An overview of the effectiveness of radiosurgery in patients diagnosed with trigeminal neuralgia with an analysis of potential predictors of good outcome.
MethodsAll patients treated with linear accelerator radiosurgery between 2004 and 2011 were analysed. A dose of 60Gy dose was administered 1 to 2mm from the root entry zone with a maximum isodose of 20% delivered to the brainstem. Clinical results for pain control and any side effects were analysed at 12 and 36 months (BNI score).
ResultsThe study included 71 patients (mean follow-up 50.5 months). Pain improvement at 12 months was observed in 68.11% of the total (28.98% with BNI score I–II; 39.12% with BNI score III) and at 36 months in 58.21% (23.88% BNI score I–II; 34.32% BNI score III). Average recovery time was 3.69 months and the relapse rate was 44.68%. Patients with typical pain displayed statistically significant differences in improvement rates at 12 and at 36 months (P<.047 and P<.002). Onset of improvement was analysed using Kaplan–Meyer plots. Statistically significant differences were observed between patients with typical and atypical pain at 36 months (P<.012) in Kaplan–Meyer plots. Side effects were recorded in 15 patients (20.89%), including 9 cases of facial numbness (13.43%); only 2 cases were clinically relevant (2.98%).
ConclusionAccording to our results, radiosurgery is an effective treatment for trigeminal neuralgia, with few side effects. Typical pain seems to be a good predictor of pain relief.
Comprobación de la efectividad de la radiocirugía en pacientes diagnosticados de neuralgia del V par y análisis de posibles factores predictores.
MétodosSe analizaron todos los pacientes entre 2004 y 2011 tratados mediante radiocirugía con acelerador lineal. Se administraron dosis de 60Gy a 1-2mm de la entrada del nervio con isodosis de 20% máxima sobre el tronco. Se analizaron los resultados clínicos de control del dolor y efectos secundarios a los 12 y 36 meses (escala BNI).
ResultadosSe incluyó a 71 pacientes en el estudio (seguimiento medio 50,50m). La mejoría del dolor a los 12 meses fue del 68,11% (28,98% BNI score I-II; 39,12% BNI score III y a los 36 meses del 58,21% (23,88% BNI score I-II; 34,32% BNI score III). El tiempo medio de mejoría fue de 3,69 meses y la tasa de reincidencia tras mejoría del 44,68%. Se aprecian diferencias estadísticas en la mejoría para pacientes con dolores típicos a los 12 y 36 meses (p<0,047 y p<0,002). Se analiza el inicio de la mejoría mediante gráficas de Kaplan-Meyer. En el análisis en función de variables se obtuvo diferencia estadísticamente significativa entre dolores típicos y atípicos a los 36 meses (p<0,012). En 15 pacientes se registraron efectos secundarios (20,89%), 9 de ellos por adormecimiento facial (13,43%) y solo 2 con relevancia clínica (2,98%).
ConclusionesDe acuerdo con nuestra experiencia, la radiocirugía es un tratamiento eficaz de la neuralgia del trigémino, con escasos efectos secundarios. El dolor típico parece un factor de buen pronóstico para la mejoría del dolor.
Trigeminal neuralgia was described as a clinical entity as early as in ancient Greece. It is characterised by chronic unilateral paroxysmal pain extending to one or more branches of the trigeminal nerve. However, a few clinical variants of this condition are currently classified as classical trigeminal neuralgia with concomitant persistent facial pain.1 Different aetiologies have been described, including compression: in the classic study by Hardy et al., compression was responsible for trigeminal neuralgia in 50% of the studied nerves.2 In trigeminal neuralgia, compression is mainly vascular (especially involving the superior cerebellar artery, although it can also be caused by a persistent primitive trigeminal artery or basilar dolichoectasia). Trigeminal neuralgia has also been associated with multiple sclerosis (MS) and presence of demyelinating plaques in the nerve root entry zone (REZ).3 However, in most cases the cause is undetermined; trigeminal neuralgia is therefore considered to be mainly idiopathic.
Many treatment approaches have been developed throughout history. Pharmacological treatment is the main approach, and it achieves good outcomes in most patients. However, approximately 25% of the patients do not respond to treatment or show poor tolerance. There are several treatment options for these patients, including microvascular decompression (MVD), thermocoagulation (THC), chemical ablation with glycerol rhizotomy (GR), balloon compression, and radiosurgery; success rates differ among treatments.4 Radiosurgery, introduced by Leksell in 1951, is a procedure with minimal complications and very little mortality which has emerged as one of the best treatment options for elderly patients and those who are not eligible for surgery. In addition, it has proved exceptionally effective for trigeminal neuralgia.
This study presents a series of patients with trigeminal neuralgia who underwent radiosurgery with a linear accelerator (LINAC) in our centre. We performed a statistical analysis of treatment outcomes according to different variables (typical pain patterns, previous treatment, etc.). Our results are presented as survival curves.
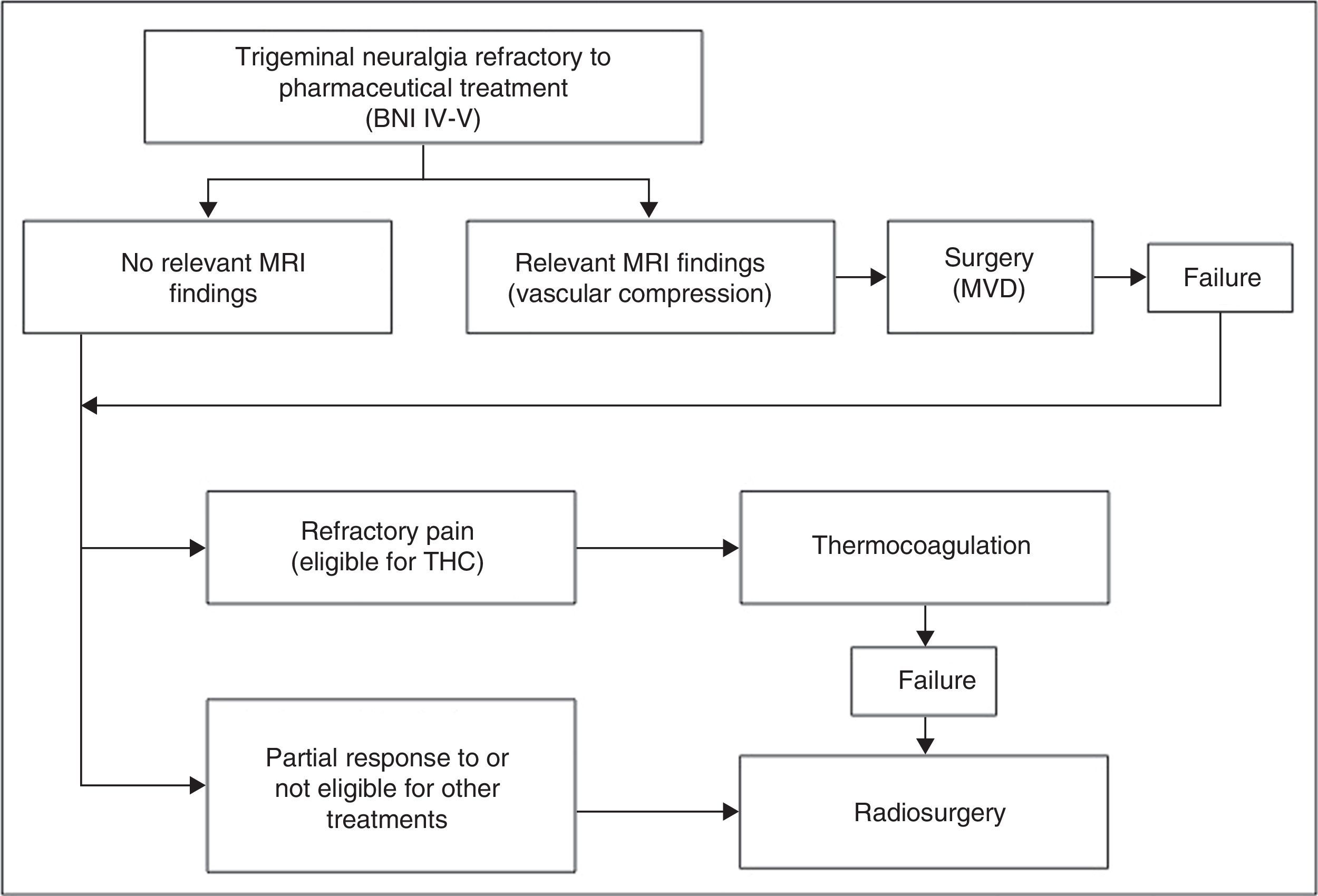
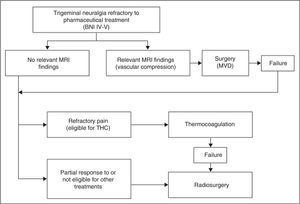
Patients and methodsPatient characteristicsBetween 2004 and 2011, 71 patients with trigeminal neuralgia were treated in the radiosurgery unit at Hospital Virgen de las Nieves in Granada. All patients included in our study underwent a single session of radiosurgery. Based on our hospital's treatment protocol for trigeminal neuralgia, all patients were assessed by the neurology department (11 patients came from other centres since our hospital is a reference centre for radiosurgery). Patients were diagnosed with trigeminal neuralgia according to the criteria of the International Classification of Headache Disorders.5 They initiated progressive pharmacological treatment (each patient received a mean of 3.8 drugs) and underwent complementary tests. The patients with relevant MRI findings and those cases refractory to pharmacological treatment were referred to our department to evaluate potential treatment options (Fig. 1). Patients who were eligible for radiosurgery were informed about the procedure and signed informed consent forms before being added to the waiting list.
We recorded different clinical and demographic variables for our patient sample. Patients were classified in 3 age groups: younger than 55, 55 to 70, and older than 70. Four patients had a previous diagnosis of MS. We classified patients by pain characteristics. Patients with recurrent paroxysmal attacks of unilateral facial pain, trigger points, no motor or sensory alterations, and a response to pharmacological treatment at some point were considered to have ‘typical pain characteristics’. We subsequently classified these patients in 2 groups: patients who had undergone surgery previously and patients who had only received pharmacological treatment. We performed brain FIESTA MRI scans on all patients to rule out structural lesions. One patient had a meningioma in a region not related to pain (this patient was also treated with radiosurgery); another patient had an arachnoid cyst with no pathological relevance. One of the patients had bilateral pain; each side was treated in different sessions.
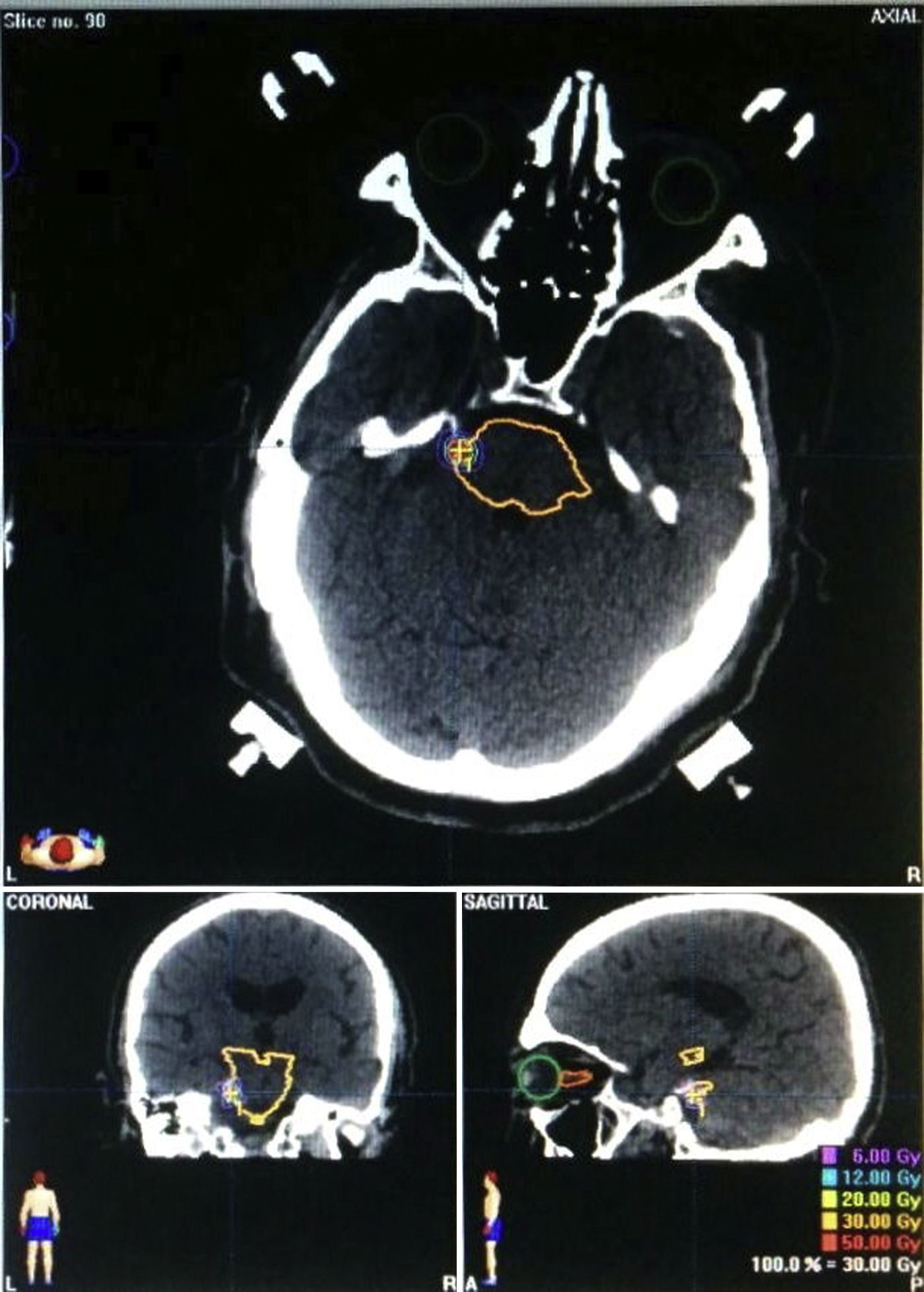
Characteristics of radiosurgerySurgeries were planned by a multidisciplinary team including oncologists, radiotherapists, physicists, radiologists, and neurosurgeons. Radiosurgery was performed with a 6MeV linear accelerator using Brainlab software. MR images were acquired and fused to CT slices, which were acquired using a stereotactic frame. We used 3-mm or 5-mm circular collimators, depending on the case. Our protocol established a maximum dose of 60Gy centred at 1 or 2mm from the REZ and using 3 to 6 arcs (Fig. 2).
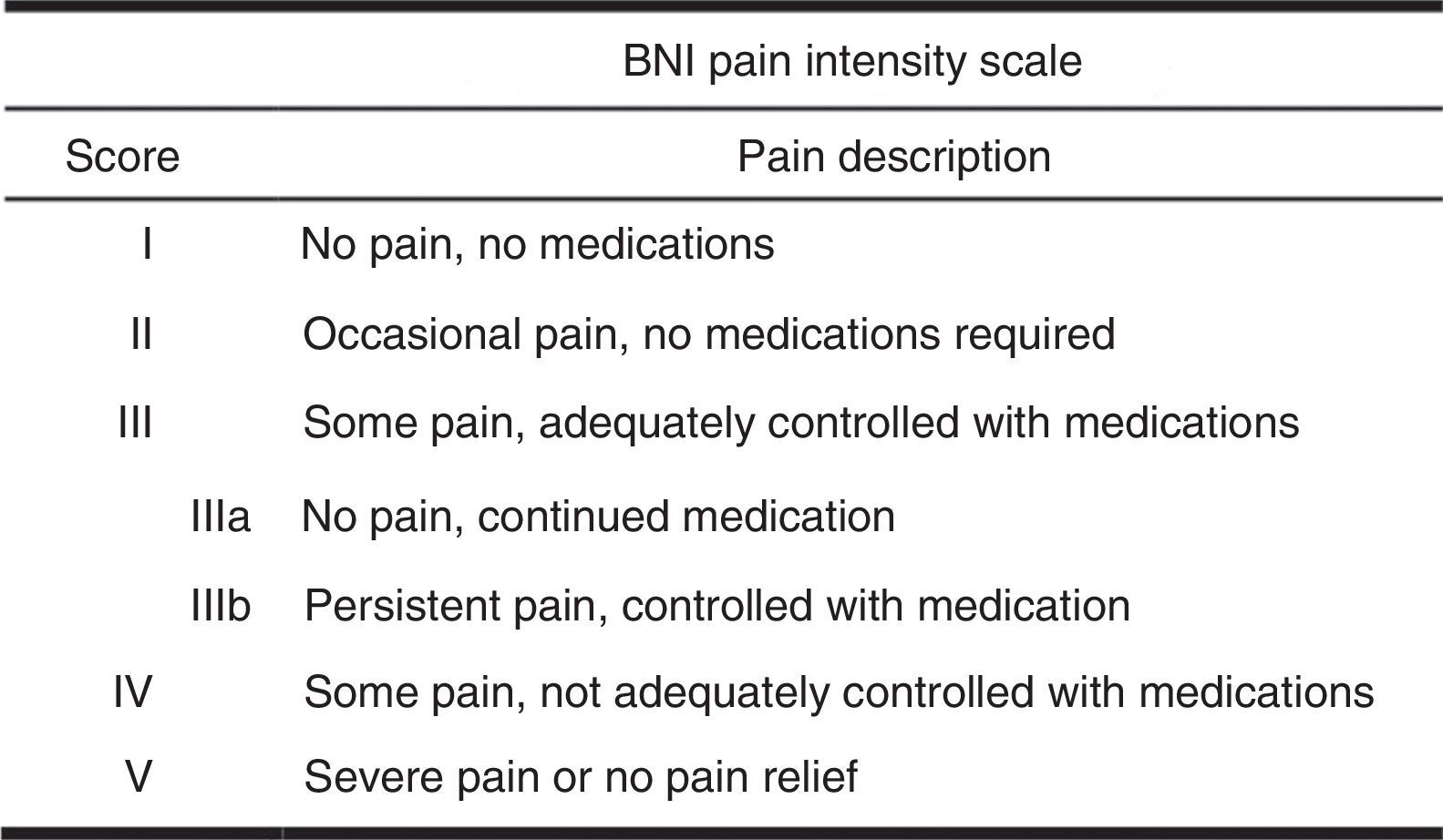
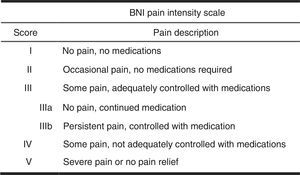
Follow-upAfter radiosurgery, patients underwent a series of follow-up MRI scans at 3, 6, and 12 months. Follow-up visits after the 12-month mark were scheduled on a yearly basis. The Barrow Neurological Institute (BNI) pain intensity scale was administered at 1 and 3 years (Fig. 3).6 Scores I and II on the BNI scale were grouped together since patients may have difficulties differentiating them. Scores I–II and III were considered to indicate treatment success whereas categories IV and V indicated treatment failure. Recourse to additional treatment for pain during the follow-up period was regarded as radiosurgery failure. During follow-up visits, we recorded onset of symptom improvement and worsening after the initial improvement, when applicable. We also gathered data about complications and secondary effects at all follow-up visits.
Statistical analysisWe performed frequency analyses for all variables. We subsequently compared means applying ANOVA for the variables sex, age group, history of MS, typical pain, previous treatments, and secondary effects broken down by treatment success at 12 and 36 months. The McNemar-Bowker test was used to compare changes in results. Data on improvement over time were plotted as Kaplan–Meier curves. We conducted a statistical analysis of improvements at both follow-up times by sex, age group, previous treatments, and typical pain using the Mantel–Cox test. P-values<.05 were considered to be statistically significant. Statistical analysis was conducted using SPSS statistical software version 20.0.
ResultsDuring the study period we identified 71 patients. Four patients (5.63%) were lost to follow-up (2 patients before 12 months and 2 before 36 months), including 2 deaths unrelated to treatment. Fourteen patients (20.3%) were younger than 55, 28 (40.6%) were between 55 and 70, and 27 (39.1%) were older than 70. We conducted a descriptive study of frequencies by the variables pain type and previous treatments and recorded demographic data and treatment outcomes at 12 and 36 months using the BNI scale (Table 1). Mean follow-up time was 50.5 months (range, 8–84 months, median=49). Mean time to onset of symptom improvement was 3.69 months (range, 0.25–32 months, median=1.5 months). Twenty-one (44.68%) of the patients showing improvements experienced subsequent exacerbations. Mean time to symptom exacerbation was 14.07 months (range, 0.5–52 months, median=8).
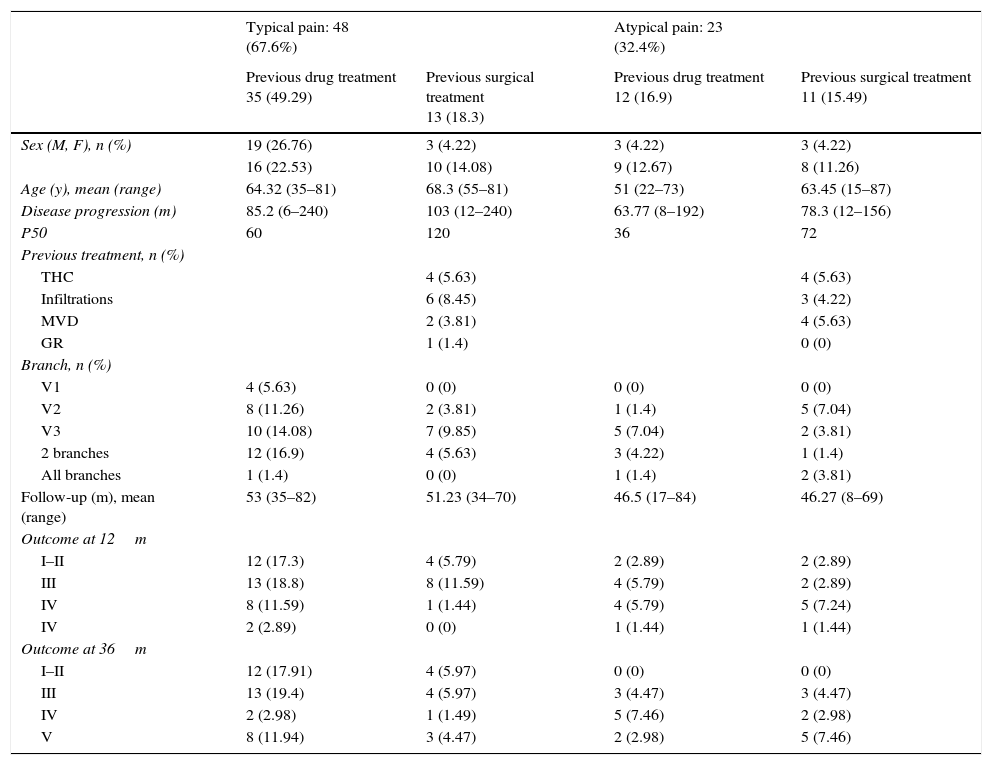
Distribution of demographic and outcome data by pain type and type of previous treatment (BNI pain intensity scale).
| Typical pain: 48 (67.6%) | Atypical pain: 23 (32.4%) | |||
|---|---|---|---|---|
| Previous drug treatment 35 (49.29) | Previous surgical treatment 13 (18.3) | Previous drug treatment 12 (16.9) | Previous surgical treatment 11 (15.49) | |
| Sex (M, F), n (%) | 19 (26.76) | 3 (4.22) | 3 (4.22) | 3 (4.22) |
| 16 (22.53) | 10 (14.08) | 9 (12.67) | 8 (11.26) | |
| Age (y), mean (range) | 64.32 (35–81) | 68.3 (55–81) | 51 (22–73) | 63.45 (15–87) |
| Disease progression (m) | 85.2 (6–240) | 103 (12–240) | 63.77 (8–192) | 78.3 (12–156) |
| P50 | 60 | 120 | 36 | 72 |
| Previous treatment, n (%) | ||||
| THC | 4 (5.63) | 4 (5.63) | ||
| Infiltrations | 6 (8.45) | 3 (4.22) | ||
| MVD | 2 (3.81) | 4 (5.63) | ||
| GR | 1 (1.4) | 0 (0) | ||
| Branch, n (%) | ||||
| V1 | 4 (5.63) | 0 (0) | 0 (0) | 0 (0) |
| V2 | 8 (11.26) | 2 (3.81) | 1 (1.4) | 5 (7.04) |
| V3 | 10 (14.08) | 7 (9.85) | 5 (7.04) | 2 (3.81) |
| 2 branches | 12 (16.9) | 4 (5.63) | 3 (4.22) | 1 (1.4) |
| All branches | 1 (1.4) | 0 (0) | 1 (1.4) | 2 (3.81) |
| Follow-up (m), mean (range) | 53 (35–82) | 51.23 (34–70) | 46.5 (17–84) | 46.27 (8–69) |
| Outcome at 12m | ||||
| I–II | 12 (17.3) | 4 (5.79) | 2 (2.89) | 2 (2.89) |
| III | 13 (18.8) | 8 (11.59) | 4 (5.79) | 2 (2.89) |
| IV | 8 (11.59) | 1 (1.44) | 4 (5.79) | 5 (7.24) |
| IV | 2 (2.89) | 0 (0) | 1 (1.44) | 1 (1.44) |
| Outcome at 36m | ||||
| I–II | 12 (17.91) | 4 (5.97) | 0 (0) | 0 (0) |
| III | 13 (19.4) | 4 (5.97) | 3 (4.47) | 3 (4.47) |
| IV | 2 (2.98) | 1 (1.49) | 5 (7.46) | 2 (2.98) |
| V | 8 (11.94) | 3 (4.47) | 2 (2.98) | 5 (7.46) |
M: male; F: female; y: years; m: months; n: number; THC: thermocoagulation; MVD: microvascular decompression; GR: glycerol rhizotomy.
At 12 months, treatment was successful in 47 patients (68.11%) and had failed in 22 (31.88%). Treatment success was achieved by 37 of the 48 patients (77.08%) with typical pain and 10 of the 21 patients (47.61%) with atypical pain. At 36 months, 39 patients achieved treatment success (58.21%) whereas treatment failed in 28 patients (41.78%). Treatment succeeded in 25 of the 47 patients (53.19%) with typical pain and 6 of the 20 patients (30%) with atypical pain.
We then used ANOVA to analyse the differences between means for the variables sex, age group, history of MS, and previous treatments as a function of treatment success at 12 and 36 months. No statistically significant differences were found except for the variable typical pain, both at 12 months (P<.047) and at 36 months (P<.002). We also conducted a statistical analysis of changes in the variables treatment outcome at 12 and 36 months using the McNemar-Bowker test; differences were statistically significant (P<.016).
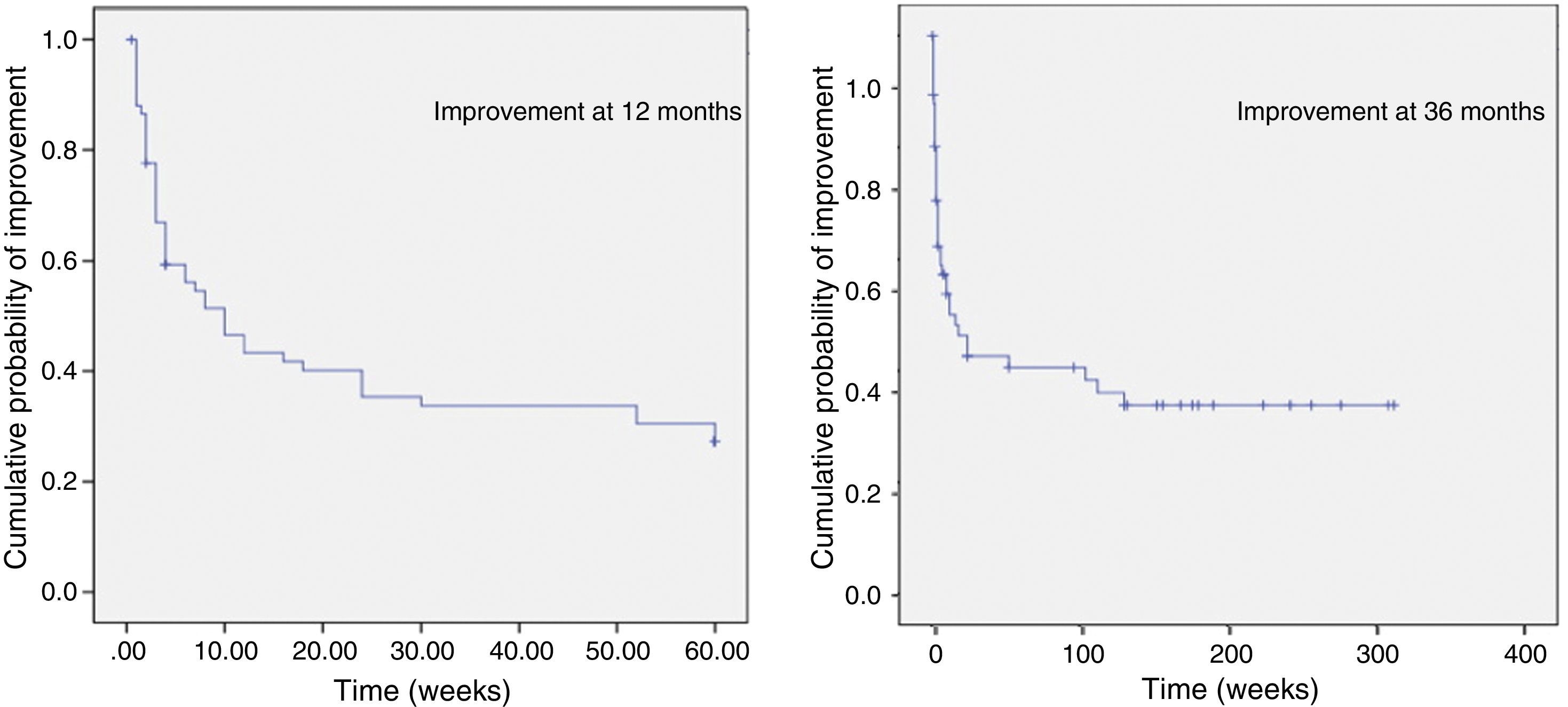
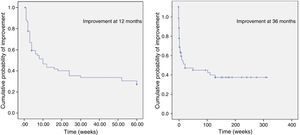
Lastly, we represented the time when symptoms began to improve in each patient, both at 12 and at 36 months, as Kaplan–Meier curves (Fig. 4). We then compared Kaplan–Meier curves at 12 and 36 months adjusted by sex, age group, previous treatments, secondary effects, and typical pain using the Mantel–Cox test. Differences were statistically significant only for the variable typical pain at 36 months (P<.012).
Complications were registered during subsequent follow-up visits. One patient exhibited radiation necrosis, which was managed successfully with conservative treatment; response to treatment in this patient was categorised as III on the BNI pain intensity scale. One patient experienced anaesthesia dolorosa after treatment and another displayed abolished corneal reflexes. Two patients developed dysaesthesia, one had dry eye syndrome, and another exhibited dry eye syndrome plus facial numbness. Nine patients, including the latter, experienced facial hypoaesthesia (13.43%), although their symptoms did not have a substantial impact on daily life. A total of 15 patients (20.89%) experienced complications. We used ANOVA to analyse differences in treatment outcomes at 12 and 36 months between patients who experienced secondary effects and those who did not; differences were not significant (P<.505 and P<.519, respectively).
DiscussionRadiosurgery for patients with trigeminal neuralgia is now a safe alternative to conventional surgical treatments. Currently available treatment options show little differences between success rates. MVD remains the gold standard treatment, especially for young patients, and it has a low rate of complications.4 The main advantages of surgery over radiosurgery are the immediacy of results, lower recurrence rates, and less probability of experiencing facial numbness.7–9 Such other invasive techniques as radiofrequency THC, balloon compression, and GR are associated with low rates of perioperative complications. However, radiosurgery has an even lower complication rate since it is performed in a single day. In addition, this treatment option is suitable for virtually all patients since it does not require sedation or general anaesthesia.9,10 In any case, radiosurgery is still considered a second-line treatment, for use in patients who are not eligible for surgery. In our centre, which implemented the protocol described above several years ago, surgery is the first treatment choice for patients displaying relevant MRI findings. However, trends are changing since patients are showing increasing interest in less invasive techniques. This tendency has also been observed by other authors7,8 and it affects the results reported in the literature: the effectiveness of radiosurgery has risen to become very similar to that of surgery. This phenomenon may be explained by the fact that patients initially selected for radiosurgery were considered to be more treatment-resistant: they were normally elderly, had received one or more treatments before radiosurgery, and displayed more severe MRI features.11 It is a well-known fact that patients who have received no previous treatments, especially MVD, are now more likely to achieve better pain relief after radiosurgery.12–15
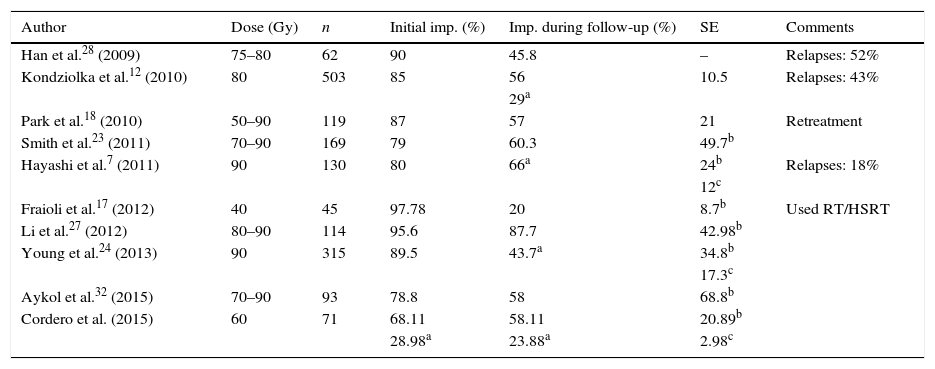
Another interesting debate revolves around the ideal target area and dose of radiation used in radiosurgery. The preliminary studies by Pollock et al.16 established a direct relation between improvements in pain management and contrast uptake in the REZ, which led many researchers to use the REZ as their target. However, other researchers continue to target the retrogasserian cisternal portion of the trigeminal nerve.17 No definitive conclusion has been reached about which target is better for radiosurgery.18 Differences in the effectiveness of Gamma Knife, Cyberknife, and LINAC are not significant. However, some studies claim that the Gamma Knife is more effective for small targets19 but LINAC is more cost-effective in terms of initial investment and maintenance costs20 since it can be used for other treatments, as in our case. Determining the dose of radiation is equally important. The mechanism by which radiation acts on the nerve is still a source of debate. Some authors, including Régis et al.,14 state that radiosurgery has the ability to select the nerve fibres that cause pain while sparing the fibres responsible for facial sensitivity. In contrast, Kondziolka et al.21 conducted a histological study of primate brains to determine the effect of radiation on nerves, and found axonal degeneration and oedema at the target after applying doses between 80 and 100Gy on the trigeminal nerve. According to other authors, such as Massager et al.,22 the most important factor is the dose of radiation delivered to the nerve root rather than the maximum dose. As a general rule, 100Gy is considered the maximum dose due to the associated risk of necrosis; most studies apply doses between 70 and 90Gy. There is a widespread belief that doses between 85 and 90Gy are optimal23,24; however, numerous studies have failed to find a significant difference between doses of 90Gy and 70Gy.14,15 Other review articles suggest that the best target is located 2 to 4mm from the REZ.25 In our series, we used a target located 1 to 2mm from the REZ and applied a dose of 60Gy, which is significantly lower than those applied in published series. However, our results are similar to those reported by other researchers in terms of overall pain relief and pain relief without medication. In our sample, pain relief was achieved in 68.11% (BNI I–II: 28.98%; III: 39.12%) of the patients at 12 months and 58.21% (BNI I–II: 23.88%; III: 34.32%) at 36 months. In a recent study including 503 patients, Kondziolka et al.12 applied a dose of 80Gy, with a maximum dose of 16Gy on the pons, and achieved pain relief in 85% of the patients (BNI I–II) just after the procedure and in 56% in the long term (mean follow-up time of 6 years); 29% of the patients required no medication. Park et al.26 administered doses between 50 and 90Gy to a series of patients and monitored them for more than 15 years on average, achieving pain relief in 57% of the sample (BNI I–III). Young et al.24 treated a series of 315 patients with a maximum dose of 90Gy and 18Gy on the pontine surface (20% isodose line), and achieved pain relief in 89.5% of the patients; 43.7% of the patients were pain-free without medication. It should be noted, however, that 21% of the patients in this study were lost to follow up. Li et al.27 conducted a study in a sample of 114 patients. Using 2 isocentres and doses between 80 and 90Gy, the authors achieved initial pain relief in 95% of the sample (BNI I–III). However, the follow-up period in this study was shorter. Smith et al.23 used a LINAC to treat 169 patients with doses between 70 and 90Gy; pain relief was achieved in 79% of the sample after completing the procedure, and in 60.3% after follow-up. In summary, the results of these studies do not differ substantially from ours considering that our sample size was smaller but follow-up was very thorough (Table 2). Pain recurrence after initial relief has also been reported in other studies. In the study by Han et al.,28 although 90% of the patients achieved initial pain relief, 52% of them experienced pain recurrence (appearing at 2–56 months). For this reason, many patients opt for additional radiosurgery sessions, with success rates above 50%.29 A similar phenomenon was observed in our series: pain reappeared in 44.68% of the patients. This is probably due to the partial effects of radiation on the nerve, or to reinnervation processes.
Summary of the main studies of radiotherapy conducted recently.
| Author | Dose (Gy) | n | Initial imp. (%) | Imp. during follow-up (%) | SE | Comments |
|---|---|---|---|---|---|---|
| Han et al.28 (2009) | 75–80 | 62 | 90 | 45.8 | – | Relapses: 52% |
| Kondziolka et al.12 (2010) | 80 | 503 | 85 | 56 | 10.5 | Relapses: 43% |
| 29a | ||||||
| Park et al.18 (2010) | 50–90 | 119 | 87 | 57 | 21 | Retreatment |
| Smith et al.23 (2011) | 70–90 | 169 | 79 | 60.3 | 49.7b | |
| Hayashi et al.7 (2011) | 90 | 130 | 80 | 66a | 24b | Relapses: 18% |
| 12c | ||||||
| Fraioli et al.17 (2012) | 40 | 45 | 97.78 | 20 | 8.7b | Used RT/HSRT |
| Li et al.27 (2012) | 80–90 | 114 | 95.6 | 87.7 | 42.98b | |
| Young et al.24 (2013) | 90 | 315 | 89.5 | 43.7a | 34.8b | |
| 17.3c | ||||||
| Aykol et al.32 (2015) | 70–90 | 93 | 78.8 | 58 | 68.8b | |
| Cordero et al. (2015) | 60 | 71 | 68.11 | 58.11 | 20.89b | |
| 28.98a | 23.88a | 2.98c |
The topic of maximum dose inevitably leads us to reflect on the complications of radiosurgery. The most frequent complications are facial hyperaesthesia and hypoaesthesia, affecting between 7% and 49% of the patients in the published series.8–10,12,13,15,17,18,27,30–32 The percentage increases considerably when the procedure is repeated.29,33–35 It seems logical to think that the frequency of facial numbness would be dose-dependent and although several studies supporting this idea have been published,16,22,30 other studies have also refuted it.10,12,33 There is controversy regarding whether facial numbness should be considered a true complication or rather an effect of the treatment since it rarely affects the patient's activities of daily living. However, other complications involving facial discomfort (dysaesthesia, hyperaesthesia, burning, etc.) may have a negative impact on daily living, especially when they affect the oral mucosa. In a series of 130 patients treated with 90Gy, Hayashi et al.7 reported pain relief in 80% of the sample, with 60% of patients remaining pain-free without medication. However, complications appeared in 24% of the patients, and discomfort in 12%. Young et al.24 also applied doses between 70 and 90Gy, achieving pain relief in 89.5% of the patients (43.7% patients were pain-free without medication); however, 17.3% of the patients experienced severe or extreme discomfort. In the study by Aykol et al.,32 78.8% of the patients achieved pain relief after the procedure and 58% maintained pain relief during follow-up. There was also a statistically significant correlation between dysaesthesia and good outcome. These data suggest that greater doses result in better outcomes, although these are accompanied by increasing complications, especially those causing discomfort. However, according to Fraioli et al.17 these results can be achieved with a much lower dose (40Gy) if radiation is delivered to the retrogasserian cisternal portion of the trigeminal nerve. In light of the above, doses applied in our hospital are lower than normal, which results in very low rates of severe complications: only 2 patients in our sample (2.98%) developed dysaesthesia affecting daily living (Table 2). In addition, and considering all the complications in general, no significant correlations were found between complications and pain relief either after the procedure or during follow-up.
A number of factors may be linked to better responses to radiosurgery treatment. These include younger age,12,14,28 pain in a single branch of the trigeminal nerve,7 pain on the right side,10 and smaller size of the trigeminal cistern.14 No history of surgery is considered an indicator of good prognosis12–15,24; for some authors, having undergone surgery on fewer occasions before radiosurgery is also an indicator of good outcome.10,12 We observed this tendency in our sample, although it was not statistically significant. Other factors, such as MS,15 have been associated with poor outcome. Although our sample included some patients with MS, numbers were not high enough to draw conclusions. Another factor potentially affecting outcomes is whether or not trigeminal pain has typical features. Some studies have reported good response to radiosurgery in patients with paroxysmal remitting unilateral pain with typical characteristics and trigger points.15 In the study by Kondziolka et al.,12 patients with atypical pain were found to respond to radiosurgery but they were significantly more likely to experience relapses. Smith et al.23 observed that improvement rates were lower in patients with atypical pain (27.3%). However, other authors have found no significant associations between pain features and response to treatment.11,36 In our series, we found a statistically significant correlation between pain relief and typical pain, both after the procedure and at 36 months. In our view, this finding reflects patients’ characteristics and previous treatments: patients with atypical pain have usually received several treatments before radiosurgery since atypical pain tends to be more treatment-resistant. As other authors have proposed, studies featuring longer follow-up periods are needed to validate treatment.4,21,22
ConclusionsRadiosurgery is seeing increasing use in trigeminal neuralgia. It is currently considered a good option as a second-line treatment due to its high success rates and low rate of associated complications. Radiosurgery achieves good rates of pain relief, especially just after the procedure; a high percentage of patients undergoing this treatment will remain pain-free without medication. Several factors, such as whether or not pain is typical, seem to be associated with better outcomes. Studies with longer follow-up periods should be conducted to determine the predictors of good outcome in radiosurgery.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cordero Tous N, Cruz Sabido Jdl, Román Cutillas AM, Saura Rojas EJ, Jorques Infante AM, Olivares Granados G. Resultados de la aplicación de radiocirugía con acelerador lineal en pacientes con neuralgia del trigémino. Neurología. 2017;32:166–174.