Parkinson's disease is a progressive neurodegenerative disorder characterised by a loss of dopaminergic neurons in the substantia nigra pars compacta, which results in a significant decrease in dopamine levels and consequent functional motor impairment.
DevelopmentAlthough its aetiology is not fully understood, several pathogenic mechanisms, including oxidative stress, have been proposed. Current therapeutic approaches are based on dopamine replacement drugs; these agents, however, are not able to stop or even slow disease progression. Novel therapeutic approaches aimed at acting on the pathways leading to neuronal dysfunction and death are under investigation.
ConclusionsIn recent years, such natural molecules as polyphenols, alkaloids, and saponins have been shown to have a neuroprotective effect due to their antioxidant and anti-inflammatory properties. The aim of our review is to analyse the most relevant studies worldwide addressing the benefits of some phytochemicals used in in vitro models of Parkinson's disease.
La enfermedad de Parkinson es un trastorno neurodegenerativo progresivo caracterizado por la pérdida de neuronas dopaminérgicas de la sustancia nigra pars compacta, promoviendo una disminución significativa en los niveles de dopamina y en consecuencia el deterioro funcional del circuito motor.
DesarrolloAunque su etiología no está bien esclarecida, se han propuesto varios mecanismos patogénicos, entre ellos destaca el estrés oxidativo. La terapia actual se basa en medicamentos que reemplazan la dopamina, sin embargo, no son capaces de detener o incluso ralentizar la progresión de la enfermedad. En la actualidad están siendo investigados nuevos enfoques terapéuticos con la intención de influir en las vías que conducen a la disfunción y muerte neuronal.
ConclusionesEn los últimos años, se ha evidenciado el efecto neuroprotector de moléculas naturales debido a sus propiedades antioxidantes y antiinflamatorias dentro de los cuales destacan los polifenoles, los alcaloides y las saponinas. El objetivo de esta revisión es recopilar los estudios más importantes a nivel mundial que establecen las propiedades benéficas de algunos fitoquímicos utilizados en modelos in vitro de la enfermedad de Parkinson.
Parkinson's disease (PD) is the second most frequent neurodegenerative disease, after Alzheimer disease, and affects approximately 1% to 2% of adults aged over 60, or 5-6 million people worldwide.1–3 The degeneration of dopaminergic neurons of the substantia nigra pars compacta and of nerve fibres projecting to the striatum significantly reduces the levels of dopamine, a neurotransmitter essential for neural modulation. This causes motor dysfunction, bradykinesia, postural instability, rigidity, and tremor, which are typical of the disease, as well as such non-motor symptoms as sleep disorders, depression, and cognitive impairment.3–7 Damage to dopaminergic neurons may be mediated by a number of pathogenic mechanisms, including mitochondrial dysfunction, apoptosis, transition metal accumulation, oxidative stress, inflammation, and protein misfolding and aggregation. Recent studies have shown that oxidative stress is the mechanism most closely linked to an increase in reactive oxygen species (ROS), which induce neuronal death by apoptosis.8–10
Pharmacological treatment for PD is based on dopamine replacement therapy, which effectively reduces motor symptoms, pain, and depression. However, these drugs are only effective for approximately 10 years; long-term use leads to the accumulation of ROS and other toxic metabolites resulting from dopamine metabolism. Furthermore, these drugs are unable to halt or slow disease progression.3,11–14 This review addresses the most relevant studies on the neuroprotective effects of some phytochemicals on in vitro models of PD.
Natural molecules with neuroprotective effects on dopaminergic neuronsNumerous plants around the world produce bioactive substances with important biological effects (antioxidant, anti-inflammatory, anticarcinogenic, antimutagenic, etc.); these compounds are known as phytochemicals. According to their impact on human health, phytochemicals may be classified into: (1) terpenoids and polyenes, (2) polyphenols, (3) organosulfur compounds, and (4) nitrogen compounds. Polyphenols constitute the largest class and include flavonoids, flavones, flavanones, isoflavones, anthocyanins, catechins, phenolic acids, tannins, phytoestrogens, stilbenes, and curcuminoids.15,16
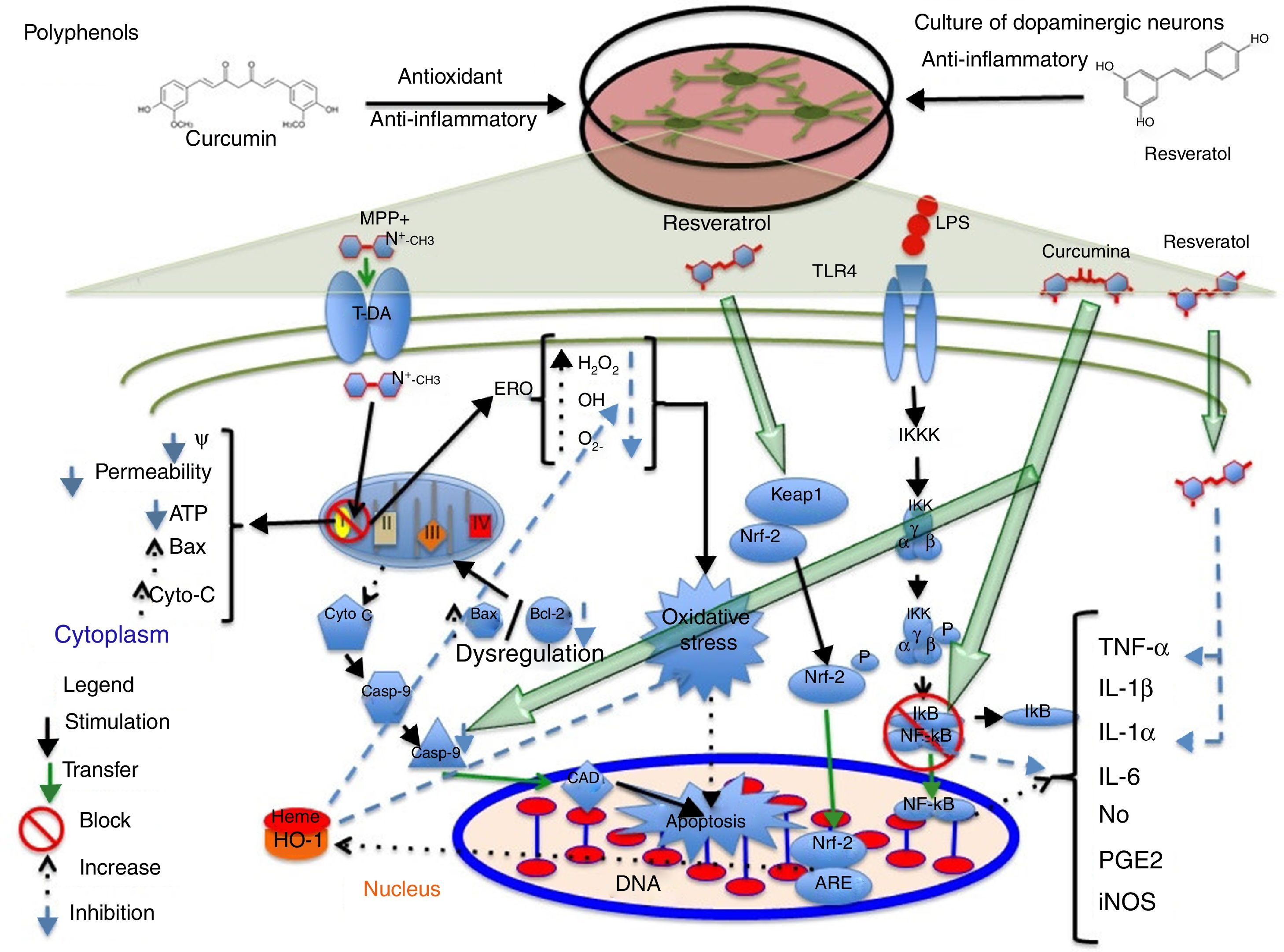
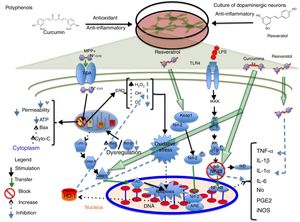
PolyphenolsPolyphenols are molecules with antioxidant and anti-inflammatory properties due to their ability to donate electrons and hydrogen atoms, and their immunomodulatory activity.17,18 They have been found to have therapeutic effects for diabetes, cancer, and cardiovascular and neurodegenerative diseases.19 Resveratrol, a type of polyphenol, is present in grapes, red wine, and other foods.20 At concentrations between 30 and 100μM, resveratrol protects midbrain dopaminergic neurons by maintaining glutathione levels and reducing ROS levels and the oxidative stress induced by 20μM of the neurotoxin 1-methyl-4-phenyl pyridinium MPP+. Resveratrol also suppresses the acetylation of p53, preventing apoptosis induced by the alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine.21 In a 2013 study by Moldzio et al.,22 resveratrol (0.01, 0.1, and 1μM) was observed to have a neuroprotective effect on rat midbrain neurons, compared to glutamate, by reducing the number of dead cells. Resveratrol promotes neurite outgrowth and prevents cell degeneration by decreasing superoxide radical (O2−) formation by 68%. Resveratrol (20μM) has recently been found to partially increase haem oxygenase-1 (HO-1) expression in SH-SY5Y dopaminergic neurons, which reduces ROS expression and oxidative stress, and induces autophagy, preventing rotenone-induced cell death.1 The available evidence also suggests that this compound increases HO-1 expression via Nfr-2 in a mouse model of cerebral ischaemia.23 Combined administration of resveratrol and quercetin to a culture of PC12 cells differentiated to dopaminergic cells significantly reduces lipopolysaccharide-mediated expression of interleukin-1 alpha (IL-1α) proinflammatory cytokines and tumour necrosis factor alpha (TNF-α), preventing neuronal death.24Fig. 1 shows a possible neuroprotective mechanism for polyphenols. Due to the antiapoptotic and free radical-scavenging activity of quercetin,25 and the protective effects of sesamin against oxidative stress,26 administration of these 2 compounds to cocultures of neuronal PC12 cells and MPP+-activated microglial N9 cells has been found to lead to a significant decrease in genetic expression and concentration of proinflammatory cytokines IL-6, IL-1 (IL-1β), and TNF-α, and to a decrease in inducible nitric oxide synthase (iNOS) expression and O2− production, rescuing dopaminergic neurons from apoptosis.27 Sesamin has also been shown to protect dopaminergic neurons derived from PC12 cells. In 2008, Lahaie-Collins et al.26 showed that a 3-hour pretreatment with sesamin before MPP+ exposure protects neurons by decreasing oxidative stress and IL-6 expression, and increasing catalase activity and tyrosine hydroxylase expression.
A possible neuroprotective mechanism for polyphenols. The image shows the action of the anti-inflammatory phytochemicals curcumin and resveratrol on molecular targets conferring neuroprotection to dopaminergic neurons in different signalling pathways which lead to the formation of proinflammatory cytokines and cell apoptosis. Curcumin acts mainly by blocking NF-κB, preventing proinflammatory cytokine production, and caspase-3, inhibiting apoptosis. Resveratrol decreases TNF-α and IL-1α levels and increases HO-1 levels; this increase protects cells from oxidative stress due to a decrease in the levels of reactive oxygen species.
Curcumin, a polyphenol obtained from Curcuma longa, has antioxidant and anti-inflammatory properties.28 At a concentration of 5μM, curcumin has a neuroprotective effect on SH-SY5Y dopaminergic neurons, reducing rotenone-induced caspase-3 activity.29 In addition to its neuroprotective properties and the significant decrease it causes in caspase-3 production in 6-hydroxydopamine-treated SH-SY5Y dopaminergic neurons, curcumin has been found to protect against oxidative stress by decreasing phosphorylated p38 expression and toxic quinoprotein formation, which increases cell viability and restores tyrosine hydroxylase (TH) levels.30 Yang et al.31 showed that 10μM curcumin has a neuroprotective effect on midbrain dopaminergic neurons, increasing dopamine uptake; decreasing the expression of proinflammatory cytokines nitric oxide (NO), prostaglandin E2 (PGE2), IL-1β, and TNF-α; and inhibiting the transcription of nuclear factor κB (NF-κB) and activator protein-1, which cause oxidative stress. However, the researchers also observed greater neuroprotection in microglial cells, which suggests that microglia in their study played a pivotal role against lipopolysaccharide-induced neurotoxicity. A group of researchers have identified a possible molecular target for curcumin that may be involved in SH-SY5Y dopaminergic neuron protection during MPP+-induced cytotoxicity (3mM MPP+). This mechanism inhibits JNK pathway activation and caspase-3 cleavage, preventing neuronal death32 (Fig. 1). When applied to MPP+-treated rat midbrain cultures, 6-shogaol (a pungent compound with anti-inflammatory properties, isolated from ginger) was found to prevent TH-immunoreactive cell loss and significantly decrease NO and TNF-α levels.33
Fustin, a flavonoid extracted from Rhus verniciflua, has anti-inflammatory34 and antimutagenic properties.35 Fustin has been found to have neuroprotective properties: SK-N-SH dopaminergic neurons pretreated for 30 minutes with fustin at concentrations above 50μM then exposed for 24 hours to 6-OHDA at 125μM displayed decreased ROS levels and higher levels of intracellular calcium (Ca2+). Fustin also prevents increases in the Bax/Bcl-2 ratio, caspase-3 activity, and p38 phosphorylation.36 Biochanin A, an isoflavone with anti-inflammatory properties, contained in Trifolium pratense, confers neuroprotection to rat midbrain dopaminergic neurons. It has been shown to effectively protect dopaminergic neurons against lipopolysaccharide-induced neurotoxicity (10ng/mL), which decreases dopamine uptake by 36.7% and reduces the cell population by 52%. At concentrations of 0.25, 1, and 2.5μM, biochanin A significantly increases dopamine uptake (by 55.9%, 77.9%, and 88.7%, respectively), protects cells against lipopolysaccharide-induced damage (62.5%, 81.9%, and 89.4%), and inhibits the production of proinflammatory cytokines TNF-α and NO; O2− production is also reduced in a dose-dependent manner.37 Acacetin is a flavone with anticarcinogenic and anti-inflammatory properties found in such plants as chrysanthemums and safflower. A primary culture of rat midbrain dopaminergic neurons exposed to 10μM MPP+ after treatment with 50-200nM acacetin showed increased numbers of TH-immunoreactive dopaminergic cells and preserved neuronal morphology, including shortening of dendrites, when compared to controls. Acacetin inhibits the production of such proinflammatory factors as NO, PGE2, and TNF-α in a dose-dependent manner.38 Baicalein is a flavonoid present in the roots of Scutellaria baicalensis. In addition to its anti-inflammatory properties, it has a potent antioxidant effect against free radicals. A study analysed resistance to 6-OHDA-induced toxicity (100μM) in vitro in a culture of SH-SY5Y dopaminergic cells pretreated with 0.05, 0.5, and 5μg/mL baicalein. The compound was shown to have neuroprotective properties at concentrations of 0.5μg/mL and above, with 64% survival compared to controls. Furthermore, pretreatment with 5μg/mL baicalein significantly reduces the percentage of apoptotic cells39 (Table 1).
List of polyphenols with neuroprotective properties.
| Compound | Concentration | Exposure duration | In vitro model | Toxicity model | Main effect | Reference |
|---|---|---|---|---|---|---|
| Resveratrol | 10, 30, and 100μM | 48h | Neonatal rat midbrain slices | MPP+ (20μM) | ↑ cell survival ↓ ROS and oxidative stress Block of p53 acetylation | Okawara et al.21 (2007) |
| Resveratrol | 0.01, 0.1, and 1μM | 48h | Mouse midbrain slices | Glutamate (5mM) | ↑ cell survival Preservation of neurite length ↓ O2− | Moldzio et al.22 (2013) |
| Resveratrol | 20μM | 24h | SH-SY5Y cells | Rotenone (20μM) | ↑ HO-1 ↓ ROS and oxidative stress ↑ autophagy | Lin et al.1 (2014) |
| Resveratrol and quercetin | 0.1μM | 3h | N9 and PC12 cells differentiated into dopaminergic neurons | LPS (1μg/mL) | ↑ cell survival ↓ IL-1α and TNF-α | Bureau et al.24 (2008) |
| Quercetin and sesamin | Quercetin 0.1μM Sesamin 1pM | 3h | N9 and PC12 cells differentiated into dopaminergic neurons | MPP+ (500μM) | ↓ IL-6, IL-1β, and TNF-α Inhibition of iNOS and O2− | Bournival et al.27 (2012) |
| Sesamin | 1pM | 3h | PC12 cells differentiated into dopaminergic neurons | MPP+ (5mM) | ↓ oxidative stress ↑ Catalase ↑ p-TH ↓ IL-6 | Lahaie-Collins et al.26 (2008) |
| Curcumin | 1, 5, and 10μM | 1h | SH-SY5Y cells | Rotenone (100μM) | ↓ Caspase-3 | Qualls et al.29 (2014) |
| Curcumin | 1, 5, 10, and 20μM | 0.5h | SH-SY5Y cells | 6-OHDA (25μM) | ↑ cell viability ↓ quinoprotein, p38, and caspase-3 ↑ p-TH | Meesarapie et al.30 (2014) |
| Curcumin | 10μM | 0.5, 1, 3, and 6h | Primary culture of rat dopaminergic neurons | LPS (5ng/mL) | ↑ dopamine uptake ↓ NO, TNF-α, PGE2, IL-1β Blocks NF-κB and AP-1 | Yang et al.31 (2008) |
| Curcumin | 1 and 5μM | 2h | SH-SY5Y cells | MPP+ (3mM) | ↑ cell viability Blocks JNK pathway and caspase-3 | Yu et al.32 (2010) |
| 6-shogaol | 0.001 and 0.01μmol/L | 1h | Primary culture of rat dopaminergic neurons | MPP+ (3mM) | ↑ survival ↓ NO and TNF-α | Park et al.33 (2013) |
| Fustin | 20, 50, 100, 150, and 200μM | 30min | SK-N-SH cells | 6-OHDA (125μM) | ↓ ROS and Ca2+ ↓ Bax/Bcl-2 ratio ↓ caspase-3 and p38 | Park et al.36 (2007) |
| Biochanin A | 0.25, 1, and 2.5μM | 30minpretreatment+24h | Primary culture of rat dopaminergic neurons | LPS (10ng/mL) | ↑ dopamine uptake and cell survival ↓ TNF-α, NO, and O2− | Chen et al.37 (2007) |
| Acacetin | 50 and 200nM | 1h pretreatment+23h | Primary culture of rat dopaminergic neurons | MPP+ 10μM | ↑ cell viability Preserved neuronal morphology (shortening of neurites) ↓ NO, PGE2, and TNF-α | Kim et al.38 2012 |
| Baicalein | 0.05–5μg/mL | 1h pretreatment+12h | SH-SY5Y cells | 6-OHDA (100μM) | ↑ survival | Mu et al.39 (2009) |
6-OHDA: 6-hydroxydopamine; AP-1: activator protein-1; HO-1: haem oxygenase-1; IL: interleukin; iNOS: inducible nitric oxide synthase; JNK: c-Jun N-terminal kinase; LPS: lipopolysaccharide; NF-κB: nuclear factor-κB; MPP+: 1-methyl-4-phenyl pyridinium; NO: nitric oxide; PGE2: prostaglandin E2; p-TH: phospho-tyrosine hydroxylase; ROS: reactive oxygen species; TNF: tumour necrosis factor.
This group of phytochemicals includes alkaloids, amines, non-protein amino acids, cyanogenic glycosides, and glucosinolates.
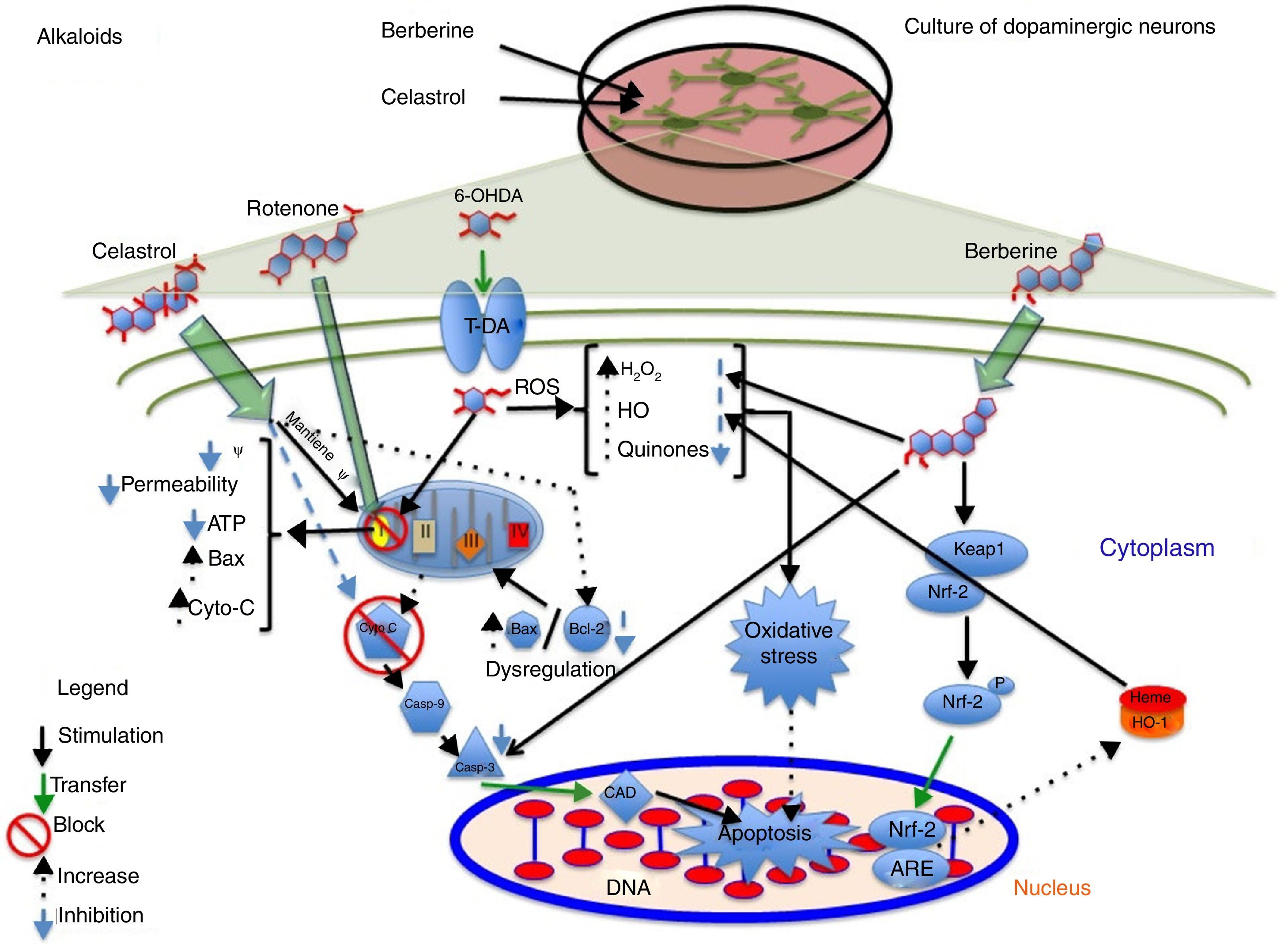
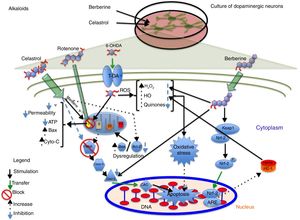
AlkaloidsTetrahydroberberine, a compound isolated from Rhizoma corydalis, used in traditional Chinese medicine, has neuroprotective properties as it blocks ATP-sensitive potassium channels.40,41 In 2010, Wu et al.41 found that tetrahydroberberine restores rotenone-induced membrane hyperpolarisation to physiological conditions (−46.1mV) in dopaminergic neurons via ATP-sensitive potassium channels, depolarising the membrane from −61.7mV to −46.7mV. The researchers also found that this mechanism involves the dopamine D2 receptor: tetrahydroberberine significantly restored hyperpolarisation and increased action potential firing in cells exposed to the D2 receptor antagonist sulpiride. Berberine, a compound isolated from Coptis xhinensis and used in traditional Chinese medicine as an antidiarrhoeal agent, has anti-inflammatory and anticarcinogenic properties, and confers cardiovascular protection. In a 2013 study by Bae et al.,42 berberine was found to protect SH-SY5Y cells against 6-OHDA-induced death. The researchers observed a dose-dependent increase in cell survival, decreased apoptosis due to reduced caspase-3 activity, and lower ROS levels after 3 hours of pretreatment with berberine. They also observed that berberine increases the expression of HO-1 mRNA by increasing the translocation of the Nrf2 transcription factor. Fig. 2 shows a possible neuroprotective mechanism for berberine.
Neuroprotective effect of the alkaloids celastrol and berberine against rotenone. The image shows the inhibition of complex I of the electron transport chain, decreased ATP production, increased ROS levels, and inhibition of the endogenous antioxidant system, which promotes oxidative stress and apoptosis. Celastrol maintains the mitochondrial membrane potential, increases Bcl-2 levels, and inhibits cytochrome C release into the cytosol, preventing apoptosis. Berberine inhibits caspase-3 cleavage and increases HO-1 via Nfr-2, preventing oxidative stress by reducing ROS levels.
Celastrol, isolated from Tripterygium wilfordii root extracts, has anti-inflammatory and anticarcinogenic properties.43 At a concentration of 10nM, the compound has been shown to prevent rotenone-induced cell death (rotenone concentration of 10μM) by decreasing ROS levels, and to block cytochrome C release into the cytosol and inhibit Bax expression. It also confers protection against loss of membrane potential (Ψ) and increases the levels of anti-apoptotic protein Bcl-22; this may be the action mechanism of celastrol (Fig. 2 and Table 2).
Alkaloids with neuroprotective effects on dopaminergic neurons.
| Compound | Concentration | Exposure duration | In vitro model | Toxicity model | Main effect | Reference |
|---|---|---|---|---|---|---|
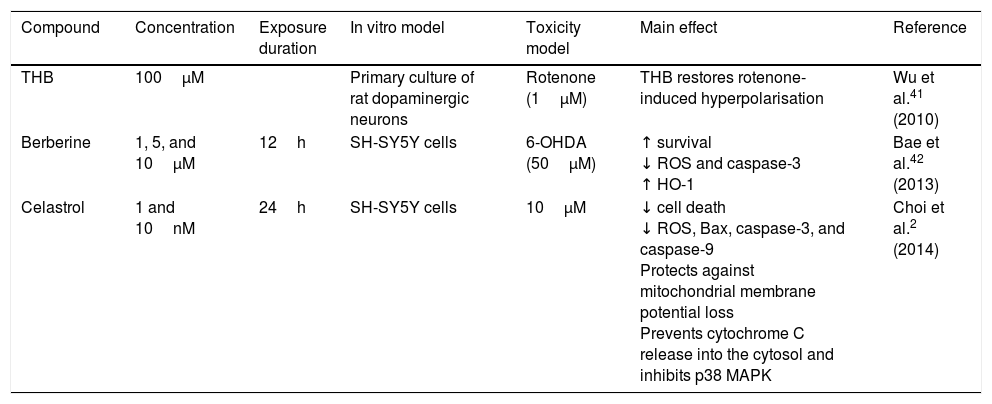
| THB | 100μM | Primary culture of rat dopaminergic neurons | Rotenone (1μM) | THB restores rotenone-induced hyperpolarisation | Wu et al.41 (2010) | |
| Berberine | 1, 5, and 10μM | 12h | SH-SY5Y cells | 6-OHDA (50μM) | ↑ survival ↓ ROS and caspase-3 ↑ HO-1 | Bae et al.42 (2013) |
| Celastrol | 1 and 10nM | 24h | SH-SY5Y cells | 10μM | ↓ cell death ↓ ROS, Bax, caspase-3, and caspase-9 Protects against mitochondrial membrane potential loss Prevents cytochrome C release into the cytosol and inhibits p38 MAPK | Choi et al.2 (2014) |
HO-1: haem oxygenase-1; MAPK: mitogen-activated protein kinase; MPP+: 1-methyl-4-phenyl pyridinium; ROS: reactive oxygen species; THB: tetrahydroberberine.
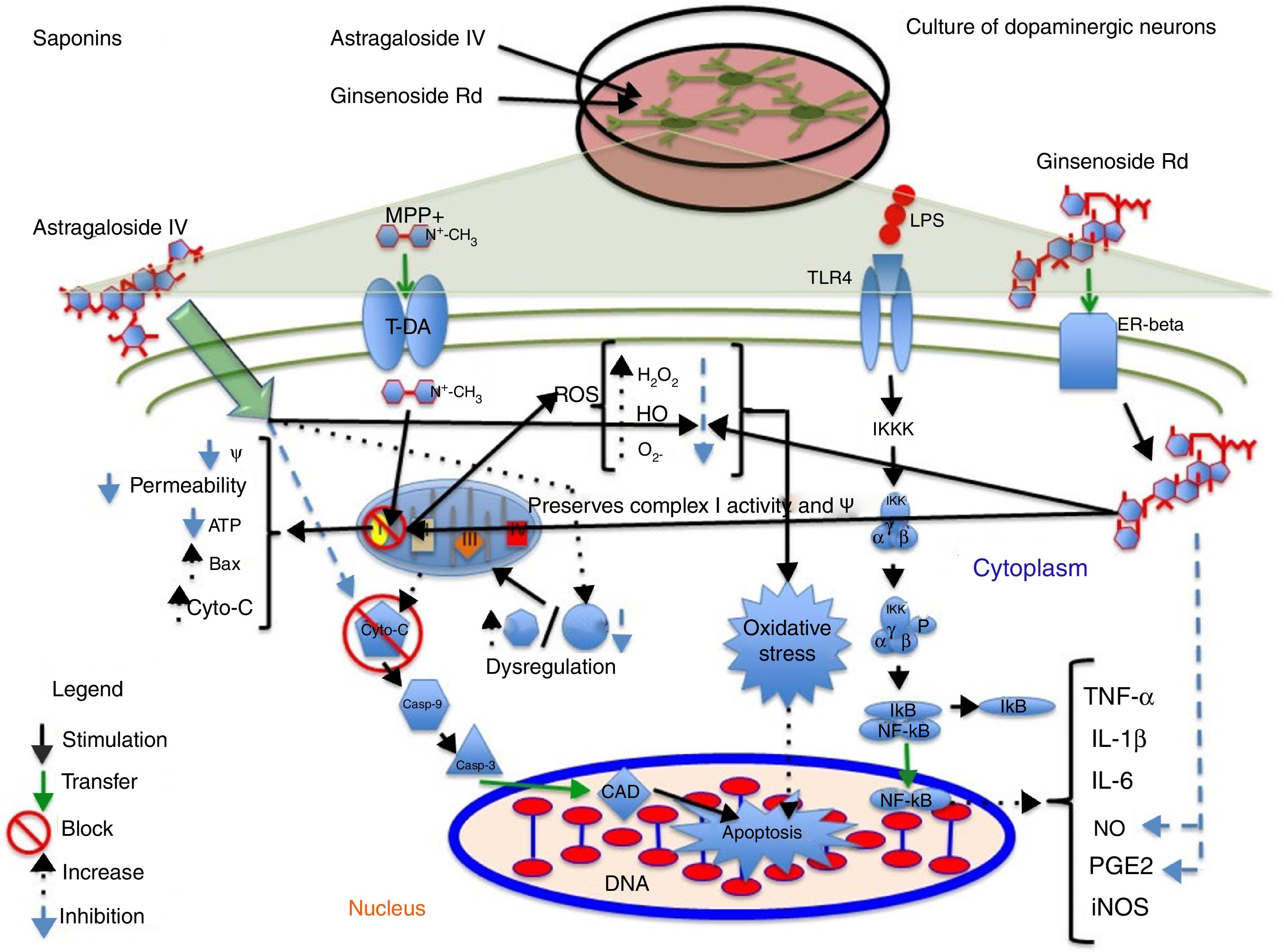
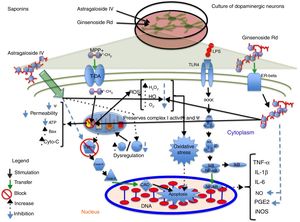
Astragaloside IV is obtained from the dried roots of Astragalus membranaceus, a herb used in traditional Chinese medicine to treat neurodegenerative diseases. Primary cultures of midbrain dopaminergic neurons exposed to 6-OHDA have shown that 100μM astragaloside IV has a neuroprotective effect (probably due to its antioxidant properties), improves cell survival, and prevents neurite loss and shortening.14 Zhang et al.44 applied the same compound to SH-SY5Y dopaminergic neurons exposed to MPP+, finding it to have a neuroprotective effect: it increases cell survival by decreasing nuclear size, chromatin condensation, and nuclear fragmentation, in addition to reducing ROS levels and the Bax/Bcl-2 ratio and inhibiting caspase-3 activity. Fig. 3 shows a possible action mechanism for this compound.
Neuroprotection of saponins in models of MPP+- and lipopolysaccharide-induced neuronal damage. Astragaloside IV reduces ROS levels and prevents oxidative stress, increasing Bcl-2 levels and inhibiting cytochrome C release into the cytosol, thereby preventing cell death. Ginsenoside Rd preserves the mitochondrial membrane potential (Ψ) and complex I activity and reduces ROS levels, preventing oxidative stress and decreasing NO and PGE2 levels, which increases cell viability.
Ginseng, which is obtained from the roots of Panax ginseng, contains over 30 ginsenosides; of these, ginsenosides Rb1, Rg1, and Rd show free radical scavenging activity and improve energy metabolism in neurons. Ginsenosides Rb1 and Rg1 have partial neurotrophic and neuroprotective effects in dopaminergic neuron cultures, and increase cell survival by decreasing lactate dehydrogenase release and preventing mitochondrial membrane potential loss. They have also been found to increase the length and number of neurites in SH-SY5Y cells surviving glutamate toxicity.45 Ginsenoside Rd (50μM) protects dopaminergic neurons against lipopolysaccharide-induced damage (100μg/mL); the compound's anti-inflammatory action increases cell survival and decreases the formation of NO and PGE2.46 Liu et al.47 recently showed that 1 and 10μM GSRd protects SH-SY5Y cells against MPP+-induced cell death by reducing the levels of lactate dehydrogenase, ROS, and Bax, and by increasing the activity of antioxidant enzymes superoxide dismutase and glutathione peroxidase. GSRd was also found to stabilise the mitochondrial membrane potential and increase intracellular ATP levels. The PI3K/Akt survival-signalling pathway was found to be involved in GSRd-mediated neuroprotection.47Fig. 3 shows a possible action mechanism for this phytochemical (Table 3).
Some saponins with neuroprotective effects on dopaminergic neurons.
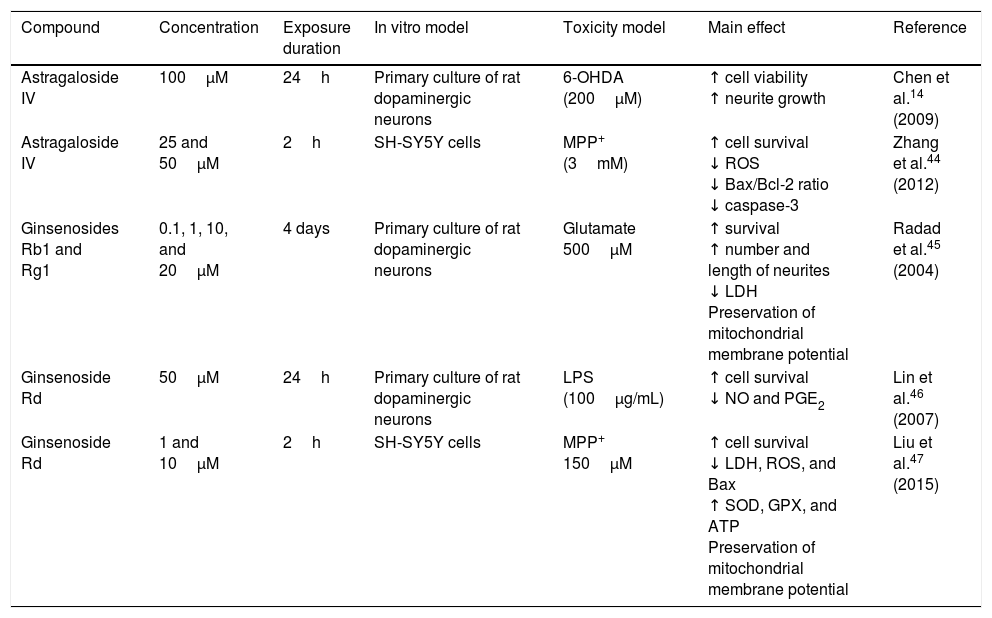
| Compound | Concentration | Exposure duration | In vitro model | Toxicity model | Main effect | Reference |
|---|---|---|---|---|---|---|
| Astragaloside IV | 100μM | 24h | Primary culture of rat dopaminergic neurons | 6-OHDA (200μM) | ↑ cell viability ↑ neurite growth | Chen et al.14 (2009) |
| Astragaloside IV | 25 and 50μM | 2h | SH-SY5Y cells | MPP+ (3mM) | ↑ cell survival ↓ ROS ↓ Bax/Bcl-2 ratio ↓ caspase-3 | Zhang et al.44 (2012) |
| Ginsenosides Rb1 and Rg1 | 0.1, 1, 10, and 20μM | 4 days | Primary culture of rat dopaminergic neurons | Glutamate 500μM | ↑ survival ↑ number and length of neurites ↓ LDH Preservation of mitochondrial membrane potential | Radad et al.45 (2004) |
| Ginsenoside Rd | 50μM | 24h | Primary culture of rat dopaminergic neurons | LPS (100μg/mL) | ↑ cell survival ↓ NO and PGE2 | Lin et al.46 (2007) |
| Ginsenoside Rd | 1 and 10μM | 2h | SH-SY5Y cells | MPP+ 150μM | ↑ cell survival ↓ LDH, ROS, and Bax ↑ SOD, GPX, and ATP Preservation of mitochondrial membrane potential | Liu et al.47 (2015) |
6-OHDA: 6-hydroxydopamine; ATP: adenosine triphosphate; GPX: glutathione peroxidase; LDH: lactate dehydrogenase; LPS: lipopolysaccharide; MPP+: 1-methyl-4-phenyl pyridinium; ROS: reactive oxygen species; SOD: superoxide dismutase.
Chunghyuldan, a herbal complex used in traditional Chinese medicine, has considerable neuroprotective properties. It contains Coptis japonica rhizome, Phellodendron amurense cortex, Scutellaria baicalensis root, Gardenia jasminoides fruit, and Rheum palmatum rhizome. From a chemical viewpoint, this mixture contains various flavonoids and alkaloids, including berberine, baicalein, wogonin, geniposide, and sennoside A. A study of primary cultures of rat dopaminergic neurons showed that 10μg/mL chunghyuldan had a neuroprotective effect against MPP+, increasing cell viability.48 Green tea polyphenols (at a minimum concentration of 10μg/mL) also protect dopaminergic neurons by blocking dopamine transporters, which prevents MPP+ uptake in a dose-dependent manner.49 Vanillyl alcohol protects NM9D dopaminergic neurons against MPP+-induced neurotoxicity. The compound reduced the number of apoptotic cells by lowering ROS and Bax levels, and also increased Bcl-2 levels. The neuroprotective effects of vanillyl alcohol were found to involve inhibition of the mitochondrial apoptotic pathway.50 Guarana (Paullinia cupana) also has antioxidant properties. Guarana dimethylsulfoxide extract has been found to protect SH-SY5Y dopaminergic neurons against rotenone, increasing cell viability (83% and 95%) and significantly reducing chromatin condensation and nuclear fragmentation (30.88% and 36.56%) at concentrations of 0.312mg/mL and 0.625mg/mL.51 Dl-3-n-butylphthalide, a natural antioxidant derived from L-3-n-butylphthalide, contained in the seeds of Apium graveolens, is a potent, novel free radical scavenger. The compound was found to improve survival of SH-SY5Y dopaminergic cells against rotenone-induced toxicity in a dose-dependent manner; it reduced apoptosis and ROS production, and prevented mitochondrial membrane potential loss.52 Mulberry fruit from Morus alba L. (Moraceae) contains large quantities of anthocyanins, which have been found to improve inflammation in rats with arthritis. In 2010, Kim et al.53 showed that mulberry fruit ethanol extract protects against 6-OHDA-induced neurotoxicity in SH-SY5Y dopaminergic neurons: the extract reduces 6-OHDA-induced cell death, inhibits ROS and NO generation in a dose-dependent manner, and inhibits apoptosis by decreasing caspase-3 activity and the Bax/Bcl-2 ratio. Mulberry fruit ethanol extract also prevents mitochondrial membrane depolarisation.53 Eicosanoyl-5-hydroxytryptamide, a compound present in coffee, has direct anti-inflammatory effects by suppressing MPP+-induced NF-κB activation, iNOS induction, and NO production. Treatment with this compound at concentrations 10-25μM showed a neuroprotective effect against MPP+ in an SH-SY5Y cell culture. Neuroprotection depends on both the anti-inflammatory and the antioxidant properties of eicosanoyl-5-hydroxytryptamide, as well as on its ability to modulate phosphoprotein phosphatase 2A methylation (Table 4).54
Neuroprotective effects of phytochemicals on dopaminergic neurons.
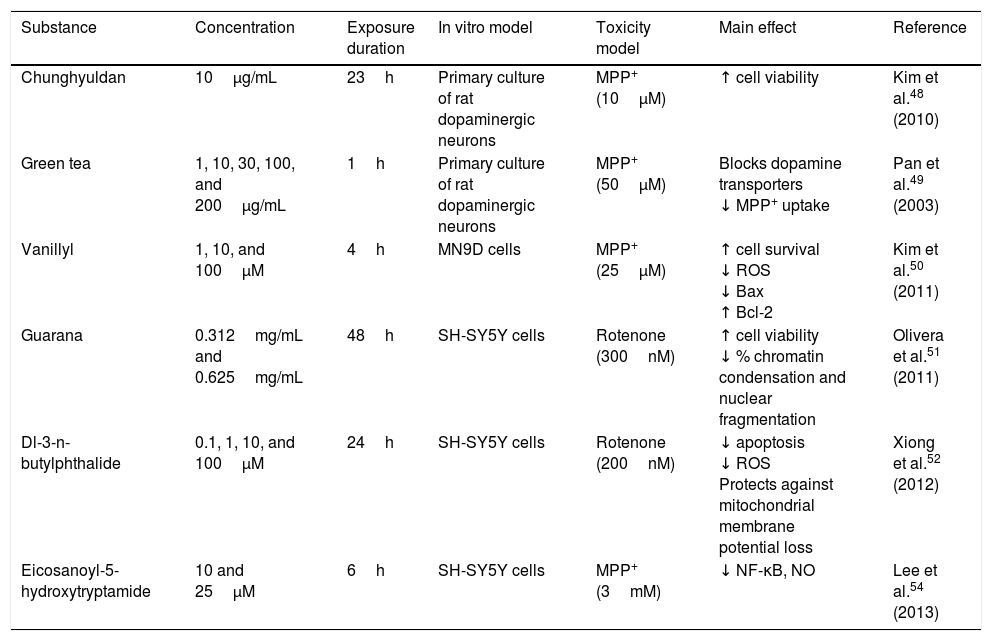
| Substance | Concentration | Exposure duration | In vitro model | Toxicity model | Main effect | Reference |
|---|---|---|---|---|---|---|
| Chunghyuldan | 10μg/mL | 23h | Primary culture of rat dopaminergic neurons | MPP+ (10μM) | ↑ cell viability | Kim et al.48 (2010) |
| Green tea | 1, 10, 30, 100, and 200μg/mL | 1h | Primary culture of rat dopaminergic neurons | MPP+ (50μM) | Blocks dopamine transporters ↓ MPP+ uptake | Pan et al.49 (2003) |
| Vanillyl | 1, 10, and 100μM | 4h | MN9D cells | MPP+ (25μM) | ↑ cell survival ↓ ROS ↓ Bax ↑ Bcl-2 | Kim et al.50 (2011) |
| Guarana | 0.312mg/mL and 0.625mg/mL | 48h | SH-SY5Y cells | Rotenone (300nM) | ↑ cell viability ↓ % chromatin condensation and nuclear fragmentation | Olivera et al.51 (2011) |
| Dl-3-n-butylphthalide | 0.1, 1, 10, and 100μM | 24h | SH-SY5Y cells | Rotenone (200nM) | ↓ apoptosis ↓ ROS Protects against mitochondrial membrane potential loss | Xiong et al.52 (2012) |
| Eicosanoyl-5-hydroxytryptamide | 10 and 25μM | 6h | SH-SY5Y cells | MPP+ (3mM) | ↓ NF-κB, NO | Lee et al.54 (2013) |
NF-κB: nuclear factor-κB; NO: nitric oxide; ROS: reactive oxygen species.
According to numerous in vitro studies, some phytochemicals confer neuroprotection against dopaminergic cell death in models of PD. This involves multiple action mechanisms, mainly the inhibition of NF-κB, caspase-3, and Bax expression, decreased ROS levels, increased expression of endogenous antioxidant enzymes, and maintenance of mitochondrial activity. This review aimed to explore the potential of a wide range of natural compounds, including polyphenols, alkaloids, and saponins, for treating PD. No neuroprotective therapy is currently available for patients with PD. A combination of several phytochemicals may constitute a new therapeutic approach since these compounds act on multiple molecular targets, slowing the degeneration of dopaminergic neurons in the substantia nigra pars compacta.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sandoval-Avila S, Diaz NF, Gómez-Pinedo U, Canales-Aguirre AA, Gutiérrez-Mercado YK, Padilla-Camberos E, et al. Efecto neuroprotector de fitoquímicos en cultivo de neuronas dopaminérgicas. Neurología. 2019;34:114–124.