Non-adherence to disease-modifying therapies (DMTs) in multiple sclerosis may be associated with reduced efficacy. We assessed compliance, the reasons for non-compliance, treatment satisfaction, and quality of life (QoL) of patients treated with first-line therapies.
MethodsA cross-sectional, multicenter study was conducted that included relapsing multiple sclerosis patients. Compliance in the past month was assessed using Morisky-Green test. Seasonal compliance and reasons for non-compliance were assessed by an ad-hoc questionnaire. Treatment satisfaction and QoL were evaluated by means of TSQM and PRIMUS questionnaires.
ResultsA total of 220 patients were evaluated (91% relapsing-remitting); the mean age was 39.1 years, 70% were female, and the average time under treatment was 5.4 years. Subcutaneous interferon (IFN) β-1b was used in 23% of the patients, intramuscular IFN β-1a in 21%, subcutaneous IFN β-1a in 37%, and with glatiramer acetate in 19%. The overall compliance was 75%, with no significant differences related to the therapy, and 81% did not report any seasonal variation. Compliant patients had significantly lower disability scores and time of diagnosis, and greater satisfaction with treatment and its effectiveness. Discomfort and flu-like symptoms were the most frequent reasons for non-compliance. The satisfaction and QoL were associated with less disability and number of therapeutic switches.
ConclusionsThe rate of compliance, satisfaction and QoL in multiple sclerosis patients under DMTs is high, especially for those newly diagnosed, less disabled, and with fewer therapeutic switches. Discomfort and flu-like symptoms associated with injected therapies significantly affect adherence.
La falta de cumplimiento terapéutico en la esclerosis múltiple puede asociarse a menor eficacia. En este estudio evaluamos el cumplimiento terapéutico, las razones para su incumplimiento, satisfacción con el tratamiento y calidad de vida (CdV) de pacientes en terapia de primera línea (terapias modificadoras de la enfermedad [TME]).
MétodosEstudio transversal, multicéntrico, de pacientes con EM que cursa a brotes. El cumplimiento en el último mes se evaluó mediante test de Morisky-Green, el cumplimiento estacional y las razones para el incumplimiento mediante cuestionario ad-hoc, y la satisfacción y CdV a través de los cuestionarios TSQM y PRIMUS.
ResultadosSe evaluaron 220 pacientes (un 91% remitentes-recidivantes); la media de edad fue 39,1 años, el 70% eran mujeres y el tiempo en tratamiento 5,4 años. El 23% estaba tratado con interferón (IFN) β-1b subcutáneo, el 21% con IFN β-1a intramuscular, el 37% con IFN β-1a subcutáneo y el 19% con acetato de glatirámero. El cumplimento global fue del 75%, sin diferencias significativas en función del TME, y no refirieron cambios estacionales el 81%. Los pacientes cumplidores presentaban significativamente menores valores de discapacidad y de tiempo de diagnóstico, y mayor satisfacción con el tratamiento y su efectividad. Las molestias y los síntomas seudogripales fueron las razones más frecuentes para el incumplimiento. La satisfacción y la CdV se relacionaron con una menor discapacidad y número de cambios terapéuticos.
ConclusionesEn pacientes con TME el grado de cumplimiento, satisfacción y CdV son altos, en especial para aquellos de diagnóstico reciente, menos discapacitados y con menos cambios terapéuticos. Las molestias y síntomas seudogripales asociados a las terapias inyectadas influyen en el cumplimiento.
Multiple sclerosis (MS) is the most frequent cause of neurological disability unrelated to trauma among young adults.1 The most common disease course (80%) is relapsing-remitting MS (RRMS), which is characterised by self-limited attacks of neurological dysfunction resulting in residual functional deficit as damage accumulates. Approximately 50% of RRMS patients will eventually present a progressive secondary course with increasing disability, called secondary-progressive MS (SPMS). SPMS disability is independent of relapses, although they can appear sporadically.2
Disease-modifying therapies (DMT) constitute the current first-line treatment option for RRMS and SPMS since they reduce relapse rate and slow disability progression. DMTs include interferon beta-1a (IFNβ-1a), interferon beta-1b (IFNβ-1b), and glatiramer acetate, which can be administered either subcutaneously (SC) or intramuscularly (IM).3 Treatment adherence is defined by the World Health Organization as the combination of compliance (taking the medication according to prescribed dosage and schedule) and persistence (taking it from the beginning until it is discontinued). Poor adherence seems to contribute to lack of effectiveness in treatments for chronic illnesses.4 Although several studies have demonstrated the importance of adhering to these treatments in order to control not only relapse rate but also MS progression,5–8 adherence rates range from 41% to 88%.9 The principal reasons for not adhering to treatments include perceived lack of effectiveness and secondary effects.10
Reduced quality of life (QoL), resulting from the physical, psychological, and social alterations occurring in MS, is also linked to motivation to adhere to a DMT11,12 and constitutes a prognostic factor for the course of the disease.13,14 Several studies of other chronic illnesses have shown that both QoL and adherence are directly related to treatment satisfaction.15–17
Our main purpose is to assess the level of therapeutic compliance of MS patients with relapses who are treated with first-line DMTs. We also aim to ascertain the reasons for non-adherence, describe seasonal compliance, determine the level of treatment satisfaction, and evaluate QoL in our patients. Furthermore, we intend to examine the connection between compliance and treatment satisfaction and to determine whether adherence, treatment satisfaction, or QoL are linked to any demographic or clinical variables.
Patients and methodsWe included consecutive patients aged 18 or older with a diagnosis of either RRMS or SPMS and presenting relapses according to the criteria established by McDonald et al.18 Patients had to have been treated with DMT for at least 6 months and attended the neurology department for a routine visit. Exclusion criteria were as follows: patients with other types of MS, patients who had changed or discontinued DMT in the previous 4 weeks, patients taking natalizumab or other immunosuppressant drugs, patients already included in another clinical trial at the time of the visit or in the preceding 6 months, and patients who were not appropriate for the study according to the researcher.
Study designThis was a multi-centre cross-sectional observational epidemiological study. Patients signed an informed consent form upon inclusion, and the primary and secondary variables were determined based on medical histories, patient interviews, and questionnaires.
Primary variableTherapeutic compliance and patients’ attitude towards treatment in the previous month were assessed using the adapted and validated Spanish version of the Morisky-Green test.19 Patients were considered compliant if they gave optimal answers to these 4 questions: (a) Did you ever forget to take your MS medication?; (b) Did you take your medication on schedule?; (c) When you felt better, did you stop taking your medication?; and (d) If you felt worse after taking your medication, did you stop taking it?
Secondary variablesWe gathered patients’ demographic data as well as past and current data from medical histories. Seasonal adherence and reasons for non-adherence were analysed using an ad-hoc questionnaire. We evaluated patient satisfaction with several aspects of the treatment (effectiveness, side effects, convenience, and global satisfaction) in the preceding 2-3 weeks using the Spanish-language version of the Treatment Satisfaction Questionnaire for Medication (TSQM).20 Each category is scored on a scale ranging from 0 to 100 (from lowest to highest satisfaction). Patient QoL at the time of the visit was measured with the Patient-Reported Indices for Multiple Sclerosis (PRIMUS).21,22 This questionnaire consists of 2 scales (activity limitations scored 0-30, and QoL scored 0-22); higher scores represent greater limitations on the first scale and better QoL on the second. Additionally, patients rated their emotional state at the time of the visit on an analogue visual scale scored 0-100 (from worst to best imaginable).
Statistical analysisWe analysed all patients meeting inclusion criteria and providing valid data for the primary variable (Morisky-Green test). Substitution methods were not employed for missing data. All statistical analyses were performed using SAS® version 9.2 for Windows. Discrete quantitative and qualitative variables were expressed as absolute frequencies and relative frequencies; continuous quantitative variables were reported as measures of central tendency and of dispersion. We performed null hypothesis tests (chi square, t-test, or Fisher exact test, as appropriate) in order to establish the connection between selected demographic and clinical variables and 4 factors: (a) adherence, (b) reasons for non-adherence, (c) treatment satisfaction, and (d) QoL. The t-test was also used to determine the relationship between compliance and patient satisfaction with treatment. All statistical tests were bilateral and the level of statistical significance was established at P=.05.
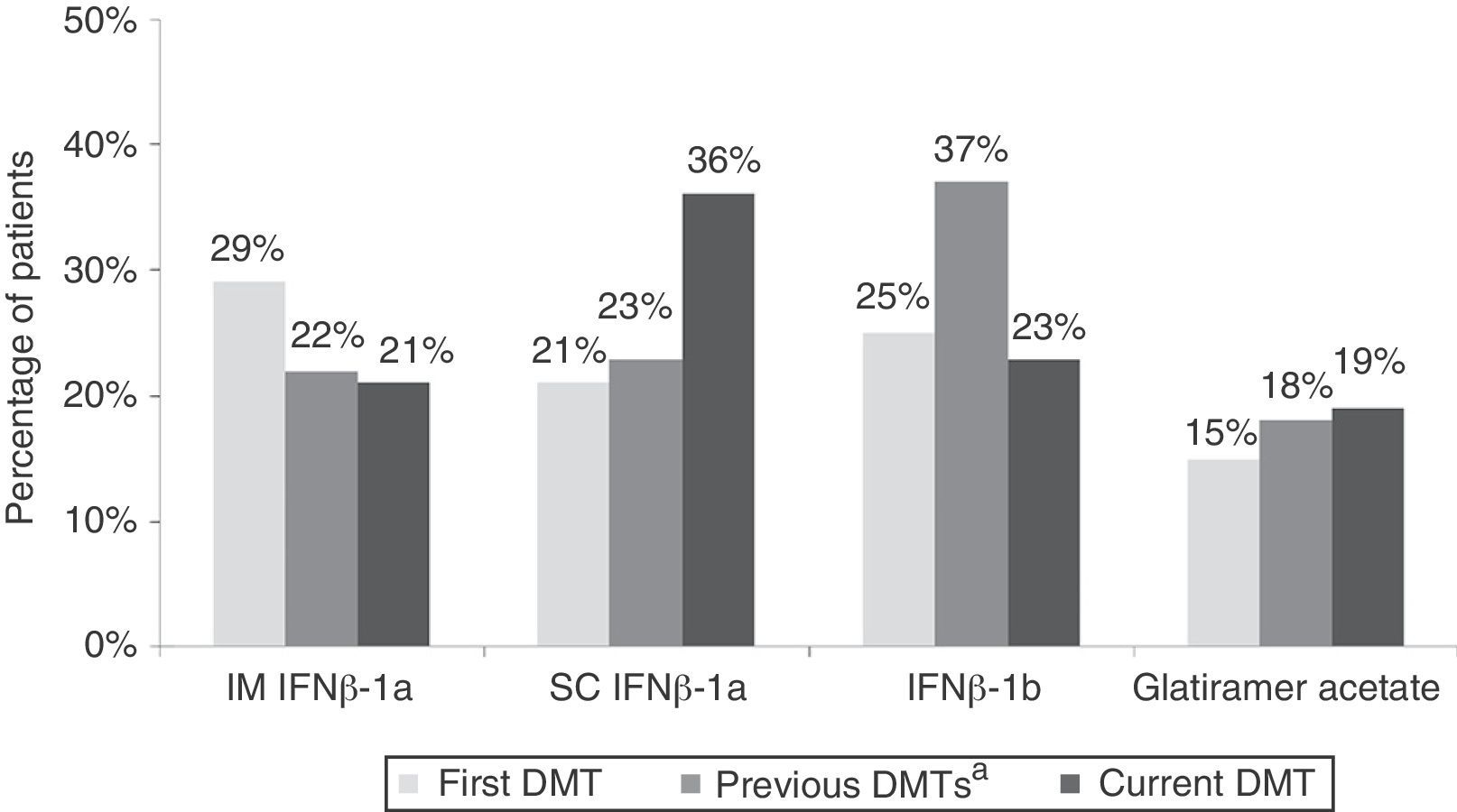
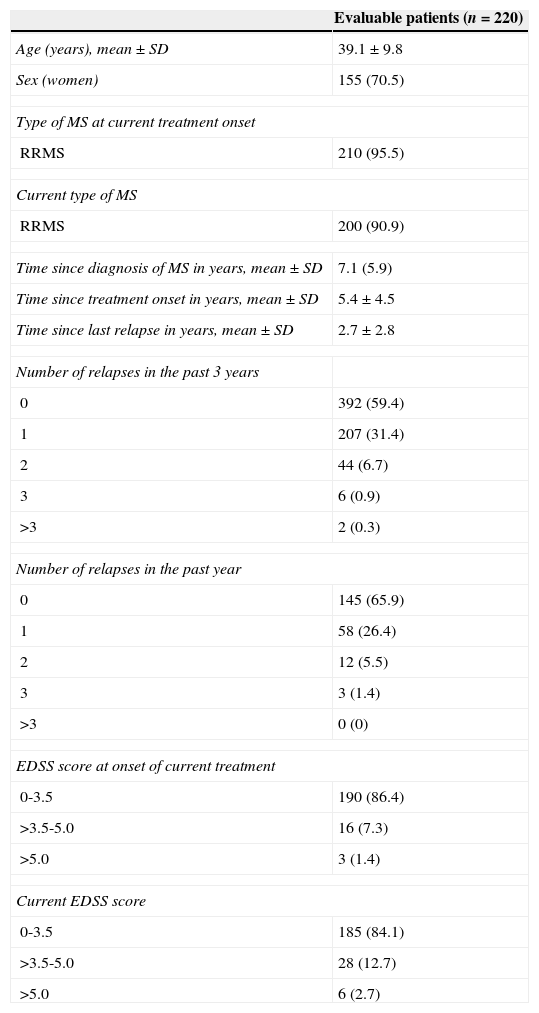
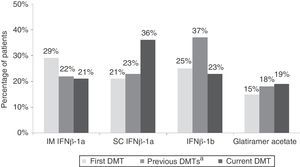
ResultsBaseline characteristics and treatmentWe included 259 patients from 32 centres located all across Spain; 220 patients were found to be evaluable. Patients’ demographic and clinical characteristics are summarised in Table 1. Fig. 1 shows treatment characteristics. Sixty percent of patients started DMT with IFNβ-1a, and 40% changed drug treatments during a mean treatment time of 5.4±4.5 years; the most common change was substituting one type of IFN for the other. The most frequent cause of drug substitution was lack of efficacy as perceived by the neurologist. At the time of the study, 57% of patients were being treated with IFNβ-1a. Mean treatment times with current DMT were 1.2±0.5 years and 5.0±4.1 years, depending on the drug.
Patients’ demographic and clinical characteristics
| Evaluable patients (n=220) | |
|---|---|
| Age (years), mean±SD | 39.1±9.8 |
| Sex (women) | 155 (70.5) |
| Type of MS at current treatment onset | |
| RRMS | 210 (95.5) |
| Current type of MS | |
| RRMS | 200 (90.9) |
| Time since diagnosis of MS in years, mean±SD | 7.1 (5.9) |
| Time since treatment onset in years, mean±SD | 5.4±4.5 |
| Time since last relapse in years, mean±SD | 2.7±2.8 |
| Number of relapses in the past 3 years | |
| 0 | 392 (59.4) |
| 1 | 207 (31.4) |
| 2 | 44 (6.7) |
| 3 | 6 (0.9) |
| >3 | 2 (0.3) |
| Number of relapses in the past year | |
| 0 | 145 (65.9) |
| 1 | 58 (26.4) |
| 2 | 12 (5.5) |
| 3 | 3 (1.4) |
| >3 | 0 (0) |
| EDSS score at onset of current treatment | |
| 0-3.5 | 190 (86.4) |
| >3.5-5.0 | 16 (7.3) |
| >5.0 | 3 (1.4) |
| Current EDSS score | |
| 0-3.5 | 185 (84.1) |
| >3.5-5.0 | 28 (12.7) |
| >5.0 | 6 (2.7) |
All variables are shown as n (%) if not otherwise indicated.
SD: standard deviation; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis.
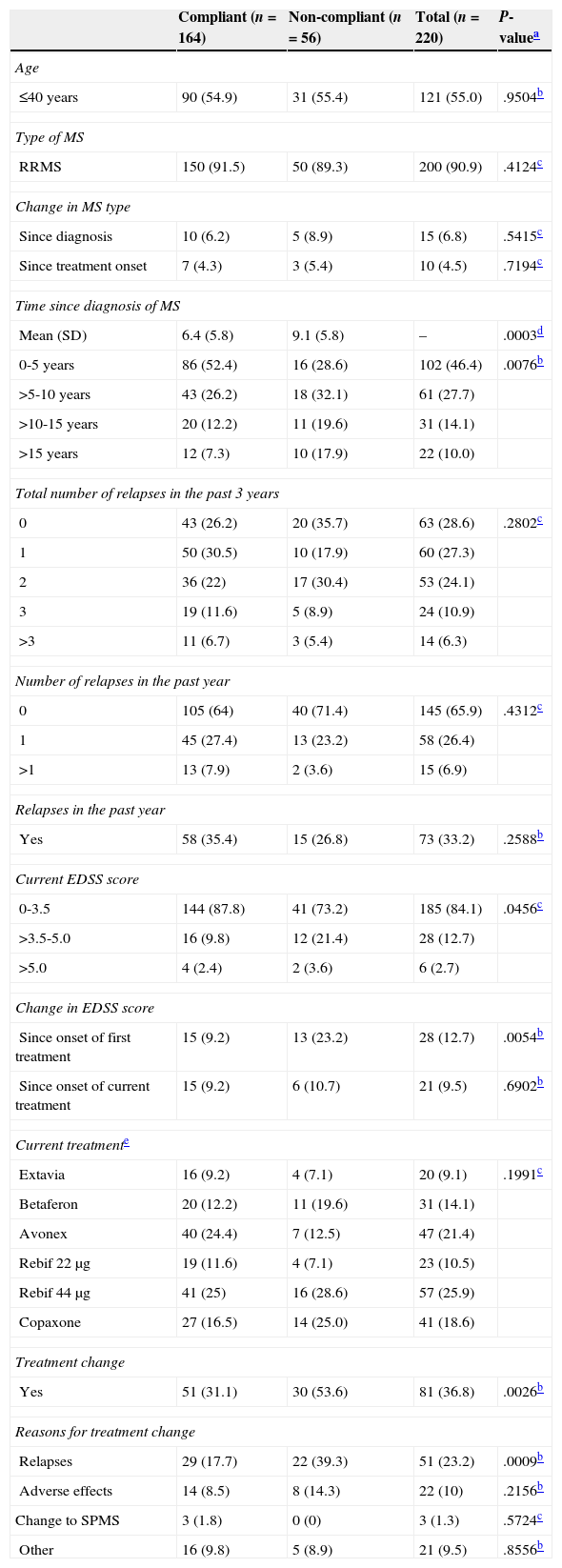
Seventy-five percent of patients were compliant. The compliant group contained a higher percentage of patients with a recent diagnosis of MS (<5 years) and less disability (0-3.5) according to the Expanded Disability Status Scale (EDSS).23 Furthermore, this group presented a lower percentage of patients whose disability had progressed since DMT onset, and fewer treatment changes; also, among patients who had changed treatment, a lower percentage of compliant patients did so due to relapses (Table 2).
Clinical and therapeutic characteristics of the patient total and the compliant and non-compliant subgroups
| Compliant (n=164) | Non-compliant (n=56) | Total (n=220) | P-valuea | |
|---|---|---|---|---|
| Age | ||||
| ≤40 years | 90 (54.9) | 31 (55.4) | 121 (55.0) | .9504b |
| Type of MS | ||||
| RRMS | 150 (91.5) | 50 (89.3) | 200 (90.9) | .4124c |
| Change in MS type | ||||
| Since diagnosis | 10 (6.2) | 5 (8.9) | 15 (6.8) | .5415c |
| Since treatment onset | 7 (4.3) | 3 (5.4) | 10 (4.5) | .7194c |
| Time since diagnosis of MS | ||||
| Mean (SD) | 6.4 (5.8) | 9.1 (5.8) | – | .0003d |
| 0-5 years | 86 (52.4) | 16 (28.6) | 102 (46.4) | .0076b |
| >5-10 years | 43 (26.2) | 18 (32.1) | 61 (27.7) | |
| >10-15 years | 20 (12.2) | 11 (19.6) | 31 (14.1) | |
| >15 years | 12 (7.3) | 10 (17.9) | 22 (10.0) | |
| Total number of relapses in the past 3 years | ||||
| 0 | 43 (26.2) | 20 (35.7) | 63 (28.6) | .2802c |
| 1 | 50 (30.5) | 10 (17.9) | 60 (27.3) | |
| 2 | 36 (22) | 17 (30.4) | 53 (24.1) | |
| 3 | 19 (11.6) | 5 (8.9) | 24 (10.9) | |
| >3 | 11 (6.7) | 3 (5.4) | 14 (6.3) | |
| Number of relapses in the past year | ||||
| 0 | 105 (64) | 40 (71.4) | 145 (65.9) | .4312c |
| 1 | 45 (27.4) | 13 (23.2) | 58 (26.4) | |
| >1 | 13 (7.9) | 2 (3.6) | 15 (6.9) | |
| Relapses in the past year | ||||
| Yes | 58 (35.4) | 15 (26.8) | 73 (33.2) | .2588b |
| Current EDSS score | ||||
| 0-3.5 | 144 (87.8) | 41 (73.2) | 185 (84.1) | .0456c |
| >3.5-5.0 | 16 (9.8) | 12 (21.4) | 28 (12.7) | |
| >5.0 | 4 (2.4) | 2 (3.6) | 6 (2.7) | |
| Change in EDSS score | ||||
| Since onset of first treatment | 15 (9.2) | 13 (23.2) | 28 (12.7) | .0054b |
| Since onset of current treatment | 15 (9.2) | 6 (10.7) | 21 (9.5) | .6902b |
| Current treatmente | ||||
| Extavia | 16 (9.2) | 4 (7.1) | 20 (9.1) | .1991c |
| Betaferon | 20 (12.2) | 11 (19.6) | 31 (14.1) | |
| Avonex | 40 (24.4) | 7 (12.5) | 47 (21.4) | |
| Rebif 22μg | 19 (11.6) | 4 (7.1) | 23 (10.5) | |
| Rebif 44μg | 41 (25) | 16 (28.6) | 57 (25.9) | |
| Copaxone | 27 (16.5) | 14 (25.0) | 41 (18.6) | |
| Treatment change | ||||
| Yes | 51 (31.1) | 30 (53.6) | 81 (36.8) | .0026b |
| Reasons for treatment change | ||||
| Relapses | 29 (17.7) | 22 (39.3) | 51 (23.2) | .0009b |
| Adverse effects | 14 (8.5) | 8 (14.3) | 22 (10) | .2156b |
| Change to SPMS | 3 (1.8) | 0 (0) | 3 (1.3) | .5724c |
| Other | 16 (9.8) | 5 (8.9) | 21 (9.5) | .8556b |
SD: standard deviation; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary-progressive multiple sclerosis.
All variables are shown as n (%) if not otherwise indicated.
Sixty-eight percent of patients reported no changes in administration patterns over time, and 81% stated that seasons did not affect their compliance. However, patients who had experienced higher relapse rates over the previous year displayed seasonal differences in treatment compliance. These patients considered summer to be the most difficult season for adherence; however, going on holiday was only occasionally regarded as an obstacle for compliance. The number of treatment changes was also related to changes in dosage over time, reduced compliance during holiday seasons, and use of medication reminders.
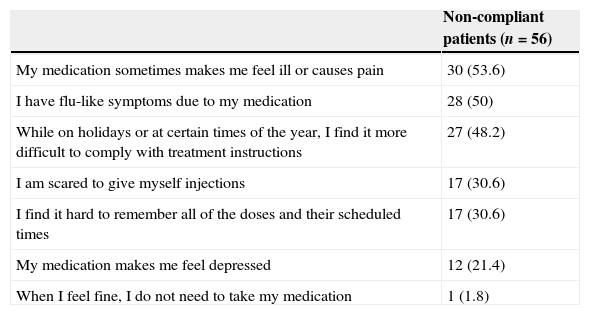
The most frequent reasons for non-adherence were secondary effects, flu-like symptoms, and difficulties in adhering to the treatment at certain times of the year (Table 3). No significant correlation was found between any of the causes reported by these patients and their clinical characteristics. While 70% of patients mentioned that their relatives helped them remember to take the medication, 75% reported using no medication reminders of any kind.
Self-reported reasons for non-adherence
| Non-compliant patients (n=56) | |
|---|---|
| My medication sometimes makes me feel ill or causes pain | 30 (53.6) |
| I have flu-like symptoms due to my medication | 28 (50) |
| While on holidays or at certain times of the year, I find it more difficult to comply with treatment instructions | 27 (48.2) |
| I am scared to give myself injections | 17 (30.6) |
| I find it hard to remember all of the doses and their scheduled times | 17 (30.6) |
| My medication makes me feel depressed | 12 (21.4) |
| When I feel fine, I do not need to take my medication | 1 (1.8) |
All values are shown as n (%). Patients were able to report more than one cause of non-adherence.
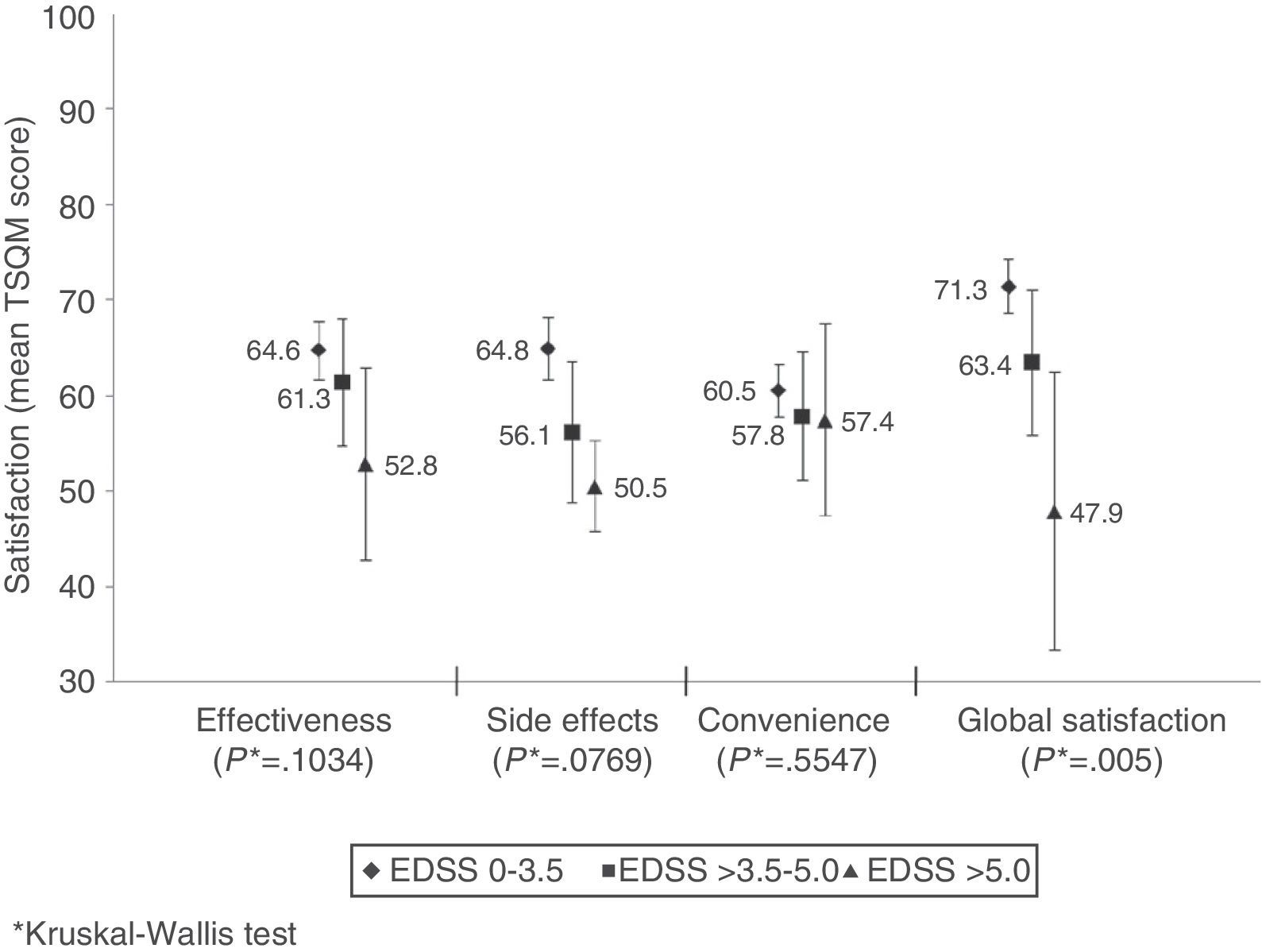
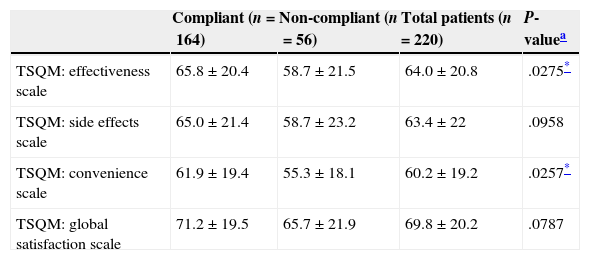
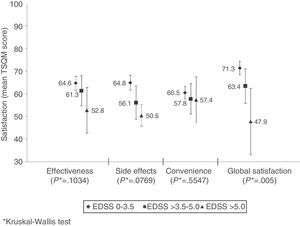
Mean global treatment satisfaction score was 70±20 for the patient total. Compliant patients showed higher mean scores than non-compliant patients on all domains of the TSQM; however, differences were only statistically significant for effectiveness and convenience (Table 4). Global satisfaction was inversely correlated with level of disability (Fig. 2). We found an inverse relation between satisfaction with treatment effectiveness and the number of treatment changes: mean scores were 66.6±19.9 in patients who changed treatment once; 61.3±20.8 in patients who changed twice, 58.8±21.9 in patients with 3 changes, and 48.1 (SD not available) in patients with 4 changes (P=.0324).
Satisfaction with first-line DMT in the patient total and the compliant and non-compliant subgroups
| Compliant (n=164) | Non-compliant (n=56) | Total patients (n=220) | P-valuea | |
|---|---|---|---|---|
| TSQM: effectiveness scale | 65.8±20.4 | 58.7±21.5 | 64.0±20.8 | .0275* |
| TSQM: side effects scale | 65.0±21.4 | 58.7±23.2 | 63.4±22 | .0958 |
| TSQM: convenience scale | 61.9±19.4 | 55.3±18.1 | 60.2±19.2 | .0257* |
| TSQM: global satisfaction scale | 71.2±19.5 | 65.7±21.9 | 69.8±20.2 | .0787 |
SD: standard deviation; DMT: disease-modifying therapy; TSQM: Treatment Satisfaction Questionnaire for Medication.
All scores are shown as mean±SD.
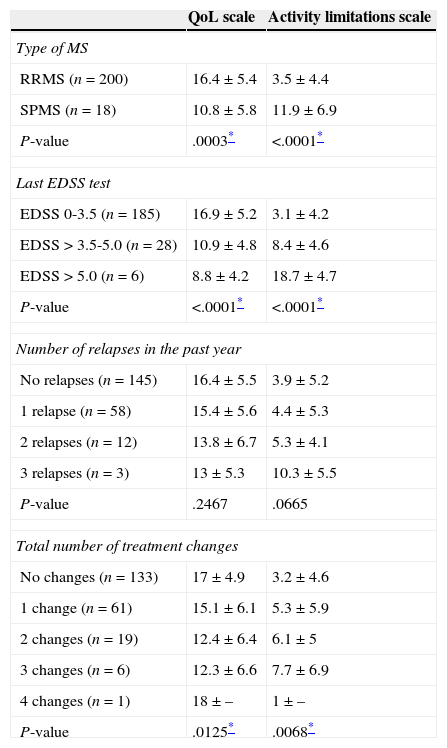
For the patient total, mean PRIMUS scores on the activity limitations and QoL scales were 4.1±5.2 and 16±5.6, respectively. RRMS patients (who showed less disability than SPMS patients) and those with fewer treatment changes presented fewer limitations for daily life activities and had higher QoL scores (Table 5). Emotional state was assessed using an analogue visual scale; mean score was 68.7±19.1.
PRIMUS questionnaire results broken down by clinical and/or treatment characteristics
| QoL scale | Activity limitations scale | |
|---|---|---|
| Type of MS | ||
| RRMS (n=200) | 16.4±5.4 | 3.5±4.4 |
| SPMS (n=18) | 10.8±5.8 | 11.9±6.9 |
| P-value | .0003* | <.0001* |
| Last EDSS test | ||
| EDSS 0-3.5 (n=185) | 16.9±5.2 | 3.1±4.2 |
| EDSS>3.5-5.0 (n=28) | 10.9±4.8 | 8.4±4.6 |
| EDSS>5.0 (n=6) | 8.8±4.2 | 18.7±4.7 |
| P-value | <.0001* | <.0001* |
| Number of relapses in the past year | ||
| No relapses (n=145) | 16.4±5.5 | 3.9±5.2 |
| 1 relapse (n=58) | 15.4±5.6 | 4.4±5.3 |
| 2 relapses (n=12) | 13.8±6.7 | 5.3±4.1 |
| 3 relapses (n=3) | 13±5.3 | 10.3±5.5 |
| P-value | .2467 | .0665 |
| Total number of treatment changes | ||
| No changes (n=133) | 17±4.9 | 3.2±4.6 |
| 1 change (n=61) | 15.1±6.1 | 5.3±5.9 |
| 2 changes (n=19) | 12.4±6.4 | 6.1±5 |
| 3 changes (n=6) | 12.3±6.6 | 7.7±6.9 |
| 4 changes (n=1) | 18±– | 1±– |
| P-value | .0125* | .0068* |
All values are expressed as mean score±SD; ‘n’ indicates the number of patients per clinical category who completed the PRIMUS questionnaire.
QoL: quality of life; SD: standard deviation; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary-progressive multiple sclerosis; PRIMUS: Patient-Reported Indices for Multiple Sclerosis.
In our setting, the percentage of adherence to first-line DMTs in patients with MS ranges from 62% to 87%.24–28 Differences in study design, the DMTs assessed, tools used to evaluate adherence, and the various working definitions of adherence make it difficult to compare studies. However, a retrospective study conducted in Spain using a methodology similar to our own (Morisky-Green test) reports an adherence rate of 68%.27 Based on the above, the percentage of compliance found in our study (75%) might be considered high, although it shows room for improvement.
Treatment adherence was associated with more recently diagnosed MS, lower disability and disability progression, and no treatment changes. On the other hand, non-adherence was shown to be directly and significantly correlated with changes in DMT due to drug ineffectiveness (appearance of exacerbations). Although evidence shows that compliance is linked to the clinical variables mentioned above,27–31 some studies have reported contradictory results, including greater adherence in patients with longer disease durations32 or after changing DMT.28,33 Compliance has also been related to type of MS26,30 and patient's age27; however, these connections were not found in our study. The directionality of the associations mentioned above is unclear, and the heterogeneous results found in the literature suggests that compliance depends on other factors as well. For example, drug self-administration, support from healthcare professionals, and medication reminders have been shown to improve adherence.30,34,35
The main reason why patients in our study do not adhere to treatments are medication-related discomfort and flu-like symptoms. Our findings are consistent with those described in a systematic review of DMT tolerability. According to this study, adverse effects were the most frequent cause of discontinuing treatment with SC IFNβ-1a and IFNβ-1b, and the second most frequent cause for discontinuing IM IFNβ-1a and glatiramer acetate. Flu-like symptoms were the most frequent adverse effect of treatment with IM IFNβ-1a and IFNβ-1b and the second most frequent of SC IFNβ-1a.36 Patients in our study had been treated with DMT for more than 5 years and for more than 1 year with their current treatment (mean values); this contrasts with the study by O’Rourke and Hutchinson which found that non-compliance due to adverse effects mainly occurred in the first months of treatment.37 However, the same study found that discontinuing the treatment at a later stage was linked to treatment ineffectiveness. Our data show a consistent connection between non-adherence and changes in relapse rate compared to the previous year. In any case, we found no significant differences in adherence associated with type of DMT.
Seasons had a considerable impact on adherence; we found a correlation between summer months and non-adherence, although this association was not statistically significant. Going on holiday was rarely cited as an obstacle, which suggests that other causes may contribute to this tendency. The warmest months are known to be associated with increased relapse rates in MS patients,38 and our study reports a connection between relapses and less regular administration patterns. In contrast with other studies including patient series,27,31,39 we found that forgetting to take the medication was relatively infrequent among non-compliant patients. On this subject, Arroyo et al. observed that forgetfulness, the most common reason for non-adherence during the first months of treatment, was later overtaken by administration-related factors.24 In our study, some of these factors (including fear of injections and perception of not needing them) were reported by 32.4% of all non-compliant patients. On the other hand, since few of our patients used medication reminders, family support may be the main reason for the low rates of forgetting doses in this sample.
Compliant patients experienced greater satisfaction with DMT in terms of convenience and effectiveness, as has been observed in previous studies.39,40 However, as seen with clinical variables associated with compliance, the directionality of this connection is unclear, and we were unable to determine it due to the study's observational design. MS requires long-term treatment involving daily to weekly injections, depending on the treatment regimen. Its benefits may be moderate compared to patients’ expectations and not noticeable in the short term. Patients who perceive their treatment as comfortable and those with more realistic expectations about effectiveness are therefore more likely to show adherence.41 Likewise, compliant patients experience lower relapse rates,28 which results in better states of health and greater treatment satisfaction. In fact, our study shows that patients with lower levels of disability presented greater global satisfaction with the treatment. The lower satisfaction related to efficacy, which is reported by patients with higher numbers of treatment changes, may be due to the fact that changes were motivated by ineffectiveness of the drug. Most changes consisted of substituting one type of IFN with another; however, we have observed that treatment satisfaction does not increase for patients changing between similar drugs, probably because these changes provide no additional effectiveness. Treatment satisfaction does increase when changes involve new therapeutic strategies and administration routes.42
Overall, patients in our study experienced few activity limitations and their QoL was quite high, especially those with RRMS; they also presented lower levels of disability and fewer treatment changes. A recent study evaluating MS population with the PRIMUS questionnaire also reported a connection between lower level of disability and greater QoL and/or fewer activity limitations, whereas RRMS was correlated with greater QoL only.43 The association between the number of treatment changes and QoL may be attributed to the connection between those changes and treatment ineffectiveness.
Our study presents the limitations inherent to observational studies. Furthermore, the Morisky-Green test, although restrictive, is an indirect measure that may at times overestimate patients’ adherence.27 Finally, the lack of association between compliance and selected clinical variables may result from the small number of patients included in some of the clinical categories, which may have affected the statistical power.
In conclusion, our population showed a high level of adherence to first-line DMTs. Seasonality apparently had little impact on adherence. However, the percentage of non-compliant patients, especially where this results from adverse effects of injections, evidences a need for improvement. Non-adherence was shown to be associated with MS progression time, disability level and progression, and number of treatment changes, which suggests that it is also linked to the limited effectiveness of current DMTs. Therefore, although treatment satisfaction and QoF were high, they both show an association with disability and number of treatment changes.
FundingThe COMPLIANCE study has received funding from Novartis Farmacéutica, S.A. for research purposes.
Conflict of interestAlbert Saiz has received professional fees (as a consultant and speaker) from Bayer-Schering, Merck-Serono, Biogen-Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, and Novartis. Sheila Mora and Julia Blanco are employed by Novartis Farmacéutica, S.A.
We would like to thank Beatriz del Val and Emili González-Pérez (Scientific Department at TFS Develop, Spain) for their assistance with the manuscript.
María Eugenia Marzo (H. San Pedro), Francisco Lacruz (H. de Navarra), Joaquín Peña (H. San Agustín), Dionisio Fernández (H. Cabueñes), Agustín Oterino (H. Marqués de Valdecilla), Miguel Ángel Llaneza (H. Arquitecto Marcide), Robustiano Pego (S. Nosa Sra. dos Ollos Grandes), María Ángeles del Real (H.G. de Ciudad Real), Ricardo Ginestal (F. Jiménez Díaz), Luis Ignacio Casanova (H.C. San Carlos), Lluisa Rubio (H.U. Príncipe de Asturias), Antonio Escartín (H. Sant Pau), Joan Massons (H. Sagrat Cor), Yolanda Blanco (H. Clínic), Francisco Barrero (H.U. San Cecilio), Jesús Foronda (H. de Jaén), Esther Dionisia Cancho (C.E. Don Benito), Ricardo Fernández (H. de Valme), Francisco Javier Carod (H. Virgen de la Luz), María Rosa García (H. Virgen de la Salud), José Manuel Flores (H. Virgen de Altagracia), Ramón Villaverde (H. Morales Meseguer), José Manuel López (H. Virgen de los Lirios), José Meca (C. Virgen de la Vega), Virginia María Araña (H.U. Doctor Negrín), Cristina Croissier (H.U. de Canarias), Virginia Meca (H. La Princesa), Jesús Cacho (H.U. de Salamanca), Wadih Bowakim (H. Río Hortega), Ambrosio Miralles (H. Infanta Sofía), José Francisco Plaza (H. del Sureste), Beatriz Romero (H. Son Llatzer).
Presented at the 64th Annual Meeting of the Spanish Society of Neurology (SEN), 20-4 November 2012, Barcelona.
Researchers contributing to the COMPLIANCE Study are listed in Appendix 1.
Please cite this article as: Saiz A, Mora S, Blanco J. Cumplimiento terapéutico con terapias modificadoras de la enfermedad de primera línea en pacientes con esclerosis múltiple. Estudio COMPLIANCE. Neurología. 2015;30:214–222.