the most used and may have a more elective action on cerebral circulation. Despite reducing the number of episodes and the intensity of the headache, prospective and retrospective studies suggest that it does not affect the time course of cerebral vasoconstriction. It may be given orally or intravenously at a dose used for prevention of vessel spasm in aneurysmal SAH. The duration of treatment should range from 4 to 12 weeks.2 New bleeding, TIA and stroke are reported in some patients, even if treated for several days.2,13,68 Other drugs include nicardipine,54 verapamil15 and magnesium sulphate (in the treatment of postpartum angiopathy).69
In the most severe cases, milrinone, nimodipine and epoprostenol administered intra-arterially, and balloon angioplasty with variable success have been used.2,29,70 These are delicate and high risk interventions, and should be restricted to patients who show clear signs of clinical progression.2,71
Glucocorticoids should be avoided because they are an independent predictor of poor prognosis.
PrognosisIn most patients, the course of this syndrome is self-limited and benign. However, complications are frequent and the prognosis may be unfavourable. In the American series, 81% of patients had brain image compatible with lesion (Table 2). Despite this, the rate of permanent neurological deficits is very low and deaths are rare.15 The recurrence rate was described as being about 8%.11 Cerebral infarct and ICH are predictors of poor prognosis.15
ConclusionRCVS is traditionally considered to have a benign and monophasic clinical course. However, it may present an unfavourable course with rapid progression of blood vessel spasm, or with hemorrhage, causing high morbidity, or even have a fatal outcome.
Multicentre retrospective and prospective studies can help to establish the true prevalence and incidence of this entity and its clinical presentation, with emphasis on atypical presentations.
The initial suspicion of RCVS should lead to clinical surveillance and intensive imaging, reaching a definitive diagnosis and ruling out other differential diagnosis, particularly those with specific treatments. The identification of potential precipitating agents, their eviction or control is essential.
This review highlights the importance of new research to further clarify the pathophysiology of this syndrome and thus to contribute to a more specific and effective therapeutic approach.
Conflicts of interestThe authors declare no conflicts of interest.