Post-stroke depression (PSD) is the most common mood disorder following a stroke, and also the main factor limiting recovery and rehabilitation in stroke patients. In addition, it may increase mortality by up to ten times.
DevelopmentPSD occurs in 1 in 3 stroke patients and more than half of all cases are neither diagnosed nor treated. Several mechanisms, including biological, behavioural, and social factors, are involved in its pathogenesis. Symptoms usually occur within the first three months after stroke (early onset PSD), and less frequently at a later time (late onset PSD). Symptoms resemble those of other types of depression, although there are some differences; PSD patients experience more sleep disturbances, vegetative symptoms, and social withdrawal. For PSD diagnosis, we recommended vigilance and use of specific diagnostic tools such as the Patient Health Questionnaire-2 (PHQ-2). The treatments of choice are selective serotonin reuptake inhibitors (SSRI). However, there are still many unanswered questions in the treatment of PSD, such as the best time to start treatment or the effects of antidepressants on cognition and motor function, among others.
ConclusionsNeurologists play a pivotal role in the care and management of patients recovering from stroke. They must be familiar with methods for early detection and treatment of PSD, as this can facilitate a patient's functional recovery and social reintegration, and improve quality of life for patients and their families.
La depresión post ictus (DPI) es el trastorno afectivo más frecuente tras un ictus y el principal factor que limita la recuperación y rehabilitación de los pacientes, además de poder incrementar su mortalidad hasta 10 veces.
DesarrolloLa DPI se presenta en uno de cada 3 pacientes con ictus y en más de la mitad de los casos no se diagnostica ni se trata. En su etiopatogenia son varios los mecanismos implicados: biológicos, conductuales y sociales. Los síntomas suelen aparecer en los primeros 3 meses tras el ictus (DPI «precoz») y menos frecuentemente más tarde (DPI «tardía»). Los síntomas son similares a los de otras depresiones, aunque con algunas diferencias, como presentar más trastornos del sue¿no, síntomas vegetativos e introversión para las relaciones sociales. Para su diagnóstico se recomienda mantener una actitud vigilante y emplear herramientas diagnósticas específicas, como el Patient Health Questionaire-2 (PHQ-2). Finalmente, el tratamiento de elección son los inhibidores selectivos de la recaptación de serotonina (ISRS). No obstante, aún son muchas las cuestiones por resolver en el tratamiento de la DPI, como cuándo es el mejor momento para iniciar el tratamiento o el efecto de los antidepresivos sobre la cognición y la función motora, entre otros.
ConclusionesLos neurólogos desempe¿nan un papel fundamental en la recuperación de los enfermos con ictus. Es necesario que estén familiarizados con la detección temprana y el trata-miento de la DPI, para así facilitar la recuperación funcional del paciente, su reinserción social y la mejora en la calidad de vida del enfermo y su familia.
Stroke has traditionally been considered a disease mainly affecting motor performance; as a result, approaches to hospital care, rehabilitation, and follow-up focus almost exclusively on this area. However, studies have recently demonstrated that there are other aspects such as cognition, behaviour, and emotion that greatly affect the impact that stroke will have on a patient's life.
Within the emotional-cognitive sphere, depression will be a determining factor for these patients. We currently know that depression is the most frequent neuropsychological complication of stroke.1,2 Nevertheless, in addition to post-stroke depression (PSD), many other neuropsychological symptoms may be present after stroke: anxiety, irritability, agitation, and emotional incontinence; changes in emotional experience; sleep disturbances; behavioural disorders such as disinhibition; apathy and fatigue; and psychotic symptoms such as delusions and hallucinations.1–3
We also know that PSD is the main predictor of poor functional outcome after stroke. Presence of PSD is associated with poorer functional and cognitive recovery, increased limitations on daily life activities, and social and interpersonal activities, poorer quality of life, and higher mortality rates (up to 10 times higher than in patients without PSD).3,4
Furthermore, we know that there are many risk factors for developing PSD, including more severe motor deficit, a higher degree of disability, and a poorer social support network. Identifying these factors permits early application of prevention and treatment strategies.1
However, although prevalence of PSD is high, it is generally underdiagnosed and usually undertreated, as we will discuss in a later section.
PSD is a frequent complication with severe negative repercussions for both patients and their carers, and yet it is clearly predictable and treatable. Therefore, it is essential to understand the risk factors for PSD and identify it in patients as soon as possible.
Prevalence of PSDEstimating the real prevalence of PSD is not an easy task due to the methodological differences between the studies that have been conducted.1,2 First, the diagnostic criteria for depression are not the same in all studies, although it must be said that most of them conducted structured interviews and followed the criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).
Second, the scales applied to assess the results also vary from one study to the other. Some use PSD-specific scales whereas some other studies use general scales, such as the Hamilton Rating Scale for Depression (Ham-D); furthermore, some studies use self-evaluation scales and others do not.
Third, inclusion criteria vary considerably. In fact, some studies excluded patients that other studies included (for example, patients with aphasia or dementia), and inclusion criteria also differed with regard to the target populations, age groups, or phases of the disease (acute- vs chronic-phase stroke).
Lastly, it has been demonstrated that prevalence rates of PSD depend on the setting where patients are examined.5
In general, prevalence rates of PSD range from 25% to 79% depending on the patient selection criteria used by each of the different studies.5,6
Community-dwelling patients have the lowest prevalence rates; in this setting, 14% of patients present major depression, and 9%, minor depression. In hospital settings, including acute-care patients and those in rehabilitation, prevalence rates of major and minor depression were 21.6% and 20.0%, respectively. Among patients discharged from hospital, prevalence rates of major and minor depression were 24.0% and 23.9% at times ranging from 3 months to 3 years after stroke. Other studies show similar rates, although the percentage of patients reporting major depression is lower than that diagnosed with minor depression.7
White et al.8 recently published data from patients with lacunar stroke only, extracted from a larger study, Secondary Prevention of Small Subcortical Strokes (SPS3). They examined the prevalence of depression, its correlation to other factors, and the course of depression over time in a cohort of 2477 patients with a history of lacunar stroke. Prevalence of depression at 4 months after stroke was 19%. Older age, male sex, and absence of cognitive impairment were the factors associated with a lower risk of depression. On the contrary, functional disability, living with a spouse or family, and presenting risk factors for stroke were associated with a high risk of depression. The study stated that likelihood of depression decreased with age at a rate of 1.12 times lower per year (95% CI: 1.06-1.17).8
However, the impact of certain factors on PSD prevalence is a controversial topic. Some studies have found a higher prevalence of PSD in patients with cognitive impairment, while others have not.1 Similarly, and contrary to the results from the SPS3 study, many authors claim that older age constitutes a risk factor for PSD, resulting in increased prevalence in elderly patients.5,6
Diagnosis of PSDDiagnosis of PSD is complex due to the frequent presence of other symptoms, especially those associated with cognitive impairment, such as aphasia, agnosia, apraxia, and changes in memory.1 Additionally, some symptoms of stroke or of depression may overlap and be indistinguishable from each other. Symptoms common to both entities include sleep disorders, difficulty concentrating, and loss of appetite. These similarities may lead to overestimating depression in stroke patients.9 On other occasions, many symptoms are not identified as the result of depression. In these cases, they are mistaken for consequences of stroke or old age (in elderly patients), which may lead to an underestimation of the diagnosis.2,10
Due to these diagnostic difficulties, between 50% and 80% of all PSD cases are not identified by doctors who are not psychiatrists.6,9
Some studies have compared several diagnostic tools in order to determine the most appropriate one. Some authors state that the Geriatric Depression Scale (GDS) is a better diagnostic tool than other scales, such as the Hospital Anxiety and Depression Scale (HADS).11 GDS is especially indicated for patients with a high level of functional ability and mild cognitive impairment.12
Quaranta et al.13 evaluated the diagnostic accuracy of the Poststroke Depression Rating Scale (PSDRS), a tool specifically designed for diagnosing PSD. They then compared the results with those of Ham-D. Both scales were reliable diagnostic tools, and they both showed good sensitivity and specificity. Cognitive impairment had a considerable effect on Ham-D, whereas PSDRS was influenced by the patient's age. Healey et al.14 compared 3 depression scales: the Beck Depression Inventory-Fast Screen (BDI-FS), Brief Assessment Schedule Depression Cards (BASDEC), and HADS. BASDEC demonstrated the greatest validity and precision. Similarly, Berg et al.15 compared different screening tools and concluded that the Ham-D, BDI-FS, and Clinical Global Impression (CGI) assessments carried out by professionals were useful for evaluating depression alongside DSM diagnostic criteria (DSM-III-R or other versions). They found no significant differences between these scales. In addition, these authors do not recommend using the Visual Analogue Mood Scale (VAMS) in patients with aphasia or other cognitive dysfunctions.
Other authors16 have demonstrated that PSD can be successfully diagnosed by using structured or semi-structured neuropsychiatric interviews following current DSM criteria. They concluded that no specific diagnostic instruments were necessary. PSD is generally diagnosed according to DSM guidelines, sometimes differentiating major from minor depression.
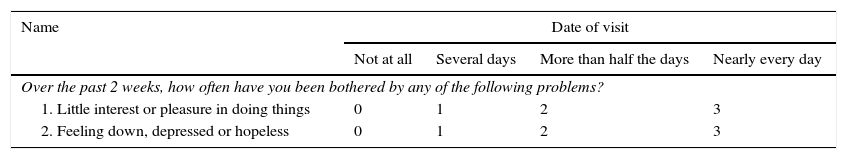
However, a study17 published in Stroke in 2012 investigated the diagnostic value of the 9-item and 2-item Patient Health Questionnaire (PHQ-9 and PHQ-2) and compared it with other scales (HADS, BDI-II, Distress Thermometer, and Kessler-10) in acute stroke patients with preserved ability to communicate. PHQ-9 includes all 9 symptoms of depression established by the DSM, whereas PHQ-2 only includes 2: anhedonia and depressed mood. The study showed a good diagnostic value for PHQ-9 scores ≥10 and for PHQ-2 scores ≥2. This group also recommends using PHQ-2 in all patients, and PHQ-9 only in those with a positive result on the PHQ-2 because this approach demonstrates the greatest sensitivity. The authors suggest these 2 screening tools for stroke patients since they are brief and easy to use, their only limitation being that they cannot be used in patients with significant cognitive and verbal impairment (Table 1).
Patient Health Questionnaire (PHQ-2)
| Name | Date of visit | |||
|---|---|---|---|---|
| Not at all | Several days | More than half the days | Nearly every day | |
| Over the past 2 weeks, how often have you been bothered by any of the following problems? | ||||
| 1. Little interest or pleasure in doing things | 0 | 1 | 2 | 3 |
| 2. Feeling down, depressed or hopeless | 0 | 1 | 2 | 3 |
Positive score >2.
DSM IV-TR18 categorises PSD as a mood disorder resulting from a general medical condition (stroke in this case). It can be classified into subtypes according to the following specifiers: depressive features, major depressive-like episode, and mixed features (manic features, depression). According to this classification, depression must be a direct physiological consequence of stroke.
Therefore, we normally use the term PSD to refer to major depressive-like episodes that follow stroke. Diagnosis of PSD requires evidence from the patient's medical history, and findings from the clinical examination and complementary tests, confirming that depression is a direct physiological consequence of stroke.
According to DSM-IV-TR diagnostic criteria, 5 or more symptoms must be present during at least 2 weeks for major depression to be diagnosed.
Pathophysiological mechanisms of PSDThe pathophysiological mechanisms of PSD are not yet fully understood. Biological, behavioural, and social factors are thought to be involved, and PSD is therefore a multifactorial process.
Biological factors can be divided into several different types: stroke location, stroke type, neurobiological pathways involved, mechanisms of inflammation and apoptosis, and genetic factors.
Stroke location and PSDThe first studies on the origin of PSD focused on the connection between cerebrovascular lesion location and development of PSD. Robinson et al.,19 for example, were the first to suggest a connection between PSD and a lesion in the left hemisphere. According to these authors, the lesions supposedly responsible for PSD were located in the left frontal and dorsolateral cortical regions and in the left basal ganglia. Many other studies have later corroborated this association.6,20 Other researchers21 found that lesions located in the left lateral frontal lobe, caudate nucleus, and putamen were significantly more likely to cause depression after acute stroke than similar lesions located in the right hemisphere. However, several other studies did not confirm this connection. A meta-analysis performed by Carson et al.22 could not support the connection between the stroke location or laterality, and risk of developing PSD. Another study23 showed that frontal or basal ganglia lesion location was a risk factor for PSD, but it found no correlation between laterality and depression. Likewise, a subsequent review of the methodological limitations of the studies addressing lesion location and PSD observed that the link between risk of developing PSD and frontal/basal ganglia lesions depended on the study population; studies in hospital inpatients showed a stronger association than those in community-dwelling patients.24 In another study, Nishiyama et al.25 found that presence of lesions in the left lenticulocapsular area, as well as hypertension and education level, was an independent predictor of symptoms of depression after stroke.
Therefore, and given that results from different studies are contradictory, mainly because of methodological biases, we can conclude that the evidence is insufficient to support a correlation between lesion laterality and risk of developing PSD.
Another structure that has been investigated extensively in connection with PSD is the amygdala. It is well-known today that the amygdala is involved in the regulation of mood and emotions. In the Sydney Stroke Study, Sachdev et al.26 showed that amygdala was smaller in patients who had suffered a stroke or a transient ischaemic attack (TIA), and especially in patients with cognitive impairment.
Stroke type and PSDThe vascular depression hypothesis proposes that ischaemic vascular lesions (acute, chronic, silent infarctions, leukoaraiosis, etc.) can damage mood and emotion regulation and provoke late-onset depression. Based on that hypothesis, some authors suggest that PSD may lead to cognitive dysfunction of vascular origin which may in turn cause new depressive symptoms, therefore establishing a reciprocal connection between depression and cerebrovascular disease.27
In the present study we aim to explain the impact of different types of stroke on the development of PSD. Firstly, white matter lesions caused by leukoaraiosis or small lacunar strokes seem to play a role in PSD pathogenesis. White matter hyperintensities have been associated with depression and executive dysfunction.28 Silent infarctions and white matter hyperintensities are frequently found in elderly patients with depression. Studies have proved that vascular risk factors and cerebrovascular lesions affect the course of depression, especially in older patients. This suggests that lesions affect not only medium- and large-calibre blood vessels, but also small-calibre vessels.29 In fact, Chen et al.30 studied PSD in patients with small subcortical strokes by comparing the 2 possible aetiologies: small vessel disease, and intracranial and extracranial large artery disease. The authors found that PSD was more frequent among patients with small subcortical strokes derived from lesions in large-calibre vessels. This suggests that cerebral perfusion plays a major role in the development of PSD.
Santos et al.31 analysed 41 autopsies of patients who had presented initial onset of depression within the first 2 years after stroke. Researchers found no connection between macroinfarct location and development of PSD for any of the locations included in the study. Lacunar strokes within the thalamus and basal ganglia were associated with a higher risk of PSD. However, microinfarcts and periventricular and diffuse demyelination did not occur more frequently in PSD patients. They concluded that the damage resulting from chronic accumulation of lacunar strokes in the thalamus, basal ganglia, and deep white matter may be a more important predictor of PSD than the localisation of a single infarct.
In a more recent study, Tang et al.32 examined the association between PSD (defined as a GDS score ≥7) and presence of cerebral microhaemorrhages (evaluated with MRI) in 235 patients at 3 months after stroke. The researchers observed that patients with PSD were more likely to present lobar microhaemorrhages than patients without PSD. Lobar microhaemorrhage was therefore considered an independent predictor of PSD.
Neurobiological pathways and PSDThe connection between PSD and lesions in different cerebral pathways has also been studied. We now know that frontal-subcortical lesions of the monoaminergic pathways reduce biogenic amine release and result in increased likelihood of depression.33
Another plausible mechanism would involve changes in glutamatergic transmission after stroke. In fact, a study34 found changes in frontal lobe glutamate/glutamine levels in PSD patients assessed by magnetic resonance spectroscopy.
On the other hand, hypothalamo-pituitary adrenal axis dysregulation has been observed in 40% of all stroke patients, and adrenocortical hyperactivity appears almost immediately after stroke onset. Additionally, the resulting persisting hypercortisolaemia has been associated with the development of depression, poorer prognosis, and increased mortality rate.35
Inflammation and PSDInflammatory processes seem to be involved in PSD pathogenesis. Cerebral ischaemia has been shown to cause increased production of proinflammatory cytokines, such as interleukin-1β (IL-1β), tumour necrosis factor α (TNF-α), or interleukin-18 (IL-18). In turn, these cytokines may cause decreased serotonin production in certain brain areas, thereby favouring depression.36 More specifically, changes in IL-18 serum levels seem to be linked to depression severity.37
A recent review by Pascoe et al.38 on inflammatory processes in stroke patients confirms that depression and ischaemia are related to inflammation. Both conditions are associated with increased circulating levels of proinflammatory cytokines. Since cytokines play a mediating role in ischaemia-related cell death and limbic region apoptosis is associated with depression, the authors concluded that ischaemic inflammation is likely to contribute to development of PSD.38 Another study39 found increased levels of cytokines IL-6, IL-10, TNF-α, and interferon-γ (IFN-γ), and elevated ratios of IL-6/IL-10 and TNF-α/IL-10, in patients with PSD compared to stroke patients without depression.
However, some authors did not observe a clear association between the increase in inflammatory markers and the development of PSD.40
In any case, other studies provide interesting information about the anti-inflammatory effects of antidepressants.41,42 These drugs can reduce depressive symptoms by acting on the inflammatory markers involved in mood regulation. More specifically, animal models have shown that antidepressants reduce the levels of pro-inflammatory interleukins IL-1 and IL-2, while increasing the level of anti-inflammatory interleukin IL-10. Additionally, they inhibit the expression of IL-6, TNF-α, and IFN-γ.
Genetic factors and PSDGenetic variability seems to be involved in PSD. For example, patients with the s/s polymorphism of the serotonin transporter-linked gene region 5-HTTLPR are 3.1 times more likely to present PSD than those with the l/l or l/χl variant. Additionally, patients with the 9/12 or 12/12 variants of the serotonin transporter locus STin2 were 4.1 times more likely to develop depression than patients with the STin2 10/10 genotype.43 A more recent study44 found a connection between the s/s variant of the 5-HTT gene-linked promoter region and post-stroke emotional incontinence. Yet another study45 stated that s/s homozygosity for serotonin transporter gene SLC6A4 may be involved in development of PSD.
Based on the above, it seems that certain genetic features determine the degree of vulnerability to depression.
Symptoms of PSDAlthough PSD can occur shortly after a cerebrovascular event, it usually develops within the first few months following a stroke.1,2 In a study of 100 stroke patients, 46% of those with depressive symptoms at 2 months after stroke also presented depressive symptoms at 12 months, whereas only 12% presented the first symptoms 12 months after stroke.46 The term ‘early-onset PSD’ has been proposed to refer to PSD occurring within the first 3 months after stroke, while ‘late-onset PSD’ refers to PSD developing after 3 months.1 In early-onset PSD, somatic depressive symptoms tend to be more numerous than psychological symptoms.27
Although depressive symptoms are usually similar, some differences have been found between PSD patients and patients with depression but no associated neurological disease. PSD patients, especially those with right-hemisphere damage, are less likely to present dysphoric depression and more likely to present vegetative signs and symptoms than patients with other types of late-onset PSD.5,47 Greater social withdrawal and fewer agitation symptoms have been reported in elderly stroke patients with depression compared to depressive patients with other conditions.5,48 A subsequent study49 found that PSD patients presented ‘inability to feel’ and ‘disturbed sleep’ more frequently than depressed patients with no prior history of stroke.
It is therefore important to perform differential diagnosis to rule out other cognitive-behavioural syndromes presenting with mood and emotional changes after stroke, since their symptoms may be similar to those of PSD.27 Those syndromes include emotional lability, athymhormic syndrome, aboulia and apathy, affective aprosody, and frontal dysexecutive syndromes. Other disorders that may appear in the absence of PSD include certain autonomic disorders, changes in sleep patterns after stroke, and vegetative symptoms such as post-stroke anxiety and subjective fatigue. Gusev and Bogolepova50 state that some 2% to 3% of first-time stroke patients may present masked depression, that is, presence of depressive symptoms but absence of depressive mood. The main manifestations of this type of depression are biological rhythm disturbances and somatisation disorders, especially vegetative-vascular dystonia, vertigo, and various algic phenomena. These symptoms, which are common to stroke and depression, may lead to overestimating depression in stroke patients.9 However, underestimation of PSD is even more frequent, since depressive symptoms are rarely identified despite the high incidence and prevalence of PSD.51
Treatment of PSDSeveral anti-depressants have proved to cause an improvement in cognitive skills and functional recovery in PSD patients. Selective serotonin reuptake inhibitors (SSRI) and tricyclic antidepressants (TAD) are the most extensively studied drug treatments.
The first study on PSD treatment, performed by Lipsey et al.,52 was a placebo-controlled study that found improvement in Ham-D scores in 11 patients treated with nortriptyline (25-100mg/d) for 6 weeks.
The first study using SSRIs53 found that patients receiving citalopram showed more symptomatic improvement (with lower scores on Ham-D at 3 and 6 weeks) than those on fluoxetine. However, 2 double-blind placebo-controlled trials found both citalopram and fluoxetine to be effective for PSD treatment after assessing 66 and 31 patients respectively over 6 weeks.54,55 Another placebo-controlled trial56 compared nortriptyline to fluoxetine in 104 patients over 12 weeks. Nortriptyline proved to be more effective than fluoxetine and placebo since it caused a more marked decrease in Ham-D scores. The study by Jorge et al.57 found that patients treated with either fluoxetine or nortriptyline presented significantly lower mortality rates than placebo-treated patients.
Rampello et al.58 found that patients on reboxetine experienced a more pronounced decrease in Ham-D scores than those treated with placebo.
More recent studies have focused on the effectiveness of SSRIs. Ried et al.59 conducted a retrospective study on mortality and found that SSRI treatment, administered either before or after stroke, was associated with a significantly lower risk of mortality during the first year after stroke (HR=0.31; 95% CI: 0.11-0.86). However, mortality rates increased after 7 years. The authors concluded that SSRI treatment was associated with better survival rates, and they therefore recommend starting or resuming SSRI treatment after stroke, especially in patients with prior history of depression or those treated with SSRIs before stroke. Another study60 found that discontinuing escitalopram treatment can increase occurrence of post-stroke depressive symptoms over the first 6 months after treatment withdrawal, compared to placebo and problem-solving therapy (PST). Jorge et al.61 conducted a double-blind placebo-controlled trial in 129 patients evaluated using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The authors found that patients receiving escitalopram after stroke showed improvement in the higher cognitive functions, specifically in verbal and visual memory functions, compared to patients receiving placebo or PST. The beneficial effect of escitalopram was independent of its effect on depressive symptoms. Similarly, it has been proven that fluoxetine improves quality of life in patients with emotional disorders, including PSD, after stroke.62 In a recent controlled trial, Cramer63 assessed the effect of fluoxetine in patients at 5 to 10 days after stroke using the Fugl-Meyer Assessment of Motor Recovery after Stroke (FMA) and found improved motor function at 90 days after stroke. These patients also showed a lower level of disability according to modified Rankin Scale scores.
The efficacy of drugs other than antidepressants has also been investigated. For example, certain stimulants, such as amphetamines and methylphenidate, have been studied in patients with severe depression and rapid cognitive decline, with contradictory results.64 In another study, PSD patients treated with the calcium agonist nimodipine showed improvement of depressive symptoms and a lower risk of recurrence.65
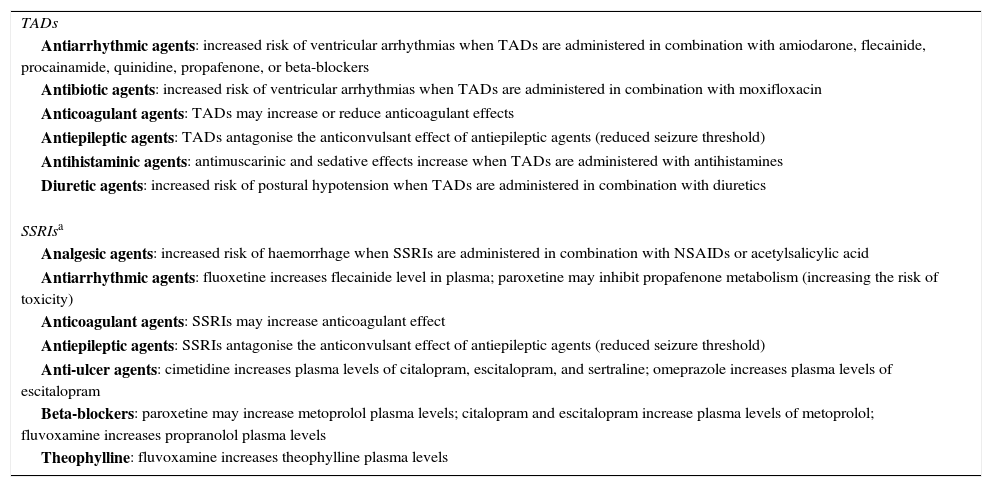
Finally, although pharmacological treatment with SSRIs is recommended for PSD, there are no studies providing conclusive data on the superiority of a specific drug.27 Nevertheless, SSRIs are safer, provoke fewer adverse effects, are relatively quick-acting with a latency period ranging from 7 to 10 days, and they also exert an anxiolytic effect. For this reason, they are considered first-line antidepressants, especially in stroke patients, who are usually elderly with underlying cardiovascular problems, and on polytherapy, which can lead to drug interactions (Table 2).
Clinically relevant drug interactions for antidepressants in elderly patients on polytherapy
| TADs |
| Antiarrhythmic agents: increased risk of ventricular arrhythmias when TADs are administered in combination with amiodarone, flecainide, procainamide, quinidine, propafenone, or beta-blockers |
| Antibiotic agents: increased risk of ventricular arrhythmias when TADs are administered in combination with moxifloxacin |
| Anticoagulant agents: TADs may increase or reduce anticoagulant effects |
| Antiepileptic agents: TADs antagonise the anticonvulsant effect of antiepileptic agents (reduced seizure threshold) |
| Antihistaminic agents: antimuscarinic and sedative effects increase when TADs are administered with antihistamines |
| Diuretic agents: increased risk of postural hypotension when TADs are administered in combination with diuretics |
| SSRIsa |
| Analgesic agents: increased risk of haemorrhage when SSRIs are administered in combination with NSAIDs or acetylsalicylic acid |
| Antiarrhythmic agents: fluoxetine increases flecainide level in plasma; paroxetine may inhibit propafenone metabolism (increasing the risk of toxicity) |
| Anticoagulant agents: SSRIs may increase anticoagulant effect |
| Antiepileptic agents: SSRIs antagonise the anticonvulsant effect of antiepileptic agents (reduced seizure threshold) |
| Anti-ulcer agents: cimetidine increases plasma levels of citalopram, escitalopram, and sertraline; omeprazole increases plasma levels of escitalopram |
| Beta-blockers: paroxetine may increase metoprolol plasma levels; citalopram and escitalopram increase plasma levels of metoprolol; fluvoxamine increases propranolol plasma levels |
| Theophylline: fluvoxamine increases theophylline plasma levels |
NSAID: non-steroidal anti-inflammatory drugs; SSRI: selective serotonin reuptake inhibitors; TAD: tricyclic antidepressants.
In any case, many questions about PSD treatment remain unanswered, such as the best moment for initiating treatment, the effect of antidepressants in areas other than emotion (for example, cognition and motor function), and the importance of antidepressants for reducing mortality in stroke patients.
ConclusionStroke has traditionally been considered a disease affecting motor function. Therefore, approaches to hospital care, rehabilitation, and follow-up have focused on this aspect almost exclusively. However, we currently know that PSD is a common problem in daily clinical practice since it is present in 1 out of every 3 stroke patients. Additionally, PSD is the main factor limiting patient recovery and rehabilitation, and increasing morbidity and mortality rates. Strangely enough, more than half of the cases are neither diagnosed nor treated. In this scenario, neurologists play a major role in caring for and managing recovery in stroke patients, and they must therefore be familiar with early detection and treatment of PSD. This way, neurologists will be able to facilitate patients’ functional recovery and social integration, and improve quality of life in patients and their families.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Espárrago Llorca G, Castilla-Guerra L, Fernández Moreno MC, Ruiz Doblado S, Jiménez Hernández MD. Depresión post ictus: una actualización. Neurología. 2015;30:23–31.