The cerebellum is a small structure of the Central Nervous System that occupies 10% of the total volume of the brain, connecting to the brain and the brainstem through the cerebellar peduncles. Its anatomical division is based on three criteria: midline, fissures, and phylogeny. Based on these criteria, the cerebellum is divided into three layers (granular, Purkinje, and molecular) that are subdivided into subzones or microcomplexes that form the fractured somatotopy or mosaic. Various studies have reported that the anatomy and physiology of the cerebellum have varied throughout evolution in each species, since it has different layers, zones, neurons, interneurons, fibers, deep nuclei, types of glial cells, and lobes in its cortex, which vary depending on the species. For many years, the cerebellum was only classified as a structure related to motor skills (coordination, planning, execution, etc.), but today it is known that it is also involved in sensory, cognitive, emotional, and even autonomic processes. These investigations have expanded the role of the cerebellum in the Nervous System. This review comprises an updated compilation of various investigations on the anatomy and physiology of the cerebellum mainly in humans and rodents.
El cerebelo es una pequeña estructura del Sistema Nervioso Central que ocupa el 10% del volumen total del cerebro, conectándose a este y al tronco encefálico a través de los pedúnculos cerebelosos. Su división anatómica se basa en tres criterios: línea media, fisuras y filogenia. Con base a esos criterios el cerebelo se divide en tres capas (granular, Purkinje y molecular) que se subdividen en subzonas o microcomplejos que forman la somatotopía fracturada o de mosaico. Diversos estudios han reportado que la anatomía y la fisiología del cerebelo han variado a lo largo de la evolución en cada especie, pues este presenta en su corteza distintas capas, zonas, neuronas, interneuronas, fibras, núcleos profundos, tipos de células gliales y lóbulos, los cuales varían dependiendo la especie. Durante muchos años, el cerebelo sólo se clasificó como una estructura relacionada con las habilidades motoras (coordinación, planificación, ejecución, etc.), pero actualmente se sabe que también está involucrado en procesos sensoriales, cognitivos, emocionales e incluso autonómicos. Estas investigaciones han permitido ampliar el papel del cerebelo en el Sistema Nervioso. Esta revisión comprende una recopilación actualizada de diversas investigaciones sobre la anatomía y la fisiología del cerebelo principalmente en humanos y roedores.
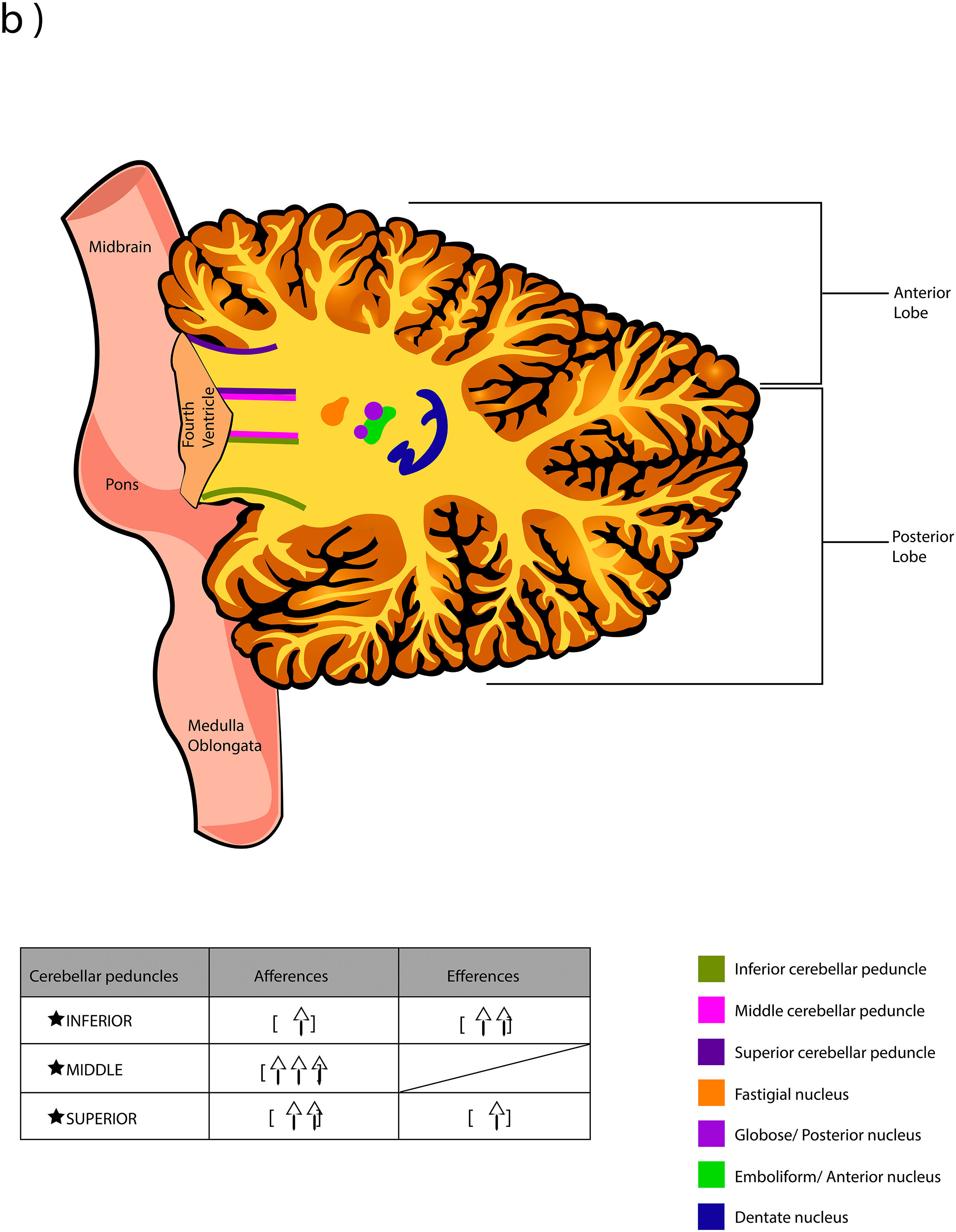
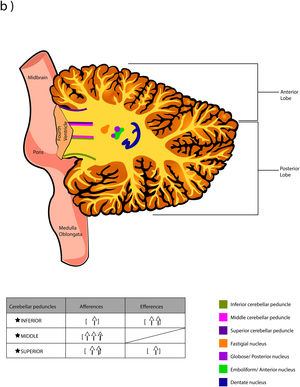
The word Cerebellum comes from the latin Cerebellum which means “small brain” and, although it only occupies 10% of the total volume, it contains more neurons than the brain. It is located in the posterior cranial fossa, under the extension of the dura mater that separates the cerebellum from the lower portion (called “the tentorium cerebelli”, latin for "tent of the cerebellum"), dorsally to the pons and medulla oblongata.1,2 It connects to the brainstem and the rest of the brain through the superior cerebellar peduncles (brachium conjunctivum or brachia conjunctiva), middle (brachium pontis or bridge arm; which is the longest of the three), and inferior (restiform body) (Fig. A.1).2–5 The cerebellum is connected with midbrain (through the superior peduncles), bridge of Varolius (by means of middle peduncles), and medulla oblongata (through the inferior peduncles).6
Neuroanatomy of the cerebellumThe adult cerebellum dimensions have been growing as the evolutionary process, for example, the cerebellar cortex area surface in a frog is approximately 10 mm2 in a rat it is around 530 mm2, in a mouse it can vary from 174 to 190 mm2, while in human it reaches about 50,000 mm2. If we were to unfold the foliar surface of the human cerebellum in the anteroposterior axis, it would extent 1 m in length and about 50 mm in width. However, despite dimensional differences, the cellular organization of the cerebellar cortex is stereotyped (almost crystalline), maintaining an arrangement and connectivity that is repeated with hardly any variation from the most primitive vertebrates, leading only to a variation in the origin and destination of the afferent pathways and in the destination of the efferent.2,7–12
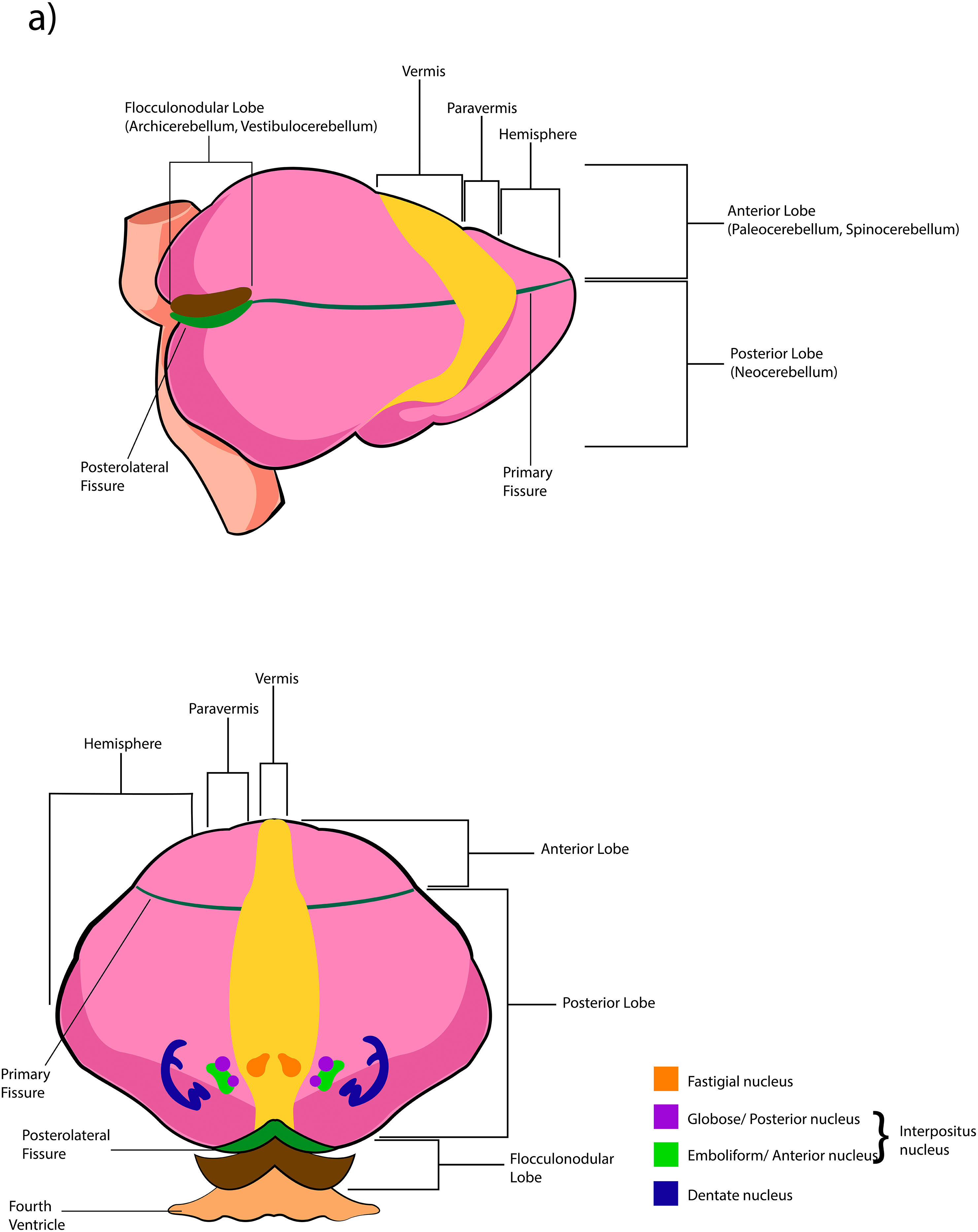
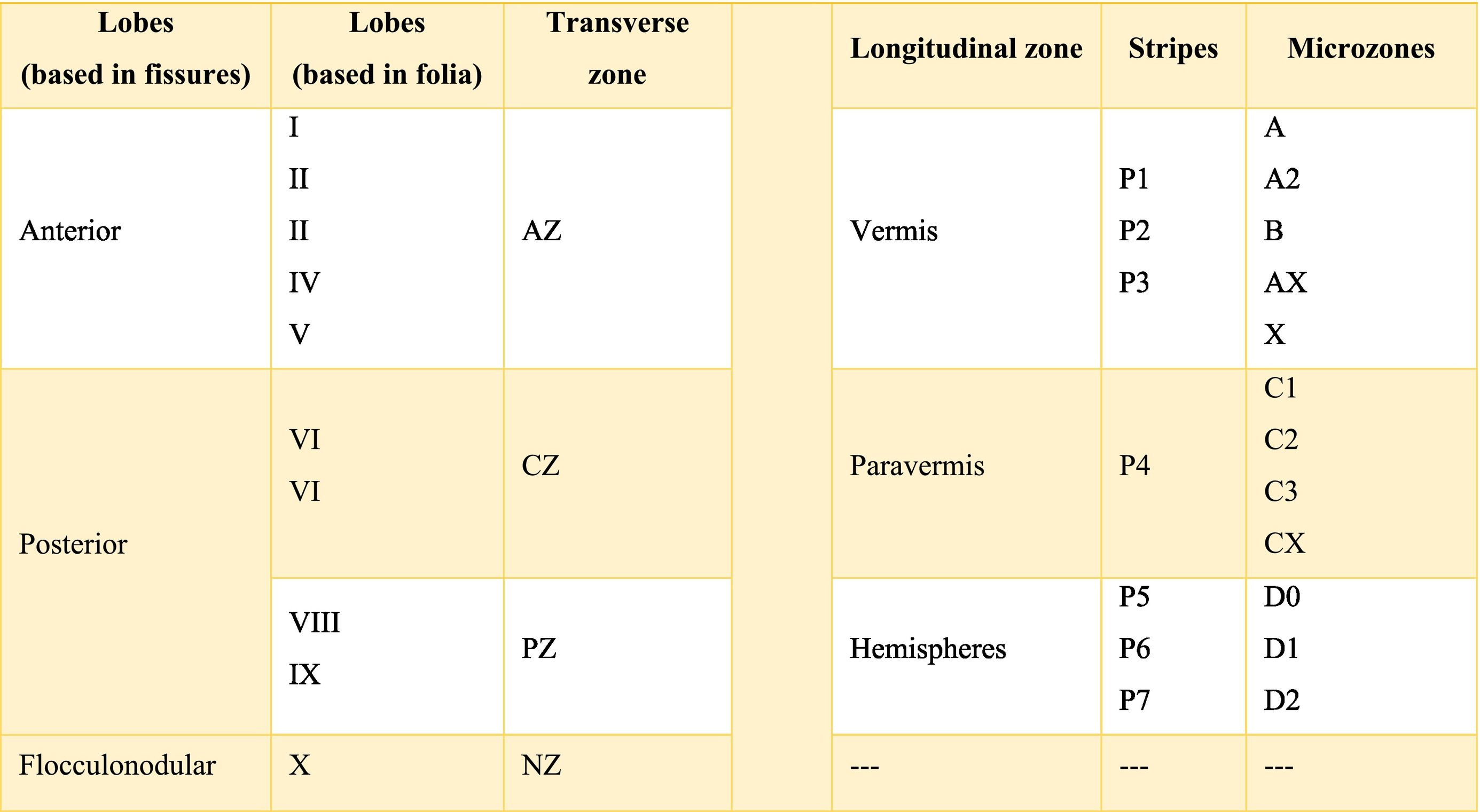
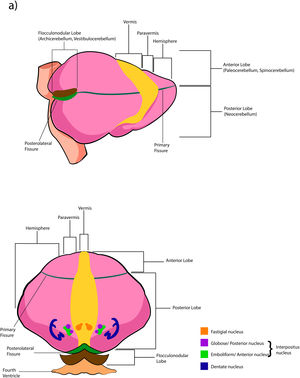
The cerebellar cortex is divided into four ways: a) based on a median line [that generates a central zone (vermis), two intermediate zones (paravermis) and two lateral zones (hemispheres)];1,2,13 b) based on fissures or grooves [giving rise to three types of lobes: an anterior one that is above the primary fissure, a posterior one found below the same fissure, and a flocculonodular one that is located under the posterior fissure];1 c) from a phylogenetic or evolutionary point of view [the oldest and most primitive zone (archicerebellum), the zone with intermediate antiquity (paleocerebellum), and the newest and most recent zone (neocerebellum)] (Fig. A.2); and d) based on depth: granular layer (deep), Purkinje layer (intermediate), and molecular layer (outer). If we put together these three criteria for cortical division, we obtain that the archicerebellum includes the flocculonodular lobe and the vermis, the paleocerebellum corresponds to the anterior lobe and the neocerebellum includes the posterior lobe and the hemispheres.2,14–18
At the same time, the vermis contains four transverse zones (although during its development, up to six zones, depending on the animal species; in this case, the mouse will be considered) defined by mediolateral gene expression limits: a) AZ or anterior zone consists of lobes I–V; b) CZ or central zone includes lobes VI–VII; c) PZ or posterior zone is made up of lobes VIII–IX and, d) NZ or nodular zone includes lobe X. The function of these zones is still unknown, but they could be linked to cerebellar compartmentalization, that is, regions or compartments of the cerebellum that were delimited during embryonic development: vestibulocerebellar, spinocerebellar, and pontocerebellar. In turn, each transverse zone is subdivided into a series of "stripes” or “bands" oriented along the rostral–caudal or parasagittal axis defined mainly by the expression of the molecular marker zebrin II.19
The zebrin II bands of Purkinje cells are named from P1 to P7 from the vermis to the hemispheres; if zebrin II is expressed then the band is called from P1+ to P7+, if the marker is not expressed the nomenclature would be P1- to P7-; on the other hand, if the presence of zebrin II is alternated, the bands P1+/- to P7+/- are formed. P1+ and P1- are immediately lateral to each other. In the vermis of the transverse zone PZ, the zebrin II+ strip of the midline is called P1+ and comprises two lateral strips on each side (P2+ and P3+). The zone P4+ is found in the paravermian region. Possibly, the specific function of these stripes is that they are related in the organization and function of the cerebellum.19
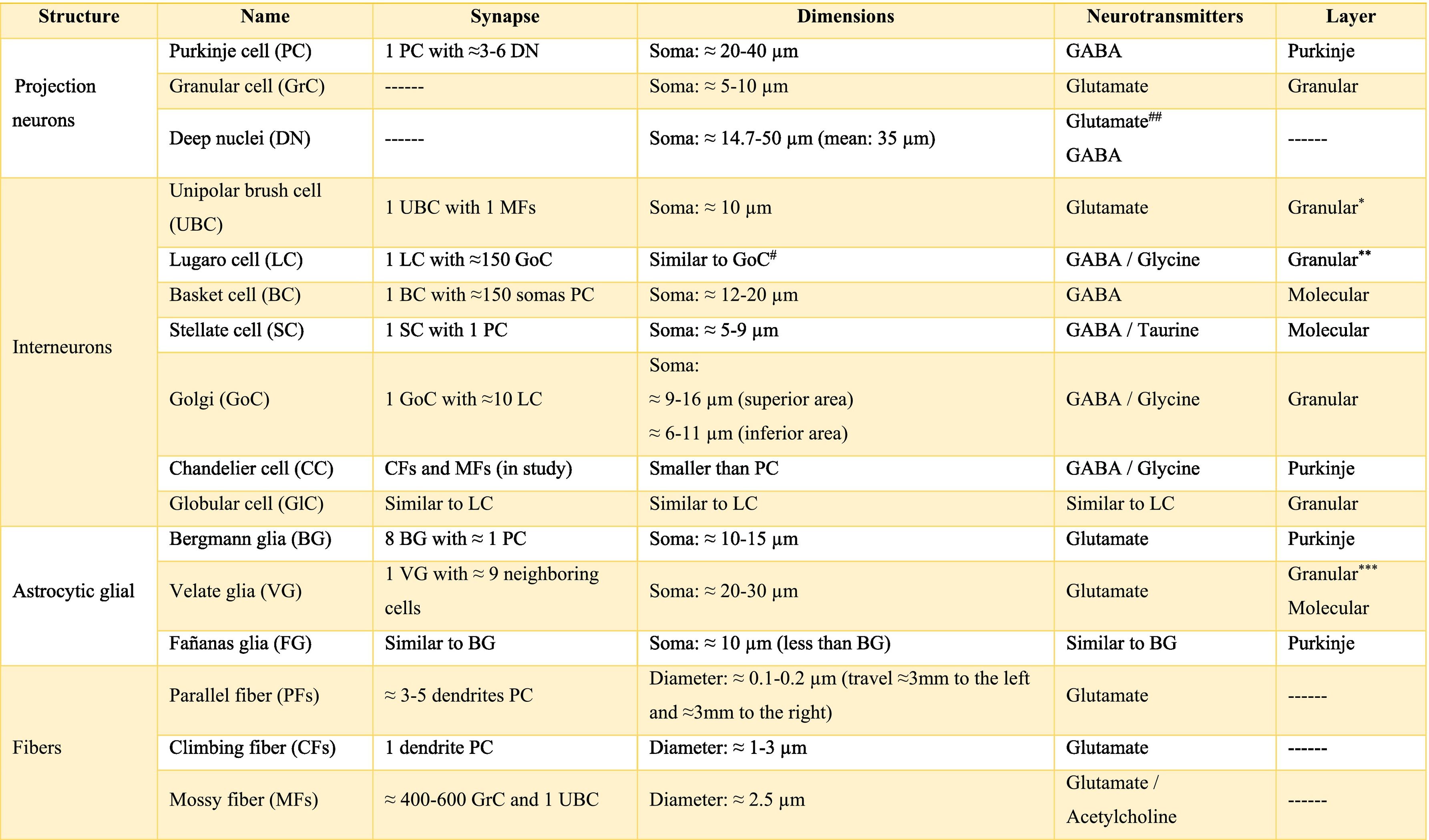
The longitudinally divided cortex (vermis, paravermis, and hemispheres) has smaller subdivisions called microzones in which Purkinje cells have afferents with other regions. In the vermis zone are the microzones A, A2, B, X, and AX; in the paravermis the C1, C2, C3, and CX; and in the hemispheres the D0, D1, and D2. Each of these microzones receives information from climbing fibers and mossy fibers, sending their efferences towards the deep nuclei. This complex of multiple representations of the same parts of the cerebellum (and any other structure) (Table A.1) to produce a pattern is called fractured somatotopic, mosaic somatotopic, or microcomplex; and postulates the idea that the response evoked in the cortical neurons of the cerebellum to a stimulus may be represented in different areas of the vermis, paravermis, or hemispheres, suggesting the participation of more than one micro-complex in the given response.19
Micro-complexes of the cerebellum in rodents*.19
* Bold values signifies p values below 0.05.
Almost all processes that involve different pathways and type of neuron required different chemicals that mediate the functionally integrated of cerebellum, these chemicals are called neurotransmitters. There is a wide range of them, the main ones are shown in Table A.2. Also, there are others such as 2-arachidonoylglycerol (2-AG) and arachidylethanolalamide or anandamide, bothendocannabinoid type, which are related to the modulation of fine-motor coordination by synapsing on presynaptic cannabinoid type 1 receptors (CB1R) located in the parallel fibers, in the Purkinje cells and in the basket cells, suppressing the release of glutamate and/or GABA, respectively. The release of these neurotransmitters allows the cerebellum to “adjust” motor learning, assisting with long-term depression (LTD).20 On the other hand, the cerebellum also receives information modulated by dopamine, serotonin, and norepinephrine, which are released in the cortex, possibly modifying the action of glutamate on Purkinje cells.6,21
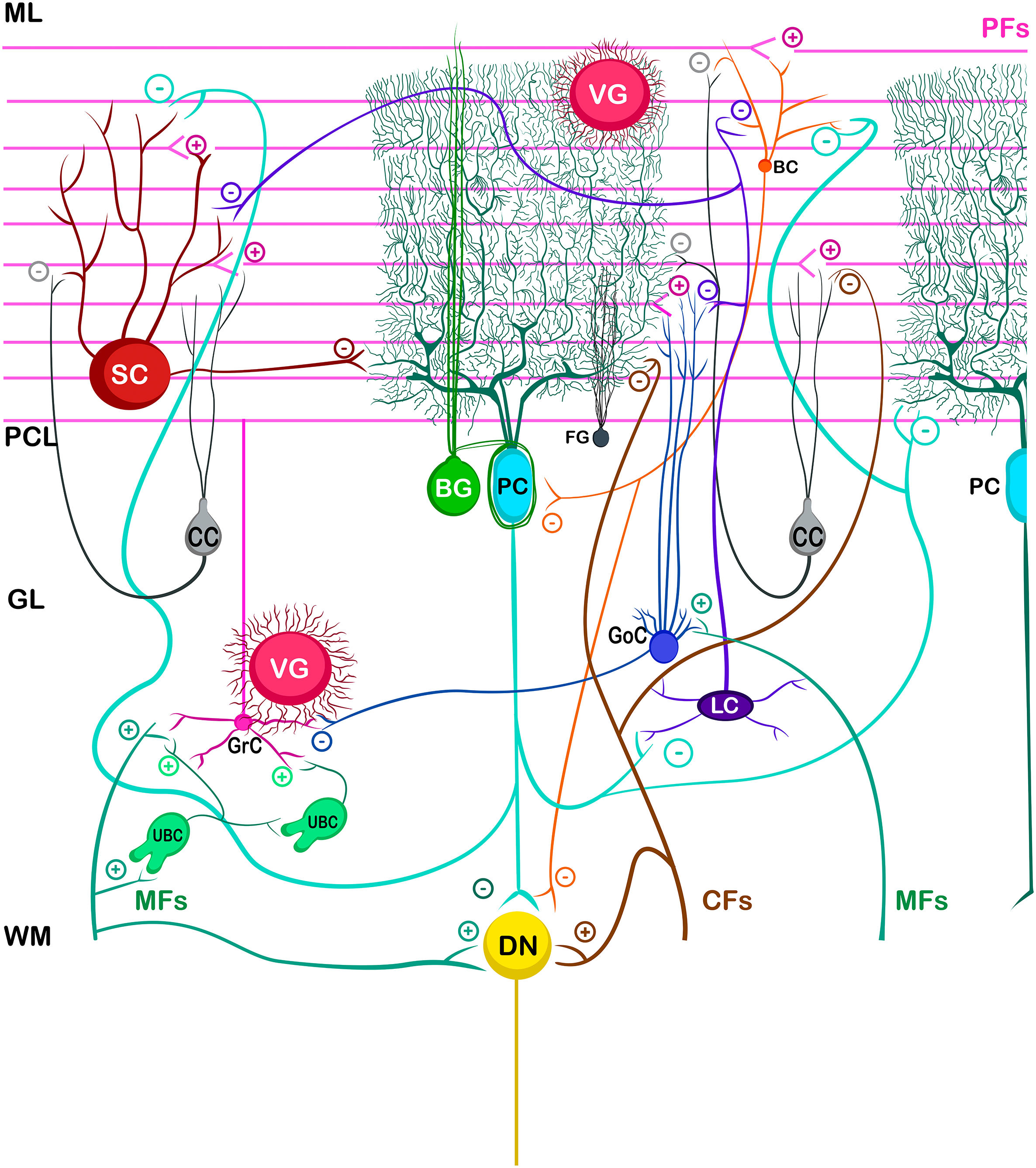
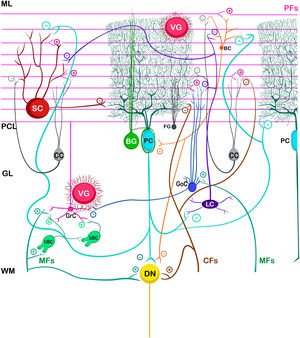
Cerebellar circuitThe basic cerebellar circuit was described more than a century ago by Cajal, This circuit has repeated itself over and over again throughout each subdivision of the cerebellum in all mammals.29 Cerebellum circuit is shown in detail in Fig. A.3 and is roughly described below:
External cerebellar circuitThe information that the cerebellum receives comes through mossy fibers and climbing fibers, and connects directly with granule cells.35,39,41 The activation of the mossy fibers produces the excitation of the granule cells which, in turn, excite the Purkinje cells and produce the inhibition of the deep nuclei. For its part, the activation of climbing fibers inhibits Purkinje cells through intense depolarization, in LTD.31 In addition, these fibers also excite stellate cells, basket cells, and Golgi cells, producing another inhibition, in this case lateral, of the Purkinje cells adjacent to the central excitation zone. The climbing fibers also send collateral projections to the deep nuclei.1,2,19
Internal cerebellar circuitGolgi cells receive information from the parallel and mossy fibers, providing inhibitory feedback to the granule cells.31,32,38 Brush cells receive afferents from mossy fibers and form excitatory synapses with dendrites of granule cells, with other brush cells, and also with mossy fibers. Lugaro cells connect with basket, stellate, and Golgi cells; but they are densely innervated by Purkinje cells.1,2,28,32,37 The somas of the candelabrum cells are located in the Purkinje layer, they have one or two long, rarely branched dendrites that rise almost vertically towards the molecular layer, as well as several short dendrites that project towards the granular layer, where they run mainly horizontally. Its axons project horizontally into the molecular layer. Since their exact connections are still unknown, it is not uncommon for them not to be considered in the current circuitry of the cerebellum; however, its dendrites are believed to synapse with parallel and climbing fibers, as well as basket, stellate, and Purkinje cells.40 Finally, globular interneurons extend their dendrites and axons into the molecular layer.32
The axons of the granule cells bifurcate in a "T" shape and stimulate the Purkinje cells in a process that is regulated by indirect activation of the stellate and basket cells through the parallel fibers, allowing both cell types to inhibit Purkinje cells.2,29,31,32,38 Likewise, Purkinje cells inhibit deep nuclei and send recurrent axonal collaterals to other contiguous cells, stellate, and basket cells, with exerting the same effect.2
The glia of Bergmann (BG) extends its processes through Purkinje bodies and covers them; making synapses in a limited way (typically five) and controlling the diffusion of glutamate through its transporters (GLT1 and GLAST) to keep its concentration low. This is important because Purkinje cells are vulnerable to excitotoxicity caused by excessive stimulation of their glutamatergic receptors AMPA (GluR1 and GluR4) and NMDA (NR2B). This stimulation is given by the afferents of the climbing, mossy, and parallel fibers. As for astrocytes, it can contain approximately 2–3 mM glutamate in its cytoplasm (unlike neurons which can contain more than 5 mM). On the other hand, although BG (as well as other types of glia) does not synthesize GABA, it presents it temporarily due to the influence of its transporters GAT1 and GAT2-3 when the basket cells come into contact with the cell body of Purkinje cells, and stellate cells with Purkinje dendrites.24
Little is known about the velate astrocytes and they are still under study; however, it is believed that they may play an important role in controlling the flow of information (for example, in the elimination of glutamate) towards the molecular layer through local interactions with neuronal synapses.34 These astrocytes have large somas and their many thick processes create a shrub-like structure, enveloping (through gap junctions) the synapses between granule cells and mossy fibers, or at the molecular level, between the synapses of Purkinje cells and the processes of BG; thus, controlling the infiltration of dendrites and axons and limiting the diffusion of neurotransmitters.33,36
Also, little is known about Fañanas astrocytes (FG) are, they are in fact, almost forgotten. Its processes extend into the molecular (lower part) and granular layers. Morphologically, they are similar but smaller than BG and although they have only been detected through a special modification of the Cajal gold sublimate technique, their morphological distinction with this technique has been almost impossible. However, after a protein labeling carried out by Goertzen and Rüdiger (2018), similarities and differences between BG and FG could be observed. Somas are located in the Purkinje layer in a dispersed way for the FG and in the form of a single row for the BG; whose shape is round and with a small diameter for the FG and epithelioid and approximately 10 μm, respectively, for the BG. At the protein level, FG is positive for potassium channels Kv2.2 and 3; while BG is positive for the potassium channel 6.1.30,36
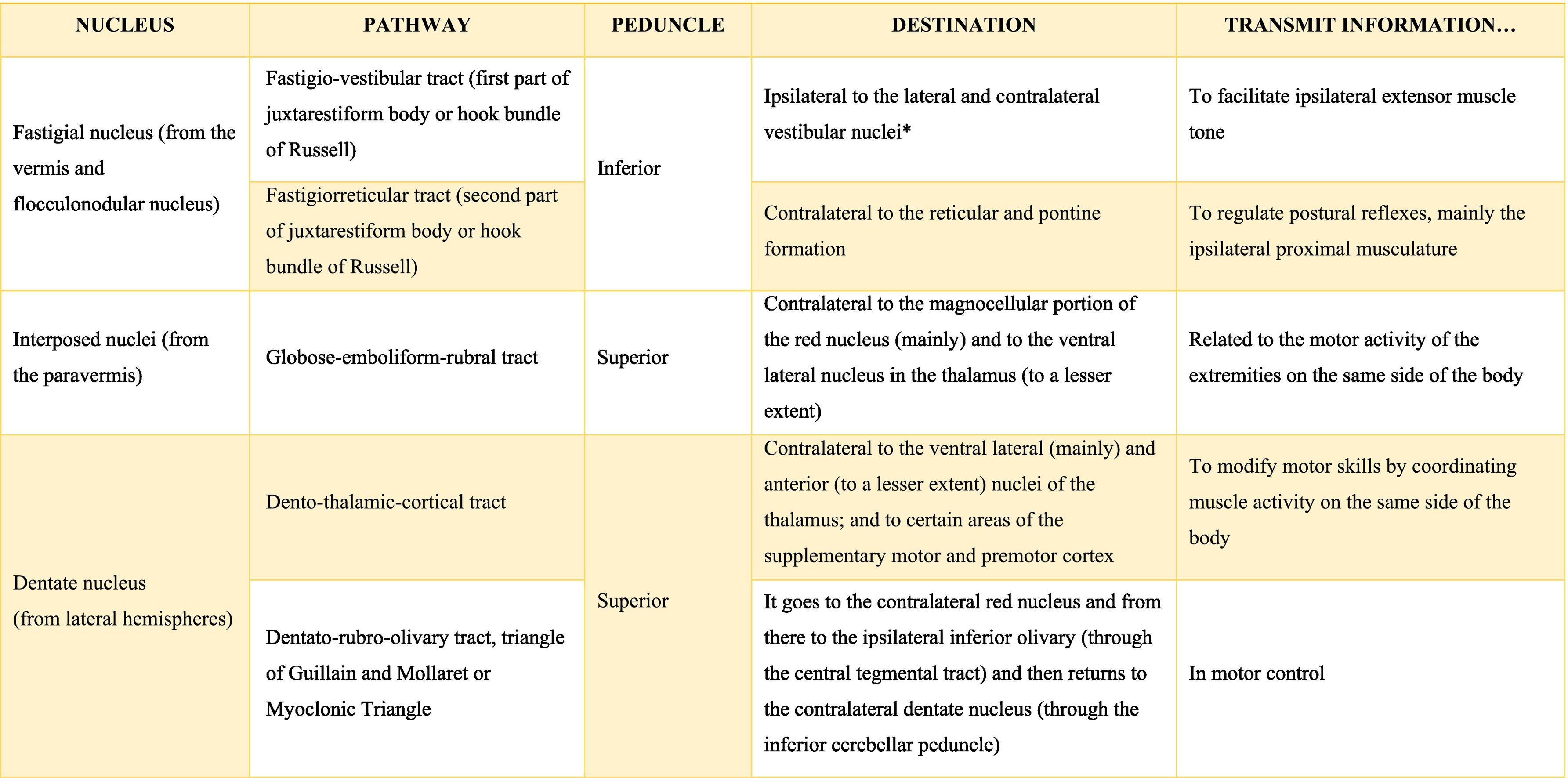
The deep nuclei of the cerebellum (intracerebellar, central, or roof nuclei) are the only information outlet from the cerebellum. They are located near the roof of the IV ventricle and are defined as masses of gray matter embedded in the white matter on each side of the midline of the cerebellum. They are made up of large multipolar neurons with simple branched dendrites6 and are located in the three cerebellar areas: the fastigial nucleus is in the vermis, the interposed nucleus (globose/posterior and emboliform/anterior) is located in the paravermis, and the dentate nucleus is in the hemispheres.25 Information they receive from the Purkinje cells located: a) in the most medial portion of the paleocerebellum and neocerebellum (vermis) projects to the fastigial nucleus; b) in the paravermal region it projects towards the interposed nuclei, and c) in the lateral zones (hemispheres) project to the dentate nucleus.2,25 The dentate nucleus is bean-shaped and is the most prominent and largest nucleus in primates (the have a dorsal and a ventral region); it corresponds to the neocerebellum and its interior is filled with white matter that consists of efferent fibers that form most of the superior cerebellar peduncle. The emboliform nucleus has an oval shape, is located medial to the dentate nucleus, and belongs to the paleocerebellum. Globose nucleus has a rounded shape, is made up of one or more rounded groups of cells, and is located between the emboliform and fastigial nuclei. Both the axons of the emboliform nucleus and those of the globose nucleus are also part, to a lesser extent, of the superior peduncle. Lastly, the fastigial nucleus is located in the midline of the vermis, it is smaller than the dentate nucleus but larger than the interposed nucleus, it belongs to the archicerebellum, and its axons are part of the inferior cerebellar peduncle.4,6,42,43
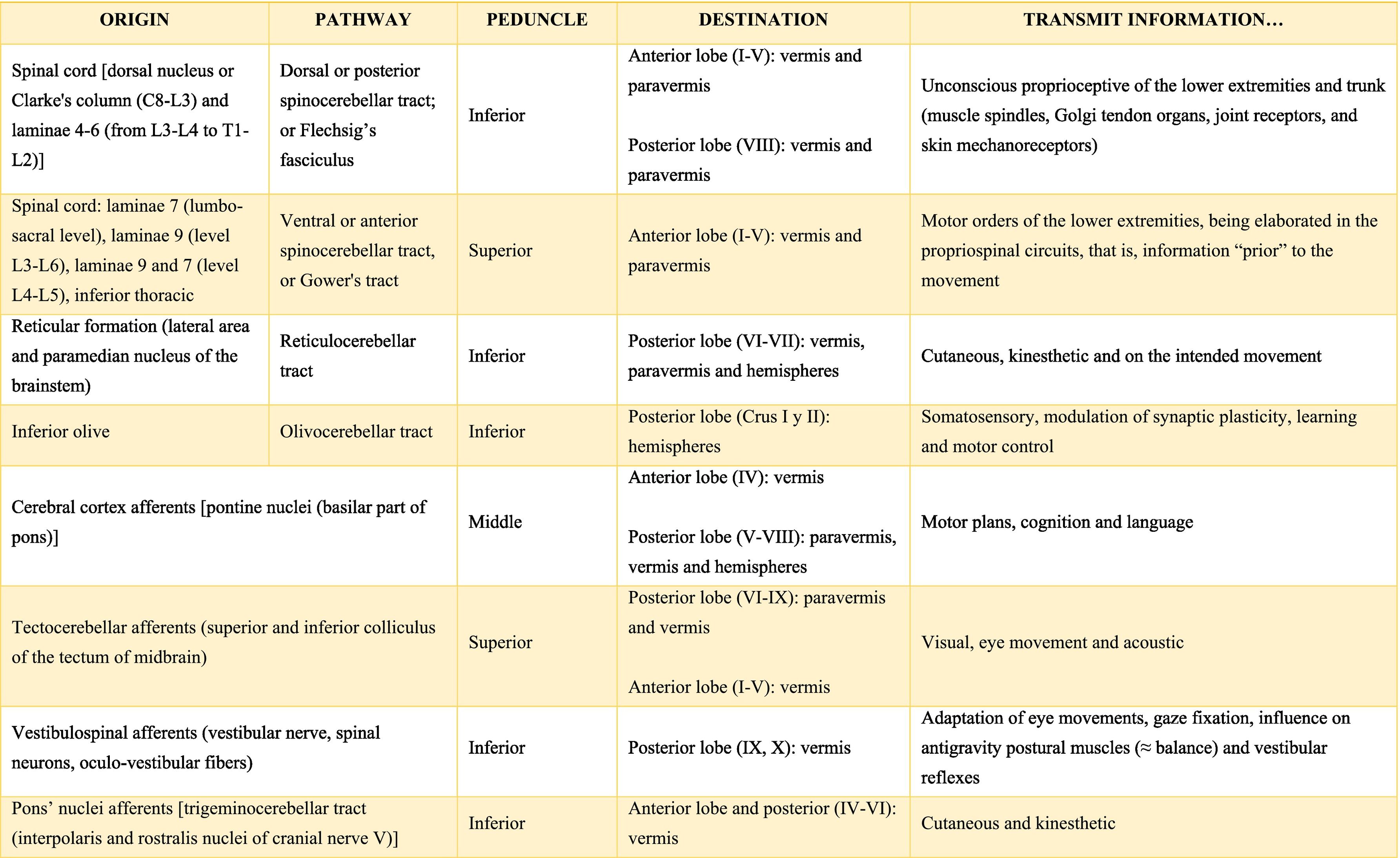
Afferent and efferent connections of the cerebellumThe cerebellum receives information from structures related to movement and motor plans that enter through the cerebellar peduncles and end in the deep nuclei (facilitating coarse adjustment) and in the cerebellar cortex (contributing to fine adjustment).2,42
The inferior peduncle receives ipsilateral information from the spinal cord (dorsal), the inferior olive, the reticular formation, the pontine, and the trigeminal nuclei. The middle peduncle contains cross afferents of the pontine nuclei. Superior peduncle also contains information on the spinal cord (ventral), the quadrigeminal tubercles, and the locus coeruleus.42 Likewise, the spinal cord sends two more afferents: cuneocerebellar and rostral spinocerebellar tracts, which are the homologues of the upper limbs of the dorsal and ventral spinocerebellar tracts, respectively.44
These afferences continuously inform the cerebellum about the state of the periphery (muscles, tendons, joints, other sensory inputs, state of the reticular formation, etc.) and the motor plans in motion (especially of the cerebral cortex through the nuclei of the pons), coordinating and adjusting motor activities by continuously comparing cerebellar efferences from deep nuclei to higher motor neuron systems (Table A.3).42
Almost all the information that is received by Purkinje cells is directed, through their axons, to the deep nuclei of the cerebellum and from there it goes to motor centers of the brainstem such as the red nucleus, the thalamus, the reticular formation, the vestibular nuclei, the motor cortex, among others; however, there are a few Purkinje axons that pass directly from the cerebellum to the lateral vestibular nucleus. The cerebellar efferent exit through the superior peduncle and, to a lesser extent, the inferior one. The middle peduncle does not show efferences.2,4,6,42 All of these efferences allow the cerebellum to perform coordination and smooth adjustment of movementconcerning motor, sensory, vegetative, and other functional activities of the Central Nervous System (Table A.4).42
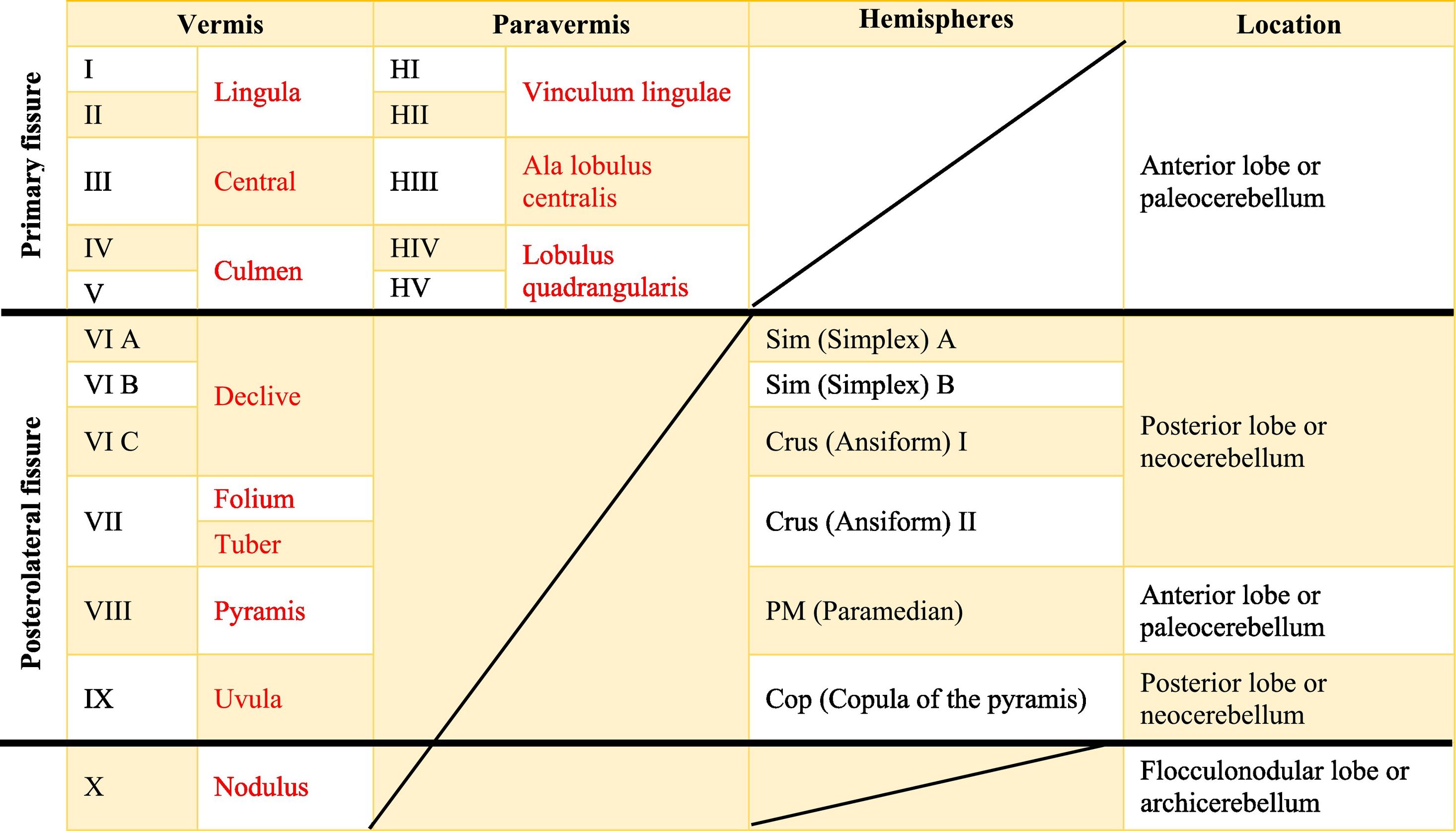
Cerebellar lobesThe dimensional or surface variation of the cerebellum in various species exhibits a very rapid and extensive growth in which its volume of gray matter begins to fold, in a process called foliation, forming leaf-like sheets, from which the term folios (folia of cerebellum). As the folios are created, certain spaces remain that divide the surface of the cerebellum, these divisions are called grooves and fissures, where the latter is deeper. The set of folios, which is between grooves and fissures, is called lobes; and although there are folios in both vermis and hemispheres (including the paravermis), the size and quantity of these are smaller at the level of the hemispheres, so the number of lobes is affected (Table A.5).1–3,29,51 Vermis has 10 lobes, while hemispheres, in its most lateral zone have 6 lobes (depending on the species). Since the number of lobes in the vermis is greater than in other areas.43,52–54
The names in red are to refer to the human, while those in black are for rats.Is there a possibility that you will leave the text in red? Since in itself that is the essence of the board foot. In the original format that was shipped, there are words in red in the table that indicate a separation between the names of the human and rat lobes.
The extent to which the lobes are further subdivided by fissures varies between species,36 in the same way, the grouping of the lobes of the hemispheres concerning the vermis varies in based on the authors. On the other hand, if we join this lobular division with the cerebellar division based on its phylogenetic, lobe X belongs to the flocculonodular lobe or archicerebellum, the lobes of I–V and VIII are located in the area of the paleocerebellum or anterior lobe, and lobes VI, VII and IX correspond to the neocerebellum or posterior lobe.2,16
Functions of the cerebellumThe regions of the cerebellum have different functions depending on their interconnections with various brain regions; being involved in sensation, movement, attention, reward/motivation, language, social processing, memory, and executive function.43 If we talk about the general functions of the cerebellum based on the division of its cortex, we have that the flocculonodular lobe is involved in the control of postural reflexes; the hemispheres participate in the control of independent movements of the limbs with precision and speed; the paravermis, on the other hand, is important for precision limb movements (such as skillful walking and/or reaching a certain goal) as it regulates information on inappropriate limb movements, that is, "motor errors". The vermis receives visual information (from the tectum), cutaneous and kinesthetic information (from the spinal cord), and is involved in depressive disorders, cognitive, and affective aspects.55 If we talk about the functions of the cerebellum based on the phylogenetic division, we have to:
PaleocerebellumIt acts as a “reinforcer” of movement by facilitating signals from the cortical and brainstem motor centers, and thereby helping to maintain adequate muscle tone, control, and regulation of extensors and flexors during changes in the position of the different joints;2 in other words, the paleocerebellum is concerned with postural and gait control.18
In this sense, it has been reported that this area (lobes I–V), could be related in the sensorimotor aspect, movement, tactile stimulation of arms, legs, hands, and fingers, as well as in orofacial movements;16,56,57 while in the aspect autonomic is possibly involved with the respiratory rate (mainly due to lack of air).58 The lobe VIII could assist in the movement and tactile stimulation of the arms, legs, hands, and fingers (mainly the index finger),16,56,57 as well as in sexual behavior.59 Regarding non-sensorimotor functions, it could influence the maintenance and storage of information.56
NeocerebellumThe sensorimotor functions of the cerebellum are like a “comparator” of the existing differences between the motor orders and their practical results, trying to reduce the imbalances that occur during movement; it also acts as a “coordinator” of the movement, making the different motor acts that make up the sequence of a complex behavior happen in harmony and without any shocks; in other words, it is linked to the functioning of rapid coordinated movements.2,16,18 By serving as a “comparator” and “coordinator” of movements, it also participates directly and actively in the learning processes of motor tasks, as well as in-memory storage.2
It has been reported that lobe VI (Sim A, Sim B, and Crus I) could be related to facial and orofacial information,16,57 to the sequential movement of hands and feet,56 as well as to the acquisition of behavioral skills related to the spatial learning processes [including the sexual aspect (disoriented and inaccurate movements during the acquisition of experience)].60,61 Otherwise, lobe VII (Crus II) could be concerned with the movement of the hands16 and with sexual aspects such as sensory stimuli, motor performance, and acquisition of experience.55,59,60 Lastly, lobe IX is considered as a possible visual guide to movement, and could also be related to tactile stimulation of the hand,16 and in sexual behavior.59
Also, the neocerebellum is linked to non-sensorimotor functions such as perceptual, cognitive, emotional processes; as well as in the speed, capacity, consistency, and adequacy of mental states.2 If we talk about the learning process, right cerebellar activation will occur during verbal fluency (word generation) and working memory tasks in right-handed subjects; while the left cerebellum will be activated during these tasks in left-handed subjects. Instead, the neocerebellum is also related to autonomous processing, including autonomic cardiovascular arousal that occurs during exercise, mental arithmetic stress tasks, and absence of air.16
Lobes VI (Crus I) and VII (Crus II) could be related to language, spatial, executive tasks (such as random number generation and complex decision-making tasks), working memory, and affective/emotional; furthermore, they could also influence their own and others’ painful experiences (such as noxious heat);16 as well as in the pathophysiology of respiration.61 The lobe IX could be associated with working memory processes.62
ArchicerebellumThe X lobe is related to the maintenance of adequate body posture both at rest and in movement2 in situations such as expectations/anticipation of rewards21 or panic, sadness, and sorrow; in addition, it also helps with balance and eye movement.16
ConclusionsRecent anatomical research has shown that the cerebellum presents different types of layers, zones, afferent–efferent connections, deep nuclei, neurons, types of glial cells, and lobes, which vary depending on the species. These discoveries are of great importance to better understand the physiology of the cerebellum. For many years, the cerebellum was only classified as a structure related to motor skills (coordination, planning, execution, etc.), but today it is known that its functions also involve sensory, cognitive, emotional, and even autonomic processes. These investigations have expanded the role of the cerebellum in the Central Nervous System. Thus, it has been shown that the cerebellum is not a small brain with only one function, but that this structure is involved in other functions.
We are grateful to the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the scholarship granted to S.Y.L.A. (Financing CONACyT-893213 doctoral fellowship).