There is a growing interest in new therapeutic strategies for the treatment of Alzheimer disease (AD) which focus on reducing the beta-amyloid peptide (Aβ) burden in the brain by sequestering plasma Aβ, a large proportion of which is bound to albumin and other proteins. This review discusses the concepts of interaction between Aβ and albumin that have given rise to AMBAR (Alzheimer's Disease Management by Albumin Replacement) project, a new multicentre, randomised, controlled clinical trial for the treatment of AD.
DevelopmentResults from preliminary research suggest that Albutein® (therapeutic albumin, Grifols) contains no quantifiable levels of Aβ. Studies also show that Albutein® has Aβ binding capacity. On the other hand, AD entails a high level of nitro-oxidative stress associated with fibrillar aggregates of Aβ that can induce albumin modification, thus affecting its biological functions. Results from the phase ii study confirm that using therapeutic apheresis to replace endogenous albumin with Albutein® 5% is feasible and safe in patients with AD. This process resulted in mobilisation of Aβ and cognitive improvement in treated patients. The AMBAR study will test combination therapy with therapeutic apheresis and haemopheresis with the possible leverage effect of Albutein® with intravenous immunoglobulin replacement (Flebogamma® DIF). Cognitive, functional, and behavioural changes in patients with mild to moderate AD will be assessed.
ConclusionsThe AMBAR study represents a new therapeutic perspective for AD.
Existe un creciente interés en las nuevas estrategias terapéuticas para la enfermedad de Alzheimer (EA) orientadas a reducir la carga de péptido β-amiloide (Aβ) en el cerebro mediante el secuestro de Aβ en el plasma, un alto porcentaje del cual se encuentra unido a albúmina y otras proteínas plasmáticas. En esta revisión, se analizan los conceptos de interacción entre Aβ y albúmina que han conducido al desarrollo del proyecto AMBAR (Alzheimer Management By Albumin Replacement), un nuevo estudio clínico multicéntrico, aleatorizado y controlado para el tratamiento de la EA.
DesarrolloResultados de estudios de investigación básica indican que Albutein® (albúmina terapéutica, Grifols) contiene niveles no cuantificables de Aβ. Asimismo demuestran la capacidad de Albutein® de unirse a Aβ. Por otro lado, en la EA existe un elevado estrés nitro-oxidativo asociado a los agregados fibrilares de Aβ que puede inducir la modificación de la albúmina, afectando a sus funciones biológicas. Resultados del estudio en fase ii confirman que el reemplazo de la albúmina endógena con Albutein® 5% mediante aféresis terapéutica es factible y seguro en pacientes con EA, produciendo una movilización de Aβ, además de una mejoría cognitiva de los pacientes tratados. En el estudio AMBAR se ensayará el uso combinado de aféresis terapéutica y hemoféresis con el posible efecto potenciador de Albutein® con reposición de inmunoglobulina por vía intravenosa (Flebogamma® DIF). Se evaluarán los cambios cognitivos, funcionales y conductuales en pacientes con EA leve o moderada.
ConclusionesEl estudio AMBAR representa una nueva perspectiva terapéutica ante la EA.
Alzheimer disease is a neurodegenerative process caused by the presence of intracellular deposits (neurofibrillary tangles of phosphorylated tau protein) resulting from the action of extracellular aggregates of Aβ peptides (Aβ) which originate senile plaques in the cerebral parenchyma.1–3 These plaques accumulate in small cerebral blood vessels to form amyloid deposits.4
There is increasing evidence of a rise in Aβ aggregate levels in the brain together with a decrease in the concentration of soluble Aβ in cerebrospinal fluid (CSF) in AD.5 Based on this observation, researchers are designing new therapeutic strategies aimed at reducing Aβ accumulation in the brain by changing the transportation of Aβ through the blood-brain barrier. Thus, sequestering Aβ in plasma could increase transport of free Aβ from CSF to plasma, thereby decreasing brain Aβ burden. This would restore the inherent balance between brain and blood levels of Aβ.6 The different types of apheresis would play a fundamental role in the new therapeutic strategies.
Plasmapheresis is a technique which separates plasma from blood cells, before the latter are returned to the bloodstream. During plasma exchange, the extracted plasma is replaced with an equivalent volume of plasma, or of colloid or crystalloid solutions. Plasma removal in therapeutic apheresis is performed to eliminate pathogenic elements.7 Experts estimate that approximately 90% of plasma Aβ is bound to circulating albumin, which favours its degradation by the liver.8 Therapeutic apheresis is especially intriguing as a potential new therapy for AD since it is based on plasmapheresis and plasma exchange combined with albumin replacement. Immunotherapy with antibodies that bind to plasma Aβ has been shown to reduce brain amyloid burden in mouse models,9 as well as in human studies.10,11
In this article, we review the dynamics of Aβ binding to albumin and the role that albumin plays in the context of nitro-oxidative stress associated with Aβ oligomers. We emphasise how these concepts may be applicable to the treatment of AD. We also present an update on the promising results from a recent phase II clinical trial of therapeutic apheresis with albumin for AD. These results have led to the launch of project AMBAR (Alzheimer Management By Albumin Replacement). This is a new clinical trial of an AD treatment consisting of a combination of therapeutic apheresis with albumin and immunoglobulin (IG), administered intravenously and at different doses. Grifols gave an in-depth presentation of this project at the 64th Annual Meeting of the Spanish Society of Neurology.
DevelopmentBinding dynamics of Albutein® to amyloid-betaThe main evidence motivating the Grifols research project on AD was as follows: (1) Soluble oligomeric Aβ, more toxic than the amyloid fibrils, shows a higher presence in brains affected by AD and is associated with cognitive impairment12; (2) a high percentage of Aβ in plasma is bound to albumin, which indicates that this protein could play a relevant role in preventing Aβ aggregation13; and (3) a dynamic equilibrium exists between levels of peripheral and central Aβ on the one hand, and Aβ clearance on the other. An alteration in this equilibrium may be a crucial event in AD pathogenesis and progression.14,15 Different agents able to bind to Aβ and shift the Aβ transport equilibrium towards plasma, such as anti-Aβ antibodies, have been and continue to be studied.10,11,16
Based on these findings and considering Grifols’ experience in plasmapheresis and manufacturing therapeutic albumin, the company decided to research a new treatment approach for AD based on therapeutic apheresis combined with volume replacement with therapeutic albumin. Since then, publications by other authors have provided additional support for this approach.17
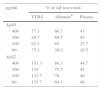
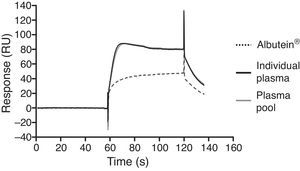
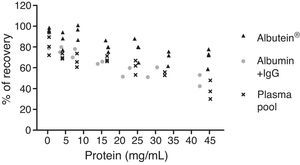
In a series of basic studies to confirm these preliminary results and refine the working hypothesis, researchers first quantified the amount of Aβ40 and Aβ42 in Albutein® 5%, 20%, and 25% (Grifols’ therapeutic albumin). They used different commercially available enzyme-linked immunoassays (ELISA) and the ABtest® (Araclon Biotech, Zaragoza), developed specifically for measuring Aβ in blood samples. The results obtained show that Albutein® does not contain detectable levels of Aβ.18 Secondly, researchers analysed the ability of therapeutic albumin to bind to synthetic peptides containing the amino acid sequence of human Aβ, using different ELISA tests as well as surface plasmon resonance (SPR).18 The results show that Albutein® is able to bind to Aβ. The accessible Aβ recovered (Aβ40 and Aβ42) after serial dilutions of buffer, plasma, and Albutein® until achieving final concentrations measurable by ELISA, decreased as the dilution's Aβ binding capacity increased: plasma>albumin>buffer (Table 1).18 Furthermore, Albutein® binding capacity to Aβ was also shown by SPR (Fig. 1). Plasma response is greater than that of purified albumins since it also contains other plasma proteins able to bind to the Aβ42 peptide, such as human immunoglobulin (IgG).10,11 Thus, ELISA testing of the amount of accessible Aβ in mixtures obtained after adding and incubating a precise amount of Aβ to solutions with increasing protein concentrations, shows that Aβ binding decreases as protein concentration increases. Broken down by solution type, the proportion is plasma>albumin+IgG>albumin (Fig. 2).18
Binding capacity of Albutein® to Aβ. Recovery of accessible Aβ (Aβ40 and Aβ42) in serial dilutions of buffer solution (TTBS), Albutein®, and plasma until final concentrations were quantifiable by ELISA.
| pg/mL | % of Aβ recovered | ||
|---|---|---|---|
| TTBS | Albutein® | Plasma | |
| Aβ40 | |||
| 400 | 77.3 | 66.7 | 43 |
| 200 | 89.7 | 69.7 | 45 |
| 100 | 92.3 | 65 | 25.7 |
| 60 | 75.3 | 30.5 | 16.5 |
| Aβ42 | |||
| 400 | 133.3 | 80.3 | 44.7 |
| 200 | 133 | 75.7 | 45 |
| 100 | 132.7 | 70 | 40 |
| 60 | 135.7 | 64.3 | 46 |
Other ongoing lines of research are assessing the potentially protective role of albumin in in vitro AD models. For example, adding Aβ aggregate to primary cultures of rat cortical neurons has been observed to reduce cell survival, but part of this neurotoxicity can be inhibited by concomitant treatment with Albutein®. Another series of in vitro studies aims at analysing how Albutein® can inhibit Aβ self-association by selectively binding to Aβ oligomers rather than to monomers and by preventing further growth of the Aβ assemblies.
Furthermore, the albumin from patients with AD is currently being described in detail; as occurs in such other diseases as hepatic cirrhosis or diabetes, albumin from patients with AD could present significant alterations at the structural or functional level.19 Lastly, preliminary results obtained using electron paramagnetic resonance spectroscopy suggest that albumin from patients with AD presents reduced functionality compared to that from healthy donors. This functionality improves after treatment with therapeutic apheresis plus Albutein® replacement.
All these results corroborate the hypothesis that albumin can be used in AD treatment. More specifically, they support the proposed therapeutic approach consisting of Albutein® and Flebogamma® DIF (intravenous immunoglobulin, IVIG) combined with therapeutic apheresis. This approach is undergoing clinical research as a new form of AD therapy.
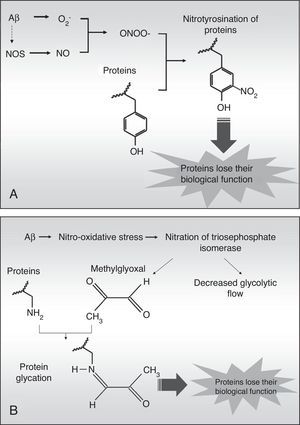
Nitrotyrosination of albumin due to nitro-oxidative stress increases its ability to bind to amyloid-beta peptide fibrilsThe high levels of nitro-oxidative stress in AD are associated with Aβ fibril aggregates, which engender free radicals.20 These aggregates induce cascades of reactive oxygen compounds inside the cells, which can easily reach adjacent tissues due to their chemical properties. The superoxide anion (O2−) is especially harmful since it can trigger oxidation processes directly, and nitration and glycation processes indirectly. The superoxide anion can react with nitric oxide (NO) from the endothelium, neurons, or glia to generate peroxynitrite (ONOO−) which is highly reactive and nitrates protein tyrosines in a process called nitrotyrosination (Fig. 3). This process has already been described in AD.21 Nitrotyrosination of proteins induces loss of their biological function, as occurs in glycation, an oxidative process directly caused by methylglyoxal, a by-product of glycolysis (Fig. 3B), or else indirectly caused by a high level of monosaccharides in the extracellular medium.19,22 Therefore, albumin can be nitrotyrosinated and glycated in a pro-oxidant medium, which would affect its biological functions.
A study investigating the effect of nitrotyrosination and glycation of abumin bound to Aβ42 was carried out in cooperation with the following institutions in Barcelona: Laboratory of Molecular Physiology and Channelopathies, Department of Experimental and Health Sciences at Universitat Pompeu Fabra, Instituto Grifols, Fundació ACE-Institut Català de Neurociències Aplicades, and the Neurology Department at Hospital Universitario Vall d’Hebron.
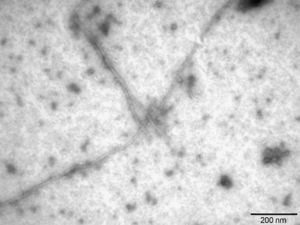
Firstly, Aβ42 fibrils were characterised using transmission electron microscopy (Sigma-Aldrich, St. Louis, MO, USA). After this step, native albumin (Grifols), albumin nitrotyrosinated in vitro with SIN-1 (Sigma-Aldrich), a peroxynitrite donor, and albumin glycated in vitro with methylglyoxal (CosmoBio Co., LTD) were incubated with soluble and fibrillar Aβ42. Samples were immunoprecipitated with anti-albumin antibody (Acris Antibodies, Herford, Germany). The Aβ42 concentration was quantified in supernatants using the Human Amyloid β assay kit (Immuno-Biological Laboratories Co., LTD, Tokyo, Japan).
The literature on AD shows that Aβ42 is produced in much lower quantities than Aβ40. Nevertheless, Aβ42 is more prone to aggregation since it is more hydrophobic and its pathological relevance in AD is therefore higher.23 In this study, Aβ42 fibrils were prepared1 and then described using transmission electron microscopy (Fig. 4), which showed Aβ42 fibrils as typical amyloid fibrils with a regular diameter and a helical and non-branching structure.
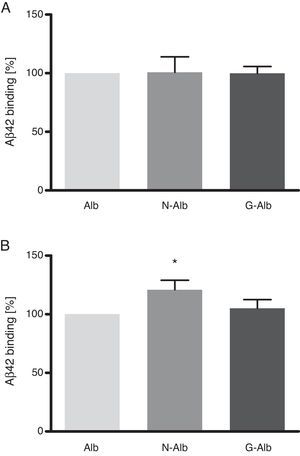
These data show that nitrotyrosination and glycation of albumin does not affect its binding capacity to soluble Aβ42 (Fig. 5A), which is the type of Aβ that is not toxic to cells. However, nitrotyrosination of the albumin did result in increased binding of the Aβ42 fibril forms (Fig. 5B; P<.05). The pathophysiological relevance of these data lies in that nitrotyrosinated albumin has a higher resistance to degradation. This situation is feasible since nitration of proteins leads to the formation of dityrosine bridges between nitrated tyrosines of different proteins. This generates protein aggregates which are difficult for protein-degrading systems to digest.24
The potential beneficial effects of therapeutic apheresis for AD25 might be associated to some extent with the removal of nitrotyrosinated albumin, and therefore, with mechanical clearance of Aβ42.
Update on results from the phase II clinical trial of therapeutic apheresis with albumin for ADThe clinical strategy proposed by Grifols includes designing different consecutive clinical trials to test the effectiveness and safety of therapeutic apheresis with albumin for AD.
The pilot study at the start of the project25 recruited 10 patients with mild to moderate AD to undergo 6 sessions of therapeutic apheresis with 5% Human Albumin Grifols (Albutein®) for 3 weeks, 2 sessions per week. At one year of follow-up post-treatment, subjects’ biochemical, cognitive, and neuroimaging variables were measured; the positive results obtained for cognition and the level of satisfaction of patients and their families led us to repeat the trial. Extending the study let us confirm the results and consolidate the experience with the treatment in this type of population. Patients included in the pilot study were offered the possibility of being treated with IVIG (Flebogamma® DIF, Grifols)16; this showed that this molecule could also have a therapeutic effect for AD. A phase II clinical trial was then launched to continue this line of research on therapeutic apheresis.25
The phase II clinical trial was designed as a multicentre randomised blind placebo-controlled parallel-group study. Its main aim was to assess whether therapeutic apheresis with Albutein® 5% was able to modify Aβ40 and Aβ42 concentrations in plasma and CSF in the treatment group between the initial and the last consultation. The secondary aim was to detect any stabilisation or improvement in cognitive skills. Our aims were to assess structural and functional changes in neuroimaging studies (magnetic resonance neuroimaging [MRI] and brain single-photon emission computed tomography [SPECT]), and to evaluate treatment safety.
Patients’ ages ranged from 55 to 85; they had mild to moderate AD and were on stable treatment with acetylcholinesterase inhibitors. They were recruited from 4 centres (2 in Spain and 2 in the USA). Patients were randomised in 2 arms (1:1): the active arm (19 subjects) and the control (20 subjects). The control group followed the same treatment regime, with the exception of therapeutic apheresis sessions, which were sham. Patients in both groups underwent a maximum of 18 sessions of therapeutic apheresis with Albutein® 5%, administered in 3 different treatment patterns: (a) 2 therapeutic apheresis sessions per week for 3 weeks; (b) one weekly therapeutic apheresis for 6 weeks, and (c) one therapeutic apheresis session every 2 weeks for 12 weeks. After the treatment period (21 weeks), both groups were in follow-up for 6 months.
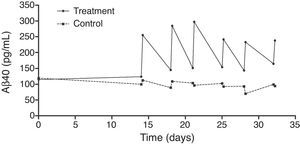
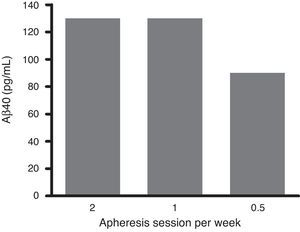
An exhaustive analysis of the results of the phase II trial is ongoing and final results are expected to be published soon. However, this update serves as a preview of some of the tendencies that have been observed. The plasma Aβ40 levels measured in the treatment group showed consistent variations in a zigzag pattern (Fig. 6) throughout the 3 periods of therapeutic apheresis (this is not the case with Aβ42), which replicated the findings observed in the pilot study. After the therapeutic apheresis sessions, Aβ40 levels returned to baseline values. During the treatment period, we observed that after each therapeutic apheresis session, plasma Aβ40 levels increased considerably in the study group compared to those in the control group. Total mobilisation of Aβ40 (amount of mobilised peptide per millilitre of plasma processed during each session) was similar between the treatment period with 2 therapeutic apheresis per week and the period with just one weekly therapeutic apheresis. Plasma Aβ42 levels showed variations in both groups but their overall pattern was not as plain as that exhibited by plasma Aβ40. Furthermore, Aβ40 and Aβ42 levels in CSF did not differ between the groups (Fig. 7).
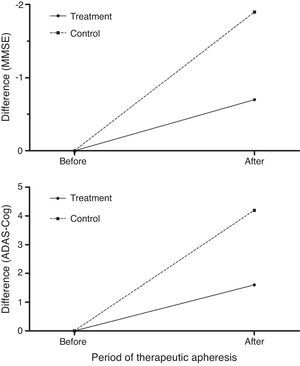
Treated patients scored better than controls on the MMSE and ADAS-Cog26,27 after therapeutic apheresis sessions (Fig. 8) but their scores had worsened at 12 months of follow-up (6 months after undergoing therapeutic apheresis for the last time).
Regarding MR neuroimaging results, a progressive loss of hippocampal volume was observed in both groups, with significant differences over the course of the study. However, no significant decreases were observed in the volume of the posterior cingulate or the total volume of the brain. In addition, SPECT images of treated patients showed that cerebral metabolism tended to stabilise immediately after the maximum intensity treatment period and worsen at the end of the treatment period. In contrast, the control group exhibited progressive decline throughout the whole study.
In terms of safety, there were no adverse events related to either Albutein® 5% or apheresis, whether severe or otherwise. We were able to confirm that therapeutic apheresis was well tolerated and presented a good safety profile in this group of patients.
Although the preliminary results of the phase II clinical trial show clinical and neuroimaging changes, they must be interpreted with caution until the complete data analysis can be published. However, the positive tendencies shown by currently available data seem to confirm the hypothesis already suggested by the pilot study: replacing endogenous albumin with Albutein® 5% through therapeutic apheresis is feasible and safe in patients with AD. It elicits consistent mobilisation of Aβ in plasma and cognitive stabilisation is observed in the patients receiving treatment. These results, together with those obtained with IVIG by Grifols, led to the design of the new AMBAR study, which we explain below.
AMBAR study: therapeutic apheresis and haemapheresis with albumin and intravenous immunoglobulinThe new AMBAR study (NCT01561053) was designed based on the promising results from earlier studies combining therapeutic apheresis with Albutein® as AD treatment and the knowledge gained since then.25 The study was registered as phase III in Europe (Spain) and as phase IIB in the United States.
The strategy of the AMBAR study is the combined use of therapeutic apheresis and haemapheresis with albumin (Albutein®) and replacement with IVIG (Flebogamma® FIG) at 3 different doses. The underlying hypothesis is that albumin will bind to Aβ, and at the same time, correct a possible immunological deficit. Haemapheresis is a type of therapeutic apheresis which uses centrifugation to separate blood cells, which are then added back to the blood of the patient. It extracts a limited volume of plasma (between 650 and 800mL), which is replaced by an albumin or Ig solution equivalent in grams to the extracted plasma.
The AMBAR study includes a first stage of intensive treatment followed by a second stage of maintenance treatment. The initial intensive treatment lasts 6 weeks with one session of therapeutic apheresis per week: 2.5–3L of plasma are extracted and replaced with the same volume of Albutein® 5%. It requires a central venous catheter, a double lumen central venous catheter on the subclavian or jugular vein, and a conventional apheresis device (centrifugation or filtration). Next, haemapheresis, considered maintenance treatment, is performed for 12 months. It consists of therapeutic apheresis of a low monthly volume of plasma (650–800mL approximately) which is replaced by Albutein® 20% (a maximum of 100mL or 200mL), and alternating with Flebogamma® DIF (a maximum of 10g or 20g) depending on the treatment branch. The albumin dose is adjusted according to the actual volume of extracted plasma (depending on the weight of the patient) and doses of Flebogamma® DIF were established for each group. Access is peripheral and the apheresis device is based on the prototype by Grifols. We should mention that the study placed special emphasis on reducing the discomforts of treatment for patients. This affected both the design of the plasmapheresis device and the alternating infusions of albumin and IG.
The AMBAR study has a multicentre randomised blinded and placebo-controlled parallel-group design which assesses cognitive, functional, and behavioural changes in patients with mild to moderate AD. It includes 3 treatment groups receiving different doses and a control group with a sham treatment. Subjects were randomised with a ratio of 1:1:1:1. The sample calculated to obtain the set results included 350 patients from centres in Spain and the United States.
The main efficacy variable will be the change from the baseline cognitive scores measured with the ADAS-Cog scale (6 measurements) in the 3 treatment branches.
As secondary efficacy variables, we will measure changes from baseline in scores on cognitive, functional, behavioural, and overall progression tests. These parameters were measured with the MMSE, neuropsychological battery, neuropsychiatric inventory, Alzheimer's Disease Co-operative Study-Activities of Daily Living Inventory, Clinical Dementia Rating Sum of Boxes, Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change, Cornell Scale for Depression in Dementia, Columbia-Suicide Severity Rating Scale, Quality of Life-Alzheimer's Disease, and Resource Utilisation in Dementia (RUD-Lite®). The trial will also assess changes in Aβ peptide concentration, plasma and CSF levels of Aβ40 and Aβ42, and CSF t-tau and p-tau levels. Furthermore, structural changes in hippocampal volume, posterior cingulate volume, and other areas of interest as shown by MRI (6 measurements) will be assessed, as well as any functional changes in the brain detected by positron emission tomography with 18F-fludeoxyglucose.
To determine treatment safety of haemapheresis with a combination of human albumin and IVIG, researchers will record vital signs, different laboratory parameters, and the percentage of interventions associated with at least one adverse event potentially related to the study procedure. During treatment periods, anxiety and agitation tests will be conducted, when appropriate, using the overt aggression scale and the agitated behaviour scale.
By the end of November 2012, 19 patients had been invited to participate in the study; 18 of them were randomised. Seven patients dropped out: 3 withdrew their consent to participate, 2 presented adverse effects (jugular vein thrombosis during intensive treatment), and 2 did not have useable veins during the maintenance phase. In fact, thrombotic complications associated with the catheter are not infrequent,28–31 and in our series, both of the latter patients were diagnosed with an underlying thrombotic disease. Eleven patients remain in the study at the time of this update.
ConclusionsResults from experimental models have confirmed that Albutein® and Flebogamma® DIF are able to bind to Aβ40, which indicates that the potential effect of both proteins may contribute to a therapeutic strategy for AD. Furthermore, clinical trials conducted to date using therapeutic apheresis with Albutein® have shown that treatment is able to modify plasma and CSF Aβ concentrations in patients with AD, and this finding has been associated with stabilisation or improvement of cognitive skills. These positive results have led to the design of the new AMBAR study which combines therapeutic apheresis procedures with Albutein® (albumin) and haemaphereresis with Flebogamma® DIF (IG). The multicentre randomised blind placebo-controlled parallel-group AMBAR study, which integrates different therapeutic components using different doses and procedures (concomitant multimodal clinical trial), will assess cognitive, functional, and behavioural changes in patients with mild to moderate AD. In short, the AMBAR study proposes new therapeutic perspectives for AD.
Conflicts of interestM Costa, AM Ortiz, JI Jorquera, M Torres, and A Páez are employed by Grifols. M Boada, FJ Muñoz, E Ramos-Fernández, and B Guivernau affirm that Grifols financed the projects whose results have been provided in this study.
The authors would like to thank the following people for their participation in these studies: Ernest Palomer, Gerard ILL-Raga, Marta Tajes, Mònica Bosch-Morató, Miguel A Valverde (Laboratory of Molecular Physiology and Channelopathies, Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona); Isabel Hernández, Mar Buendia, Lluís Tárraga (Fundació ACE. Institut Català de Neurociències Aplicades, Barcelona); Javier Olazarán (Neurology Department, Hospital General Universitario Gregorio Marañón, Madrid); Fernando Anaya (Nephrology Department, Hospital General Universitario Gregorio Marañón, Madrid); Joan Muñoz, Joan Ramón Grifols, Pilar Ortiz (Banc de Sang i Teixits, Barcelona); Isabel Roca, Gemma Cuberas (Department of Nuclear Medicine, Hospital General Universitari Vall d’Hebron, Barcelona); Lourdes Rubio, Gustavo Torres (Clínica Corachán, Barcelona); Ángel Bittini (Department of Nuclear Medicine, Hospital General Universitario Gregorio Marañón, Madrid); Juan Guzmán de Villoria (Radiology Department, Hospital General Universitario Gregorio Marañón, Madrid); Isidre Ferrer (Institute of Neuropathology, Hospital Universitario Bellvitge, Barcelona). Mercè Boada, Francisco J Muñoz, Eva Ramos-Fernández, and Biuse Guivernau would like to thank Grifols for financing the projects whose results have been presented in this study. The authors would also like to thank Jordi Bozzo (Grifols) for helping draft and edit this manuscript.
Please cite this article as: Boada M, Ramos-Fernández E, Guivernau B, Muñoz FJ, Costa M, Ortiz AM, et al. Tratamiento de la enfermedad de Alzheimer mediante terapia combinada de aféresis terapéutica y hemoféresis con albúmina e inmunoglobulina intravenosa: fundamentos y aproximación terapéutica al estudio AMBAR (Alzheimer Management By Albumin Replacement). Neurología. 2016;31:473–481.