Infertility is a clinical condition that affects around 15% of reproductive-aged couples worldwide. Around half of these cases are due to male factors, the most owing to idiopathic causes. The increase of reactive oxygen species (ROS), which leads to oxidative stress (OS), has been discussed in the last years as a possible cause of male idiopathic infertility. Superoxide anion (O2•−) and nitric oxide (NO) can react with each other contributing to the formation of peroxynitrite (ONOO−). This molecule can then act on spermatozoa proteins, leading to nitration of protein tyrosines – addition of a nitro (NO2) group – that is then manifested by the formation of 3-nitrotyrosine (3-NT). In turn, 3-NT may be responsible for the alteration or inactivation of the protein function.
This review will focus on the description of spermatozoa ROS, namely O2•−, NO and ONOO− and in their contribution to protein tyrosine nitration, namely by 3-NT formation. Previous results about the effect of ONOO− and 3-NT in spermatozoa will be presented, as well as, the methods that can be performed to detect the protein oxidation by these species. The impact of measuring, at the clinical level, 3-NT, considered a marker of OS, in spermatozoa will be discussed.
According to World Health Organization, infertility is defined as a “disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse”.1 Currently, this clinical situation affects around 15% of the reproductive-aged couples worldwide, of which around half are directly or indirectly related with male infertility.2,3
In the last years, the increase of oxidative stress, which corresponds to an imbalance between the levels of reactive oxygen species (ROS) and the levels of antioxidant systems, where the ROS are increased, has been indicated as a factor that leads to male infertility.3–7
Reactive oxygen species are molecules and free radicals with one unpaired electron derived from molecular oxygen, which, in the ground state, is a bi-radical with two unpaired electrons with the same spin in the outer shell. As the electrons have the same spin, oxygen can only react with one electron at a time. However, if there is a change in any spin of the two electrons, which occurs when one of the two unpaired electrons is excited, the two electrons with opposing spins can quickly react with other pairs of electrons, especially through double bonds, resulting in powerful oxidant species.8
Human spermatozoa are characterized by a paucity of cytoplasm, which leads to a lack of an adequate reserve of defensive enzymes. Furthermore, human spermatozoa membranes are mainly constituted by polyunsaturated fatty acids. The combination of these two characteristics makes these cells very sensitive to oxidative attack by ROS that are generated by neutrophils or by defective or immature spermatozoa.9–11
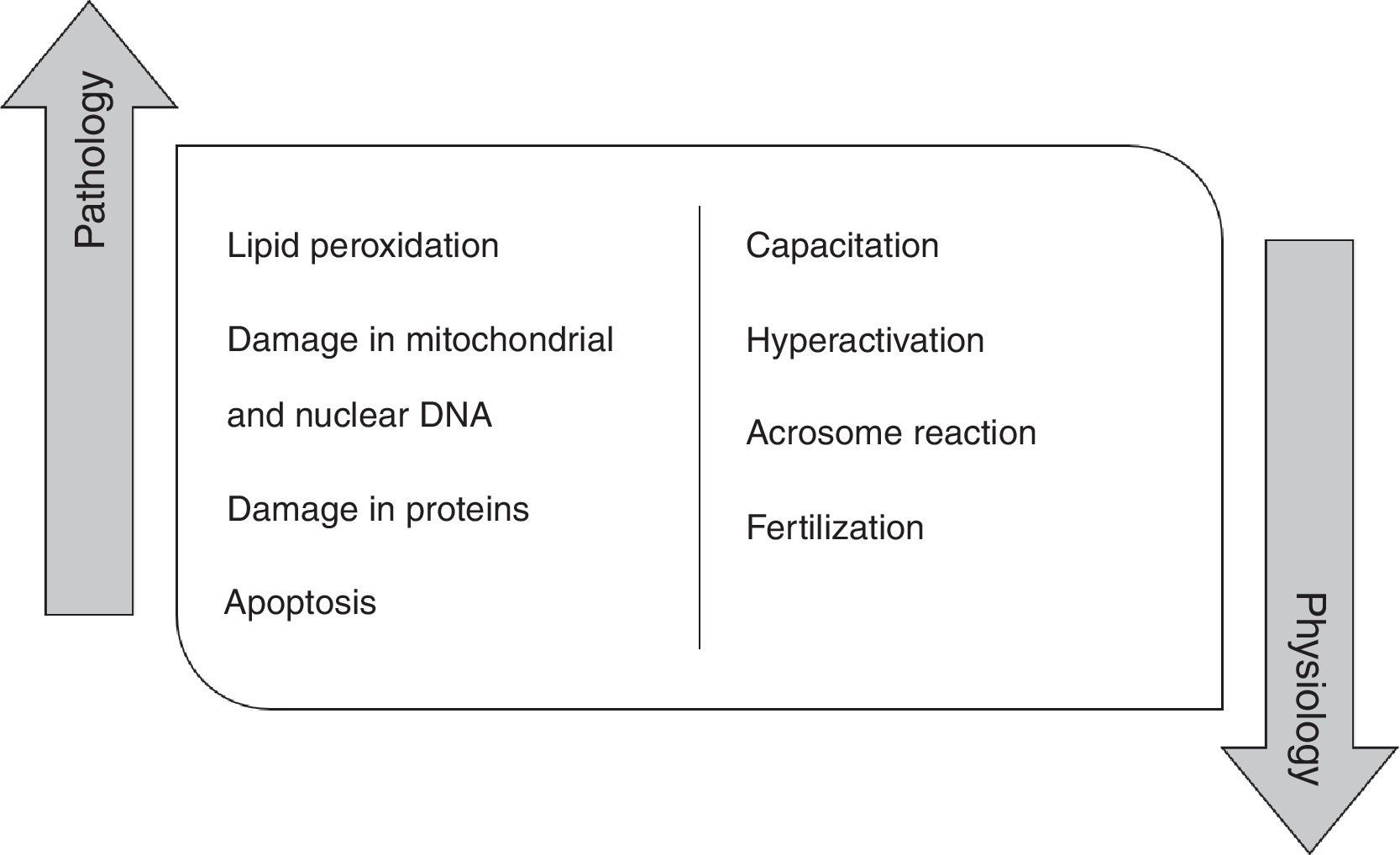
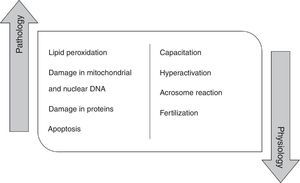
High concentration of ROS and reactive nitrogen species (RNS) induce pathologic effects in the spermatozoa biomolecules, namely in proteins, lipids and mitochondrial and nuclear DNA. In fact, the peroxidation of phospholipids of the plasma membrane promotes alterations in its fluidity, causing a loss of motility, as well as, a decrease of membrane enzymes and ionic channels activity, contributing to fertility impairment.4,12,13 ROS are also responsible for damaging mitochondrial and nuclear DNA in human spermatozoa due to their action on phosphodiester backbones and DNA bases, leading to its fragmentation.14,15 These species can also damage proteins, interfering with enzymatic activity or with structural protein function. At last, ROS promote a decrease in the number of spermatozoa by activating apoptosis 14. Indeed, it has been shown that antioxidants improve rat sperm viability during storage and in several disease condition, including diabetes.16–18
However, ROS do not play just a pathological role in the male germ cells (Fig. 1). Currently, it is believed that these molecular species have a physiological role when they are in an ideal concentration. Low ROS levels regulate fertilization, acrosome reaction, hyperactivation, motility and capacitation.19
This review will focus in the description of the ROS contributing to the protein oxidation, namely by tyrosine nitration. First, we will make a short introduction on superoxide anion (O2•−), nitric oxide (NO) and peroxynitrite (ONOO−), followed by a discussion on the contribution of these ROS to protein tyrosine nitration and the effects in the spermatozoa. Lastly, we will describe the methods that can be applied to evaluate the presence of peroxynitrite and 3-nitrotyrosine in the sperm cells and discuss their utility at the clinical level since they are considered markers of oxidative stress.
Reactive oxygen species involved in 3-nitrotyrosine formationSuperoxide anionSuperoxide anion is the product of the one-electron reduction of oxygen, which can be produced both enzymatically and non-enzymatically.8 Enzymatically, the sources of O2•− include NADPH oxidases, which are located on the cell membrane of several cells, such as polymorphonuclear cells, macrophages and endothelial cells20,21 and cytochrome P450-dependent oxygenases.8,22 Another enzymatic source of O2•−, but also of hydrogen peroxide (H2O2), corresponds to the proteolytic conversion of xanthine dehydrogenase to xanthine oxidase.23 In turn, the transfer of a single electron to oxygen by reduced coenzymes or prosthetic groups or by reduced xenobiotics corresponds to the non-enzymatic production of O2•−. In the majority of human tissues, the mitochondrial electron transport chain is the primary source of O2•−.8 Furthermore, O2•− can also result from oxidation of peroxide (O22−).24
The standard reduction potential for the conversion of molecular oxygen to O2•− is highly dependent on the nature of the medium. Mitochondria is the principal source of O2•−, since 0.2–2% of the oxygen consumed is transformed to O2•−.25,26 The electron transfer chain, namely complexes I and III, is responsible for the majority of the O2•− produced during cellular respiration. Superoxide anion is then released in the matrix and in the intermembrane space.8,26 Due to its anionic character (pKa=4.7), O2•− has a limited capacity to diffuse through membranes.26–28
Nitric oxideNitric oxide (NO) is a short-lived and highly reactive free radical that is produced in all mammalian cells during the oxidation of l-arginine to l-citrulline, by a family of NO synthase (NOS) isoforms.11,29,30
Three different NOS isoforms have been reported. Neuronal or brain NOS (nNOS), first described in neuronal tissues, and endothelial or constitutive NOS (eNOS or cNOS), first described in endothelial cells, which are Ca2+-calmodulin-dependent isoforms. Inducible NOS (iNOS), originally found in macrophages, is expressed only in response to inflammatory cytokines and lipopolysaccharides and is an isoform Ca2+-calmodulin-independent.11,30–33
The described isoforms can be found in human spermatozoa, namely in its head and/or flagellum regions, and their presence and activity depends on the maturity of male germ cells.34–36 However, as the iNOS produces higher NO levels and remains active for a longer time period, when compared to nNOS and eNOS, it is responsible for a more negative effect in the sperm function.31,32
The activation of cNOS is dependent on calcium levels, so when the intracellular levels of calcium increase, a cascade that leads to cNOS activation and to NO synthesis is initiated. In this process, the intracellular calcium binds to calmodulin, forming a calcium-calmodulin complex. This linkage also regulates the binding of calmodulin to the “latch domain”, which permits electron transfer from NADPH via flavin groups within the reductase domain to a haem-containing active site, thereby facilitating the conversion of O2 and l-arginine to NO and l-citrulline.37–39 In turn, activation of iNOS does not require alterations in intracellular calcium levels, because this isoform contains calmodulin tightly bound to each subunit of the enzyme, resulting in the permanent activation of the enzyme.39,40 Furthermore, calcium chelators have been shown to reduce cNOS activity significantly. Other factors can also regulate NOS activity, such as pre- and post-translational and transcriptional mechanisms.33,34 In sperm, which is virtually devoid of transcription, the post-translational modifications may be preponderant. Constitutive NOS can be linked to cell membranes due to the possession of a site for myristylation.43 Phosphorylation of cNOS by protein kinase C (PKC) or protein kinase A (PKA) is associated with the detachment of the enzyme from the cell membrane and loss of activity.44
Nitric oxide has the ability to diffuse through cell membranes, reaching the intracellular targets, where it can act through two different signaling pathways: cGMP-dependent signaling and cGMP-independent signaling.30,33,45 The first pathway involves the activation of soluble guanylyl cyclase (sGC), generation of 3′,5′-cyclic guanosine monophosphate (cGMP) and finally the activation of specific cGMP-dependent enzymes, such as protein kinases, channels and phosphodiesterases.45 The other pathway occurs through covalent post-translational modification of target proteins, such as S-nitrosylation, S-glutathionylation and tyrosine nitration.46–48 This last pathway needs a higher NO concentration to occur when compared with the activation of sGC.30
PeroxynitriteNitric oxide and O2•−, when at physiological conditions, are present at very low concentrations.49 These two molecules may combine to form peroxynitrite (ONOO−), by a reaction that occurs at 6.7×109mol−1/l/s and that is considered irreversible due to its highly exothermic nature.49–51
In contrast to NO and O2•−, ONOO− is not a free radical because the unpaired electrons of these radicals have combined to form a new N–O bond; however, it is a strong oxidant and nitrating agent.49,52
Like NO, ONOO− can diffuse for long distances into the cells and can even cross cell membranes due to its relative stability.52 This molecule can react with the biomolecules of the cells, namely proteins,53–56 lipids57 and DNA,58 but also with the sulfhydryl and tyrosyl groups on cells structures.59–61 These reactions may affect the function of signaling systems or also lead to the formation of NO−-releasing compounds. With respect to biomolecules, ONOO− can lead to the nitration of tyrosine residues in proteins, which gives rise to 3-nitrotyrosine (3-NT) that is known to compromise protein structure and function and to block tyrosine phosphorylation, a crucial event in signal transduction pathways. Peroxynitrite can also promote the oxidation of redox metal centers, DNA and lipids, and lead to nitrosation of cysteine residues in proteins.10,11,62
Peroxynitrite has a dual role with both deleterious and beneficial effects,63,64 which are dependent on the environment where it is inserted.65,66 The concentration of ONOO− is also a factor that is responsible for its beneficial or deleterious effects.49 At low concentrations, ONOO− is responsible for the stimulation of a metabolic response in human erythrocytes, which leads to an increase on the tyrosine phosphorylation and on lactate production. In turn, high concentrations of ONOO− are responsible for the inhibition of tyrosine phosphorylation and glycolysis.67
Tyrosine is an aromatic amino acid present in most proteins.68,69 This amino acid is mildly hydrophilic due to the rather hydrophobic aromatic benzene ring carrying a hydroxyl group. Tyrosine is mainly surface-exposed in proteins, being available for modifications, such as nitration, resulting in the formation of 3-nitrotyrosine (3-NT) or tyrosine nitrated proteins.69–72
As previously referred, ONOO− has the ability to react with several amino acids, which includes tyrosine, phenylalanine and histidine. These amino acids are modified through intermediary secondary species.54,69,73–75 Tyrosine nitration occurs with a maximum scale in physiological pH (pH 7.4); in turn, when the environment is under acidic or basic conditions, the nitration decreases.52,59 Furthermore, the presence of carbon dioxide and bicarbonate has a strong influence in peroxynitrite-mediated reactions and enhances nitration of aromatic rings (e.g., tyrosines).76,77 Another factor that accelerates nitration is the presence of transition metal ions, which can be present in the free form, such as Cu2+, Fe3+, Fe2+, or as complexes involving protoporphyrin IX (heme) or certain chelators – ethylene diamine tetraacetic acid (EDTA).78–80
Peroxynitrite and related RNS have the ability to promote the nitrosative stress, which is resultant from the oxidation and nitration of the aromatic side-chains of some amino acids, namely tyrosine and tryptophan.81,82 Oxidative stress promotes protein damage by direct oxidation of protein side-chains by ROS and RNS, but also by adduction of secondary products of oxidation of sugars (glycoxidation) or polyunsaturated fatty acids (lipid peroxidation).69,83 In turn, the protein oxidative damage can also occur due to alternate oxidants, such as hypochlorous acid (HOCl) and circulating oxidized amino acids, such as tyrosine radical generated by metalloenzymes (e.g. myeloperoxidase).69,84 The rates of ROS formation, antioxidants levels, but also the ability to eliminate ions of oxidized proteins, are responsible for the accumulation of oxidized proteins.69,85
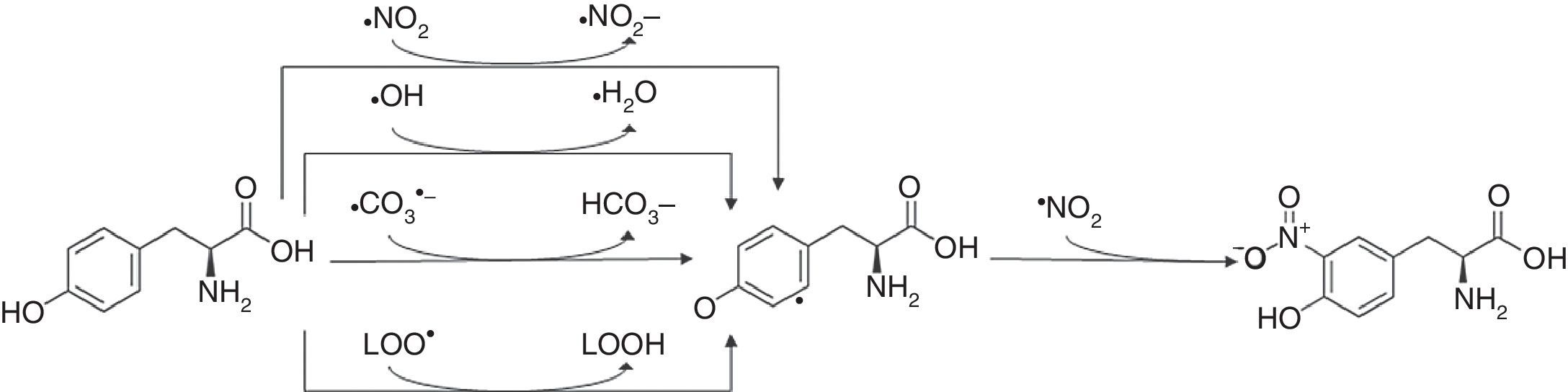
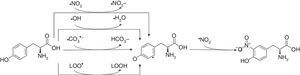
3-Nitrotyrosine corresponds to a post-translational protein modification and has been used as a marker of oxidative or nitrosative stress, mediated by ONOO− and other nitrogen free radical species (Fig. 2). The nitration of proteins may result in their inactivation. 3-Nitrotyrosine occurs through the action of a nitrating agent and consists in the addition of a –NO2 group in ortho position to the phenolic hydroxyl group of tyrosine.26,69,86,87 This process is performed in two different steps. Initially, tyrosine suffers oxidation, losing one hydrogen atom from the phenolic ring, producing tyrosyl radical (Tyr•), that can be performed in mitochondria by several one-electron oxidants such as carbonate radical (CO3•−) (k=4.5×107M−1s−1) hydroxyl radical (•OH) (k=1.3×1010M−1s−1), nitrogen dioxide (•NO2) (k=3.2×105M−1s−1) and in membranes by lipid peroxyl radicals (LOO•) (k=4.5×103M−1s−1). Carbonate radicals that react with tyrosine residues are produced by decomposition of nitrosoperoxycarboxylate (ONOOCO2), which is the product of the reaction between ONOO− and CO2. The protonated form of peroxynitrite generates •OH and •NO2, due to hemolytic decomposition. The second step of 3-nitrotyrosine formation is characterized by the addition of •NO2 (k=3.9×109M−1s−1) to the Tyr• in a radical–radical termination reaction.23,44,85,86
3-Nitrotyrosine formation. The reaction of tyrosine with peroxynitrite leads to the formation of 3-nitrotyrosine.
Protein tyrosine nitration is not a random process where all tyrosine residues are nitrated; in fact, this process exhibits a certain degree of selectivity.90 Then, the nitration of protein tyrosine residues, present mostly in specific functional domains of the proteins, promotes protein structure and conformation alterations impacting protein function.69,80,50,91
Besides the formation via peroxynitrite, nitrotyrosine can also be produced by peroxidases-catalysed oxidation of nitrite (NO2−) and tyrosine by hydrogen peroxide (H2O2).92 This ROS performs a two-electron oxidation of the heme-peroxidase forming Compound I, which is reduced monovalently by NO2− leading to the production of NO2 and Compound II, which will be then reduced to the resting enzyme by another molecule of NO2− producing a second molecule of NO2.26,93 Nitrogen dioxide can promote nitration of tyrosine, free or protein-bound.26,86,94
The effect of protein tyrosine nitration in sperm cellsWe have studied the formation of 3-NT in spermatozoa and tried to correlate it with seminal parameters, such as concentration, motility or morphology, as well as, with the oxidative balance of the spermatozoa. The results of this analysis did not show any correlation between the seminal parameters and the presence of 3-NT. In turn, nitration of proteins showed a negative correlation with the presence of the antioxidant protein superoxide dismutase (SOD), which means that when SOD levels increase there is a decrease in 3-NT levels. This correlation can be justified by the fact that SOD promotes the conversion of O2•− in hydrogen peroxide (H2O2), decreasing the levels of O2•−, which leads to an exponential decrease in the formation of ONOO−.95
Vignini et al. showed a significant correlation between the ONOO− concentration and the percentage of total sperm motility in 69 infertile patients affected by idiopathic asthenozoospermia and 29 normal fertile donors. Furthermore, by Computer Assisted Semen Analysis (CASA) evaluation, they also found a significant linear negative correlation between peroxynitrite concentration and curvilinear velocity (VCL), straight progressive velocity (VSL) and linearity coefficient (LIN). The results of this group also demonstrated that compared to asthenozoospermic infertile patients, normospermic volunteers have lower levels of peroxynitrite, higher values of kinetics parameters and a decrease in tyrosine nitration.10
Salvolini et al. evaluated the presence of NOS and tyrosine nitration in 29 infertile patients with idiopathic asthenozoospermia and 26 age-matched normospermic fertile donors. They have shown that the immunohistochemical expression of constitutive NOS isoforms was higher in sperm cells of normospermic donors. Furthermore, they observed that asthenozoospermic patients had negative results for the presence of eNOS in sperm samples. However, these patients also showed that the immunostaining for iNOS and nitrotyrosine was more intense and the percentage of positive cells was significantly higher when compared with normospermic donors. Another evaluation performed by this group demonstrated that asthenozoospermic samples had more diffuse and intense NOS staining than normospermic samples, which indicates that NOS activity was higher in spermatozoa from asthenozoospermic patients.11
Herrero et al. reported that human spermatozoa from healthy volunteers display a reproducible pattern of protein tyrosine nitration after an incubation for 8h under favorable conditions to the capacitation process. Several proteins became increasingly tyrosine-nitrated in the first 15min of incubation, followed by a decrease in the nitration rate after this time. They also observed that the exposure of spermatozoa to controlled concentrations of ONOO− (between 2.5 and 50μmol/l) contributes to the increase in their motility and their response to become capacitated. However, depending on ONOO− concentration, this ROS can also promote the protein tyrosine nitration and phosphorylation.49
Thus, at low concentrations ONOO− modulates crucial sperm function. Nevertheless, asthenozoospermy is associated with higher levels of peroxynitrite, tyrosine nitration and NOS activity, mainly iNOS.
Methods to analyze 3-NTNitrated proteins have an increased immunoreactivity, with a wide range of antibodies that recognize this post-translational modification. Thus, some methods, such as Western blotting, immunoprecipitation and immunochemistry, may be performed.26,96
In recent years, the enzyme-linked immunosorbent assay (ELISA) method has been applied to analyze the presence of nitrated proteins in biological fluids and homogenates. To quantify the presence of nitrated proteins using the ELISA method, standard curves need to be delineated through the binding of the nitrated tyrosine antibody to the immobilized antigen, with serial dilutions of nitrated BSA. However, this method is only semiquantitative since antibodies for nitrated tyrosine in various proteins may differ from that of nitrated bovine serum albumin (BSA). Lastly, this method showed that the lower limit of detection was 0.02ng of nitrated tyrosine per milligram of protein.97,98
Another method that can be performed to quantify nitrated proteins is the UV–vis spectroscopy, which uses the characteristic spectral shift of these proteins in alkaline solutions. The pKa of the phenolic group in nitrated tyrosine is 7.5, which is lower than that for tyrosine (pKa=10). Both forms of tyrosine have a maximum absorbance at 280nm; however, nitrated tyrosine has additional peaks situated at 365nm, in acidic solutions (pH around 5), and at 430nm, in basic solutions (pH around 9.5), allowing the calculation of nitrotyrosine concentration by an increase of the absorbance at 430nm.98,99
Nitrated proteins can also be identified by high-performance liquid chromatography (HPLC) or other high resolution method for separation of peptides, coupled with tandem mass spectrometry (MS). In this method, peptides from complex samples, such as cellular, tissue or organelle homogenates, are first subjected to proteolytic digestion and then separated according to their physical properties, such as charge, size or hydrophobicity. After this first separation, a second dimension of separation is performed using sensitive MS. In this technique, per each run, it is proceeded to the selection of the five most abundant parent masses for the fragmentation form MS/MS spectra, where the peptide sequence information is obtained.26 It is important to note that to perform this technique, the samples need to be prepared involving the acid hydrolysis of proteins to amino acids, which can represent a problem in what concerns the verification of the complete hydrolysis of the protein into single amino acids and to the possible creation or destruction of nitrotyrosine during hydrolysis.98,100 Despite its good limit of detection, LC is not sensitive for the analysis of small amounts of proteins, which corresponds to a concentration less than 100μg.98
Methods to evaluate the levels of peroxynitrite and 3-NT in the spermatozoaIn the last years, several studies focused on the evaluation of the amount of peroxynitrite and the formation of 3-NT, specifically in the spermatozoa, using different techniques. The methodology used in each of these techniques for the analysis of sperm samples is described below.
Fluorimetric assaysFluorimetric assays can be performed to evaluate the peroxynitrite levels in sperm samples. In these assays, the samples need to initially be diluted to a concentration of 5×106mL−1, using phosphate-buffered saline (PBS) (20mM, pH 7.4).
To determinate peroxynitrite production, 2,7-dichlorofluorescein (DCF) fluorimetric assay can be used. Dichlorofluorescin diacetate (DCFDA) is a membrane-permeable nonfluorescent dye that is hydrolyzed by esterases, within the sperm cytoplasm, into a free acid, DCFH. ONOO− oxidizes DCFH to a strongly fluorescent dye.
A DCFDA-free base is prepared by mixing 10mmol/L of DCFDA with 0.01M NaOH, at room temperature, for 30min. This mixture is then neutralized with 25mmol/L PBS at pH 7.4. Due to its fluorescent characteristics, after being prepared, the solution needs to be maintained on ice and in the dark, until be used. The DCFDA-treated samples are then incubated in NO buffer with 100μM l-arginine at 37°C, in a dark room. The mixture is washed with PBS at pH 7.4 and centrifuged for 2min at 214×g. Supernatant fluorescence is measured in a spectrofluorometer, at an excitation wavelength of 475nm and at an emission wavelength of 520nm.10
DAF-FM fluorescenceTo detect the presence of ONOO− with high sensitivity, a membrane-permanent probe may be used, namely DAF-FM. This probe has also been reported to detect NO, however the required levels of this radical to activate this probe are beyond the concentration found in most biological tissues (>7.7μM).
The probe can be made up as 1mM stock solution in dimethyl sulfoxide (DMSO) and added to sperm suspensions to give a final concentration of 10μM. The cells should be incubated at 37°C before being centrifuged at 300×gand resuspended in PBS. Confocal microscopy is conducted using an excitation wavelength of 488nm and a 522/35nm emission filter. Control incubations incorporated cells that had not been incubated with DAF-FM to ensure that neither the cells nor the treatments to which they were exposed resulted in spontaneous fluorescence at the excitation of emission wavelengths used with this probe.10
Nitrotyrosine ELISAAs previously referred, nitrated plasma proteins may also be assayed by sandwich enzyme-linked immunosorbent assay (ELISA). In this technique, the wells of a microplate are coated with an antibody to nitrotyrosine in PBS, and then the microplate is incubated at 4°C. After incubation, wells must be blocked with 1% bovine serum albumin (BSA), in order to prevent the formation of unspecific bindings, and then washed. Following, the antigen, namely nitrated proteins, is added and the microplate incubated with biotinylated HM11 (Hbt), correctly diluted in PBS containing 0.1% BSA for 1h.
After binding of nitrated proteins to antibodies, the next step is the detection of this binding and measurement of the amount of nitrated proteins present in the sperm samples. With this purpose, one aliquot of streptavidin±horseradish peroxidase conjugate, with the correct dilution, is added to each well, followed by incubation at 37°C. After incubation, the microplate is washed with washing buffer. Lastly, 0.1ml of O-pheylenediamine substrate solution is added to each well and an enzymatic reaction between this substrate and the peroxidase previously added produces a yellowish color that allows the measurement of the amount of nitrotyrosine. To obtain reliable results the enzymatic reaction needs to be stopped, which is done adding sulfuric acid (H2SO4). After the reaction has been stopped, the amount of nitrotyrosine is measured through measurement of the absorbance at 490nm in an ELISA plate reader.101
Slot-blotFor 3-NT expression measurement using Slot-blot, the spermatozoa samples must initially be diluted in 1× TBS in order to obtain a final protein concentration of 1ηg/μL. A volume of 100μL of these samples is slot-blotted into a nitrocellulose membrane, which is then incubated with 10% methanol, for its permeabilization, and washed in water and 1× TBS-T.
After these steps, 5% milk in 1× T-TBS is added to block the nitrocellulose membrane. The blocked membrane is then incubated with primary antibody anti-nitrotyrosine, washed two times with 1× T-TBS, and finally incubated with the respective secondary antibody. Once incubation is completed, the membrane is washed again for two times with 1× T-TBS and, then, visualized through enhanced chemiluminescence (ECL) or infrared imaging systems.
SDS-PAGE and Western blottingSDS-PAGE followed by Western blotting can be an alternative to Slot-blot. To visualize plasma proteins a polyacrylamide gel needs to be performed, which will then be used for blotting onto a transfer membrane. Once the transfer is done, and in order to prevent the occurrence of unspecific bindings, the membrane is blocked with 5% non-fat milk dissolved in 1× TBS-T for 1h. After blocking, the membrane is incubated with an antibody to nitrotyrosine, washed, and then incubated with the respective secondary antibody. The nitrated proteins are finally visualized through enhanced chemiluminescence (ECL) or infrared imaging systems.
ConclusionThis review focused in the formation of ONOO–, which is produced through the interaction between O2•− and NO, and in its binding to spermatozoa proteins, contributing to protein tyrosine nitration due to 3-NT formation. The presence of ONOO−, protein nitration and 3-NT was correlated with several seminal parameters, such as antioxidant proteins in sperm cells, namely SOD, and with spermatozoa motility. The levels of protein nitration in sperm may be clinically relevant as a marker of oxidative stress. Furthermore, several methods to detect directly or indirectly the presence of protein tyrosine nitration were described. Those may be straightforwardly implemented in a clinical setting environment for a better understanding of the oxidative stress alterations in sperm.
Conflicts of interestThe authors declare no conflicts of interest.