To assess effectiveness of 5-aminolevulinic acid (5-ALA, Gliolan®) in patients treated for malignant glioma under typical daily practice conditions in Spain, using complete resection rate (CR) and progression free survival at 6 months (PFS6).
Materials and methodsRetrospective review of data from 18 neurosurgery departments that were categorised as either using or not using 5-ALA. The study included adult patients with suspected malignant gliomas for whom the intended treatment plan included complete resection followed by radiotherapy and chemotherapy with temozolomide. Postoperative MRI and clinical data representing at least 6 months were required for inclusion. Rates of CR and PFS6 were compared between patients with 5-ALA treatment and those without.
ResultsThe study included 251 evaluable cases. CR and PFS6 rates were significantly higher in the group of patients treated surgically with 5-ALA: CR, 67% versus 45%, P=.000; PFS6 for patients with grade IV tumours, 69% versus 48%; P=.002. The differences retained their significance and magnitude after adjusting for all covariates including age, functional status, and whether gliomas were located in eloquent areas.
ConclusionsIn this retrospective series, use of 5-ALA during habitual surgical procedures in Spain was associated with a higher complete resection rate for malignant glioma and increased PFS6 for grade IV glioma.
Evaluar la efectividad del ácido 5-aminolevulínico (5-ALA, Gliolan®) mediante la tasa de resecciones completas (RC) y supervivencia libre de progresión a los 6 meses (SLP6) en pacientes intervenidos quirúrgicamente de glioma maligno, en condiciones de práctica médica habitual en España.
Material y métodosRevisión retrospectiva en 18 servicios de neurocirugía, divididos en centros que usan habitualmente 5-ALA y centros que no. Se incluyó a pacientes adultos con sospecha de glioma maligno, en los que la intención de tratar incluyó resección completa y posterior radioterapia y quimioterapia con temozolomida. Era necesaria la existencia de resonancia magnética posquirúrgica y datos clínicos al menos durante 6 meses. Se comparó la diferencia entre pacientes con o sin 5-ALA en la tasa de RC y en la SLP6.
ResultadosSe obtuvieron 251 casos evaluables. La tasa de RC y la tasa de SLP6 fueron significativamente mayores en el grupo de pacientes operados con 5-ALA: RC, 67% frente a 45%, p=0,000, y SLP6 en el caso de los gliomas de grado IV, 69% frente a 48%; p=.002. Estas diferencias se mantuvieron relevantes y significativas tras ajustarlas por todas las covariables estudiadas, que incluyeron edad, estado funcional y localización en área elocuente o no.
ConclusionesEn esta serie retrospectiva, el uso de 5-ALA en la cirugía del glioma maligno en la práctica habitual en España se asoció a un incremento en la tasa de resecciones completas y, en el caso de los gliomas de grado IV, a un incremento en la supervivencia libre de progresión a los 6 meses.
Treatment of malignant glioma remains an unresolved problem. Studies of glioblastoma (GBM) have found that complete resection (CR) of the part of the tumour showing contrast uptake in the MRI study is associated with survival benefit.1–3 Nevertheless, complete resection was achieved in only a small percentage of these cases until recently. A 2008 literature review found rates between 30% and 47% in well-known centres of reference around the world.4 The low rate of CR is largely due to the difficulty of identifying certain areas of the tumour in the surgical field. In one study correlating the surgeon's impression with post-surgical MRI results, surgeons believed they had achieved CR in 54% of the cases, whereas MRI showed a CR rate of only 18%.5
Fluorescence induced by 5-aminolevulinic acid (5-ALA, Gliolan®) in high-grade gliomas affords the neurosurgeon a clear view of the tumour in the surgical field.6 In one randomised study of GBM, the technique demonstrated an increase in CR rate from 36% to 65%,7 accompanied by an increase in 6-month progression-free survival rate (PFS6) of 21%-41%. This study includes various histological types because it is not possible to distinguish between GBM and other grade IV gliomas, or between grade III and IV gliomas, prior to surgery. The benefit in the CR rate can be observed for all cases, whereas benefit to PFS6 was only analysed for GBM cases due to differences in survival rate for distinct histological types and the low number of cases of grade III glioma in the sample.
The result is difficult to generalise for two reasons. The first is that each hospital identifies different types of patients as candidates for surgery, a practice which cannot be standardised. The second is that, at the time this study was carried out, radiotherapy with concurrent temozolomide was not yet standard first-line treatment for GBM.8
This study aims to explore the use of this surgical tool in our setting. Firstly, we will quantify the effect on the CR rate of 5-ALA guided surgery in patients with high-grade gliomas who are candidates for surgery according to normal clinical practice in Spain. Secondly, we will quantify the impact on PFS6 for GBM in a setting in which radiotherapy with temozolomide is now standard treatment, also according to normal clinical practice in Spain.
Global survival (GS) is the outcome variable of choice for assessing the efficacy of any treatment. Nevertheless, at the time the study was designed, we did not have enough cases of 5-ALA treatment in Spain, as this would have required a follow-up time of between 12 and 24 months after surgery. At the same time, experience with Gliolan® is still very recent given that it has only been available for compassionate use since July 2008. Gliolan® was authorised by Spanish regulatory authorities in October 2010 and began to be marketed in 2011. A GS study would also require a much larger sample (570 patients in the study by Stupp et al.8). Such a sample would not be manageable for the purposes of our study even if a large population of patients treated with Gliolan® did exist.
Materials and methodsThis study was reviewed and approved by the Clinical Research Ethics Committee of the Regional Government of Navarra. It was also authorised by the Spanish Agency for Medicines and Medical Devices (AEMPS). Additionally, the study was revised by ethics committees at each of the participating hospitals as a requirement for acceptance by the provider and/or signing the contract with the hospital. These steps were carried out according to Order SAS/3470/2009 of 16 December regulating observational post-authorisation studies of drugs intended for human use.
This observational, retrospective, multi-centre study included 2 groups consisting of patients whose interventions did or did not employ 5-ALA (Gliolan®, Laboratorios Gebro Pharma S.A., Barcelona) during first-line surgical treatment for malignant glioma (grades III and IV according to the WHO classification system).
The 2 study groups were formed by sending viability questionnaires to 27 Spanish hospitals offering neurosurgery and then selecting one group of centres in which surgical excision with 5-ALA guidance was available and used habitually and a second group in which the fluorescence-guided technique was unavailable and had never been used.
We obtained two different comparable patient groups by establishing the inclusion and exclusion criteria shown in Table 1. These criteria include requirements for clinical indication of 5-ALA in hospitals that use the drug, in addition to basic follow-up data. By using the same criteria for patients in hospitals that did not use 5-ALA, we attempted to select highly homogeneous groups. According to the inclusion and exclusion criteria, patients who underwent surgery without 5-ALA guidance would have done so if their hospitals had been using that technique. The study included patients who underwent surgery between July 2011 and January 2011 to ensure a 6-month minimum follow-up period.
Patient selection criteria.
| Inclusion criteria |
| Adult patients (≥18 years) with clinical diagnosis of highly malignant glioma (grades III and IV) according to WHO criteria and presence of a tumour that caused symptoms and displayed contrast uptake in the presurgical MR imaging study. |
| Patients who underwent initial surgical tumour resection (after July 2008) in which total macroscopic excision was attempted. |
| Patients with an indication of adjuvant radiotherapy treatment along with temozolomide as intention-to-treat. |
| Patients with at least one postsurgical MRI taken a maximum of 28 days after surgery and necessarily before onset of radiotherapy. |
| Patients with available information spanning at least 6 months after surgery or who died within that period (whichever occurred first). |
| Exclusion criteria |
| Hypersensitivity to 5-aminolevulinic acid hydrochloride or porphyrins |
| Acute or chronic porphyria |
| Pregnancy |
Electronic case report forms (CRF) were sent to researchers at each of the hospitals. We collected the following information for each patient: date of surgery, age, functional status according to the Karnofsky performance status scale (KPS), tumour localisation, preoperative tumour volume or maximum diameters, preoperative neurological status, postoperative neurological status and subsequent progression if a deficit was present, date of postoperative MRI, postoperative tumour volume or maximum diameters, postoperative adjuvant therapy, clinical status in follow-up examinations at 3, 6, 9, and 12 months, and date of progression or death if applicable. The end point for follow-up was October 2011. Variables describing tumour localisation included whether the area was eloquent, non-eloquent, or undetermined; if the tumour affected the ependyma or crossed the midline; and the main lobe it occupied.
Postoperative MRI was used to determine whether or not CR had been achieved; researchers rated results as CR, incomplete resection, or undetermined. The accepted standard for evaluating residual tumour size consists of taking MRI scans with and without contrast in the first 3 days after surgery. However, viability questionnaires indicated that this method is not commonly available in many Spanish hospitals, so we accepted MRI scans performed before the onset of radiotherapy (RT). The analytical framework included a separate category for uncertain MRI results and an analysis containing a subgroup with early MRI results (scan performed 5 days or less after the surgery) and one with late MRI results (scan performed between 6 and 28 days after surgery).
Main study variablesThe main study variables were CR rate and PFS6 in GBM. ‘CR’ was defined as the proportion of patients whose grade III and IV gliomas were excised using 5-ALA-guided surgery or traditional white-light based microsurgery and whose postoperative MRI scan prior to radiotherapy onset showed no contrast enhancement. ‘PFS6’ was defined as the percentage of patients with grade IV glioma who had not experienced disease progression or death by any cause within 6 months of surgery. ‘Disease progression’ was defined as the appearance of new contrast-enhanced lesions larger than 1cm, increase in tumour size of 25% or more in MRI scan, clinical or neurological deterioration, or need to increase the corticosteroid dose.9 Only those patients with GBM were included in the PFS6 analysis since survival rates for patients with grade III gliomas are quite different. Furthermore, their sample was too small to be examined in a separate analysis.
Statistical methodsIn all statistical tests performed on outcome variables, the significance level was established at P=.05. The main study variables were analysed using the chi-square test, and each variable's association with the treatment group was estimated using logistic regression. Data were analysed using SPSS software version 19.
Before completing the main analysis, we completed a descriptive and comparative analysis of the patients’ demographical and clinical characteristics.
To verify the homogeneity of the group of patients who underwent 5-ALA guided surgery and those who underwent traditional white-light based microsurgery, researchers applied the non-parametric Mann-Whitney U test for numerical variables and the chi-square test or Fisher's exact test for categorical variables. Raw data analysis was completed by adjusting for the covariables that were collected.
In order to meet the pharmacovigilance measures required by law for observational studies, and considering the retrospective nature of this study, a descriptive analysis of the adverse effects linked to the product, and those potentially linked to the procedure, was completed using frequency tables. The chi-square test was used to compare the group with 5-ALA guidance to the group with traditional white-light based microsurgery.
The study's sample size was calculated using an estimated 26% increase in CR and a 16% increase in PFS6 for the group with 5-ALA guided surgery, based on results by Stummer et al.7 According to this estimate, we calculated an optimal sample size of 142 assessable cases in each of the 2 treatment groups and established an alpha error of 0.05 and a power of 0.80.
ResultsThis study collected data from a total of 264 cases. In the end, 13 were ineligible; 12 did not meet inclusion criteria because the date of surgery fell after January 2011, and the date of the postoperative MRI scan was not recorded in the last case. Once ineligible cases had been excluded, researchers analysed a total of 251 cases (95.1%), of which 131 belonged to the 5-ALA guidance group and 120 belonged to the traditional white-light-based microsurgery group.
Most tumours were assigned a histological diagnosis of glioblastoma multiforme; these tumours accounted for 85% of the white-light microsurgery group and 93.1% of the 5-ALA® guided group.
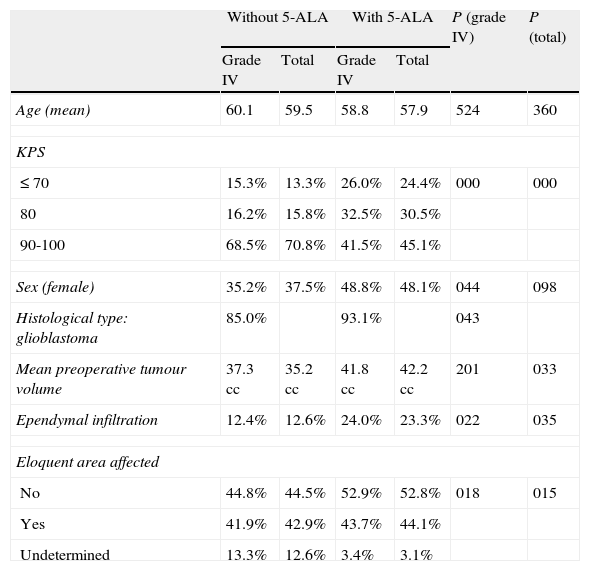
Patients’ main prognostic factors are listed in Table 2. Some significant differences are clearly apparent. In the group of patients who underwent surgery without 5-ALA, there were clearly more patients with a good functional status (KPS 90-100) and a higher percentage of patients with an uncertain degree of impact on the eloquent area. In the group of patients with 5-ALA guided surgery, preoperative tumour volume was somewhat larger and more tumours had infiltrated the ependyma.
Patient baseline characteristics and prognostic factors.
| Without 5-ALA | With 5-ALA | P (grade IV) | P (total) | |||
| Grade IV | Total | Grade IV | Total | |||
| Age (mean) | 60.1 | 59.5 | 58.8 | 57.9 | 524 | 360 |
| KPS | ||||||
| ≤70 | 15.3% | 13.3% | 26.0% | 24.4% | 000 | 000 |
| 80 | 16.2% | 15.8% | 32.5% | 30.5% | ||
| 90-100 | 68.5% | 70.8% | 41.5% | 45.1% | ||
| Sex (female) | 35.2% | 37.5% | 48.8% | 48.1% | 044 | 098 |
| Histological type: glioblastoma | 85.0% | 93.1% | 043 | |||
| Mean preoperative tumour volume | 37.3 cc | 35.2 cc | 41.8 cc | 42.2 cc | 201 | 033 |
| Ependymal infiltration | 12.4% | 12.6% | 24.0% | 23.3% | 022 | 035 |
| Eloquent area affected | ||||||
| No | 44.8% | 44.5% | 52.9% | 52.8% | 018 | 015 |
| Yes | 41.9% | 42.9% | 43.7% | 44.1% | ||
| Undetermined | 13.3% | 12.6% | 3.4% | 3.1% | ||
P (grade IV): significance level for differences between the 2 groups with grade IV tumours.
P (total): significance level for differences between both groups for all tumours.
Regarding the main outcome variables, the CR rate confirmed by MRI in patients who underwent 5-ALA guided surgery was higher than in the white-light group at 67.2%, compared to 45%, this difference was highly significant (P=.000). Similarly, the proportion of patients with grade IV tumours and PFS6 was higher in the 5-ALA group at 69.1% vs 48.1% in the traditional surgery group. This difference was also statistically significant (P=.002).
Results from main variables were similar and also significant after adjustments for known prognostic factors (age, KPS, presence or absence in eloquent areas) and the known potential covariables recorded in this study (Tables 3 and 4).
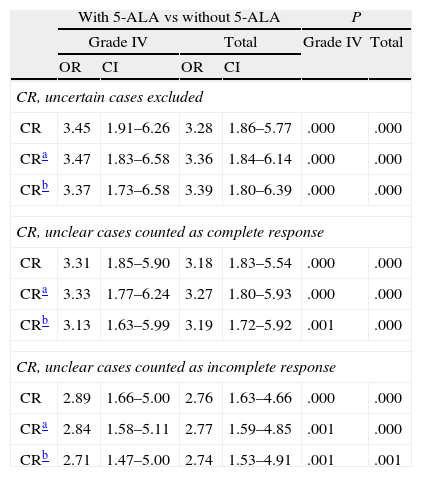
Odds ratio for complete resections with use of 5-ALA.
| With 5-ALA vs without 5-ALA | P | |||||
| Grade IV | Total | Grade IV | Total | |||
| OR | CI | OR | CI | |||
| CR, uncertain cases excluded | ||||||
| CR | 3.45 | 1.91–6.26 | 3.28 | 1.86–5.77 | .000 | .000 |
| CRa | 3.47 | 1.83–6.58 | 3.36 | 1.84–6.14 | .000 | .000 |
| CRb | 3.37 | 1.73–6.58 | 3.39 | 1.80–6.39 | .000 | .000 |
| CR, unclear cases counted as complete response | ||||||
| CR | 3.31 | 1.85–5.90 | 3.18 | 1.83–5.54 | .000 | .000 |
| CRa | 3.33 | 1.77–6.24 | 3.27 | 1.80–5.93 | .000 | .000 |
| CRb | 3.13 | 1.63–5.99 | 3.19 | 1.72–5.92 | .001 | .000 |
| CR, unclear cases counted as incomplete response | ||||||
| CR | 2.89 | 1.66–5.00 | 2.76 | 1.63–4.66 | .000 | .000 |
| CRa | 2.84 | 1.58–5.11 | 2.77 | 1.59–4.85 | .001 | .000 |
| CRb | 2.71 | 1.47–5.00 | 2.74 | 1.53–4.91 | .001 | .001 |
CI: 95% confidence interval; OR: odds ratio; CR: complete resections.
When examining CR rate, researchers had the option of rating MRI-based evaluations of residual tumour presence after surgery as ‘uncertain’. This option was recorded in the CRF. The analysis compared CR rates obtained using the following 3 scenarios: excluding uncertain results, grouping uncertain cases with complete resection, or grouping them with incomplete resection. The CR rate obtained with 5-ALA guidance was significantly higher for patients with grade IV tumours and in the total sample, according to all of the above scenarios. In the model of excluding doubtful cases, the non-adjusted odds ratio for all tumours was 3.3 (CI 1.9-5.8), displaying an observable and very significant treatment effect (P=.000). After adjusting for age, KPS and effect on eloquent area, the odds ratio was 3.4 (CI 1.8-6.1, P=.000); adjustments for all significant variables yielded an OR of 3.4 (CI 1.8-6.4, P=.000). Table 3 displays the odds ratio of the effect of 5-ALA guidance on CR for all scenarios and grade IV tumours only, with both adjusted and unadjusted data. As we see, this effect is robust and significant for all scenarios included in the analysis.
The study also compared CR rates with and without 5-ALA guidance using only those results from cases in which MRI was performed in the first 5 days after surgery: 118 in the 5-ALA group and 80 in the conventional surgery group. The difference was similar to results in the total sample and also significant (72.9% with 5-ALA vs 50% without 5-ALA; P=.003); there were no changes related to the interpretation scenarios for unclear MRI results.
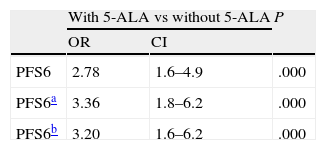
For PFS6, results were similar to those obtained for CR (71.2% with 5-ALA vs 52.5% without 5-ALA, P=.010). The unadjusted odds ratio for PFS6 was 2.8 (CI 1.6-4.9) with a marked and very significant treatment effect (P=.000). After adjusting by age, KPS, and effect on eloquent area, the difference was even more pronounced with an odds ratio of 3.4 (CI 1.8-6.2) and P=.000; adjustment by all significant variables (age, KPS, effect on eloquent area, and treatment with single chemotherapy drug) yielded an OR of 3.20 (CI 1.6-6.2), P=.001.
In line with both of the study objectives, we analysed subgroups formed according to the main prognostic factors, as described in a later section. When interpreting these data, we should be mindful that some subgroups are so small that statistically significant differences cannot be anticipated.
Differences in the CR rates remained similar for different KPS score intervals (KPS≤80: 68.8% vs 46.9%, P=.047; KPS>80:80.0% vs 48.8%, P=.000). Differences in PFS6 followed a similar pattern; they could be observed in all intervals, although they only reached statistical significance in the largest subgroup, containing patients with the best functional status (KPS<60: 50% vs 33.3%, P=1.0; KPS 70-80:61.3% vs 46.9%, P=.19; KPS 90-100: 84.7% vs 51.8%, P=.000).
For tumours located in non-eloquent areas, the CR rate was higher in patients who underwent 5-ALA guided surgery, at 74.6% vs 63.3%, but the difference was not significant (P=.216). For tumours located in close proximity to eloquent areas, the difference was substantially larger, 75.9% vs 35.8% (P=.000) In contrast, PFS6 showed similar and statistically significant differences for tumours located in non-eloquent areas (80.6% vs 54.7%, P=.003) and those in eloquent areas (64.3% vs 42.9%, P=.032).
We also analysed neurological complications in the immediate perioperative period and at one month after surgery. During the immediate postoperative period, the group with 5-ALA guidance displayed a significantly higher rate of 2 neurological complications: aphasia and hemianopsia. Aphasia was detected in 26.9% of this group, compared to 10% in the group that had undergone conventional surgery (P=.048). While many of these complications had resolved one month after surgery, they persisted in 10.5% of the 5-ALA group and 5% of the conventional surgery group. Neurological deficit was also more frequent before surgery, but the difference was not statistically significant (24.8% vs 19.8%). There was also a significant difference in the frequency of hemianopsia (25.4% vs 5%, P=.008), but almost all cases resolved within the first month (the condition persisted in 2.3% and 2.5%, respectively). As in cases of aphasia, more patients in the 5-ALA group presented hemianopsia prior to surgery (15.2% vs 8.1%; the difference was not statistically significant at P=.109). All other neurological complications displayed similar frequencies in both groups.
DiscussionDespite the fact that the sample was somewhat smaller than the target size, results from this study are statistically significant and confirm the primary objectives as much as the study's methodological constraints will allow. An analysis of potential limitations indicates that, since this study is observational and retrospective rather than prospective and controlled, results depend on the quality and quantity of the information obtained. Nevertheless, our results are relevant to daily clinical practice, and this is precisely why data were gathered. Results reflect the situation in Spain and they are strongly applicable, based on the fact that the viability questionnaire was sent to most large hospitals nationwide, including centres offering neurosurgery in all of Spain's autonomous communities. The main limitation preventing some hospitals from participating is the fact that a routine MRI scan is not standard practice.
In this particular study, we might also question the reliability of the researcher's assessment of the degree of resection. Logically enough, this measure is not as reliable as using a single evaluator. This being the case, we should point out that results are consistent for all potential models, regardless of whether the effect is measured with unadjusted data or with data adjusted by age, KPS, and effect on the eloquent area. Results remain consistent when adjusted by all associated variables at once. CR rate results are also coherent with PFS6 results for all adjustment models. This coherence supports the validity of these results, although PFS6 may be affected by other factors that cannot be analysed in a retrospective, multi-centre, non-blinded study like this one.
CR results observed in daily clinical practice in Spain were quite similar to those obtained in the randomised prospective study by Stummer et al.7 The CR rates in this study were 67% vs 45%, compared to 65% vs 36% in the Stummer study. Although the 2 studies are not easily comparable, concordance between these data sets confirms that use of the product yielded the exact results anticipated a priori.
These results also indicate that benefits from temozolomide treatment (TMZ) were added to those of resection. In an earlier randomised study with 5-ALA,7 patients underwent surgery and radiotherapy only. This study predates RT+TMZ being accepted as standard treatment. PFS6 rates in our study are clearly higher, in both groups, than rates observed by Stummer (69% with 5-ALA vs 48% in our study; 41% with 5-ALA vs 21.1% in the Stummer et al. study). This indicates that including TMZ in treatment increased PFS by about 27%; furthermore, we find a 20% increase in PFS attributed to 5-ALA guidance. The PFS rate observed among patients treated with 5-ALA in this study is superior to the rate recorded in the Stupp et al. study (53.9%) which defines the current standard.8 This finding is especially important for confirming the applicability of benefit outcomes of 5-ALA to the current situation in Spain and other countries, since some researchers question whether the utility of CR will decrease when effective chemotherapy treatment is used.
One of this study's limitations is that we cannot rule out the presence of cases of pseudoprogression, given that RANO criteria had not yet been established when these patients were undergoing treatment. However, there do not seem to be any reasons to explain why the rate of pseudoprogression would differ from one group to another. Furthermore, the 2005 study by Stupp et al., which serves as a reference in this field, does not explore the phenomenon of pseudoprogression, and our results would therefore be comparable.
Improving overall survival rates is the end goal for all measures intended to treat this type of tumour. This being the case, we might contemplate whether this should be the objective of a study like this one. We must be mindful that all treatments received by the patient after the initial surgery, and the different responses to those treatments, act as confounders in this study. To counteract this effect, researchers will need a very large patient sample and a much longer follow-up period than the time elapsed since the product was made available in Spain. In addition to the above, we believe it is important to stress that while 5-ALA is a chemical substance and has the legal status of a drug, it is in fact a surgical tool. It has no effect on the tumour apart from helping the surgeon excise the lesion more completely. Based on the above, we believe that the main study objective is to determine if the tool serves its purpose. By concluding that 5-ALA is an effective tool, this multi-centre observational study coincides with a number of previously published studies with different approaches.10–14 The utility of CR forms part of a much larger debate in neuro-oncology that covers all the tools a neurosurgeon may use. Currently, level 2b evidence shows that this benefit exists1 and the leading study groups agree that CR is associated with increased survival.2,3 Data suggest that, in addition to providing intrinsic benefits, CR offers the best baseline situation for an adjuvant treatment to be successful.15
While 5-ALA helps surgeons achieve larger margins, these results also show that such procedures are associated with a higher risk of neurological damage. Doctors know that malignant gliomas are diffuse tumours that lack defined borders and infiltrate the surrounding parenchyma, and that 5-ALA clearly indicates the solid tumour in addition to a part of the infiltrated area around it in which fluorescence is less intense.14,16–18 The product's recommendations for use remind us that its demonstrated benefit is associated with excision of the most intensely fluorescent areas; mild fluorescence corresponds to infiltration and extreme caution should be used in lesions adjacent to eloquent areas. Likewise, intraoperative neurophysiological use is recommended for procedures involving complete resection of lesions near eloquent areas.11,19 This study did not contemplate whether or not neurophysiological monitoring was used. The higher rate in deficits in the 5-ALA group confirms that the product is recommended for high-risk cases. Most neurological deficits were transient and studies have indicated that patients who underwent incomplete resection presented early neurological impairment due to disease.20 As a result, the balance between the risk of an immediate deficit and a future benefit should be carefully weighed by the neurosurgeon and the patient on a case-by-case basis.
In conclusion, use of 5-ALA guidance for malignant glioma resection in normal clinical practice in Spain is associated with statistically significant increases in the complete resection rate and PFS6 rate in GBM. This increase is clinically relevant and remains statistically significant after adjusting data for numerous covariables.
FundingThis study was financed by Laboratorios Gebro Pharma S.A.
Conflicts of interestJordi Galván, Cristina Arza, and Cristina Romáriz are employed by Laboratorios Gebro Pharma, S.A. Cristina Vidal, John Slof, and Ricardo Díez Valle have received professional fees from Laboratorios Gebro Pharma, S.A.
We would like to thank the researchers participating in the VISIONA study: Dr Ricardo Díez Valle; Dr Pilar Teixidor i Rodriguez; Dr Gloria Villalba Martínez; Dr Jose Manuel Cabezudo; Dr Luis Miguel Bernal García; Dr Josep J. González Sánchez; Dr Enrique Ferrer Rodríguez; Dr Miguel Ángel Arraez Sánchez; Dr Gonzalo Olivares Granados; Dr Álvaro Toledano Delgado; Dr Gerardo Conesa Bertrán. Dr Juan José Acebes Martín; Dr Gerard Plans Ahicart; Dr Avelino Parajón Díaz; Dr Carlos Botella Asunción; Dr Ricardo Prat Acin; Dr Marta del Álamo de Pedro; Dr Luis Ley Urzaiz; Dr Juan A. Barcia; Dr Juan R. Brin; Dr José María Torres Campa-Santamarina; Dr Ángel Maíllo Sánchez, and Dr Jesús Merino Peña.
Please cite this article as: Díez Valle R, Slof J, Galván J, Arza C, Romariz C, Vidal C, et al. Estudio observacional retrospectivo sobre la efectividad del ácido 5-aminolevulínico en la cirugía de los gliomas malignos en España (Estudio VISIONA). Neurología. 2014;29:131–138.
A summary of this study was presented orally at the 17th Congress of the Spanish Society of Neurosurgery in May 2012.