The need for safe health care, in which the care and treatment of the patient does not cause any injuries in addition to those already arising from their baseline disease, has led to the present study. Our objective has been to determine the frequency and describe the neurological syndromes attributable to drugs, their preventability and the levels of medical care involved.
MethodsObservational study. Cohort of subjects referred from Primary and Specialised Care between December 2008 and January 2010 due to neurological symptoms attributable to drugs, and previously known neurology patients who began to have symptoms other than those of the baseline disease, also caused by drugs. The notifications were recorded in a questionnaire. Frequency distributions, central tendency measurements, χ2 or Fisher tests and non-parametric tests were performed.
ResultsThe prevalence of adverse neurological events was 0.586% of the total sample. Of the 105 patients selected, the most frequent adverse events were: 25.7%, akinetic-rigid syndrome, 18.1%, dyskinetic syndrome, 11.4% neuro-psychiatric symptoms, and 10.5% confusional syndrome. The most commonly recorded pharmacological groups were, in decreasing order: anti-epileptic, dopaminergic, antidepressant, neuroleptic, antivertiginous and prokinetic drugs. We describe the most susceptible population and the statistically significant relationships between the presence of certain pharmacological groups and neurological syndromes.
ConclusionsThe low prevalence detected may be due to the study design, although adverse neurological events accounted for 2.84% of the admissions to a Neurology Unit. Understanding the epidemiology should help us to identify the safest approaches, apply them correctly to the population at a higher risk, and reduce healthcare needs and consumption of medical resources.
La necesidad de una asistencia sanitaria segura en la que los cuidados y tratamientos no supongan daños diferentes a los derivados de la enfermedad de base, ha motivado este estudio. Nuestro objetivo ha sido determinar la frecuencia y describir los síndromes neurológicos atribuibles a fármacos, su evitabilidad y los niveles asistenciales implicados.
MétodosEstudio observacional. Cohorte prospectiva de todos los sujetos derivados desde atención primaria y especializada, en el período de diciembre de 2008 a enero de 2010, por síntomas neurológicos atribuibles a fármacos y enfermos neurológicos conocidos con clínica distinta o agravada de la enfermedad de base causada por fármacos. Las notificaciones quedaron reflejadas en un cuestionario. Se realizaron distribuciones de frecuencias, medidas de tendencia central, pruebas de la χ2 o Fisher y pruebas no paramétricas correspondientes.
ResultadosLa prevalencia de efectos adversos neurológicos respecto a la muestra total fue 0,586%. De los 105 pacientes seleccionados, los principales efectos adversos fueron: 25,7% síndrome rígido-acinético; 18,1% discinético; 11,4% síntomas neuropsiquiátricos, y 10,5% síndrome confusional. Los grupos farmacológicos más registrados fueron, en orden decreciente: antiepilépticos, dopaminérgicos, antidepresivos, neurolépticos, antivertiginosos y procinéticos. Describimos la población más susceptible y las asociaciones estadísticamente significativas entre la presencia de determinados grupos farmacológicos y síndromes neurológicos concretos.
ConclusionesLa baja prevalencia detectada puede deberse al diseño del estudio, aunque los efectos adversos neurológicos suponen el 2,84% de los ingresos en una unidad de neurología. Conocer la epidemiología permitirá identificar los abordajes más seguros, aplicarlos correctamente a la población de mayor riesgo y reducir necesidades asistenciales y recursos médicos.
There is an increasing interest in the risks and adverse events within the health field, as patient safety has emerged as a fundamental goal in healthcare. With this in mind, the World Health Organization launched the “World Alliance for Patient Safety” and defined the quality of care as follows: proper diagnosis and treatment for the patient (scientific and technical quality), according to current medical knowledge and biological factors (achievable optimal state of health), with a minimum cost in resources (efficiency), minimum exposure to the risk of additional harm (risk management), and maximum patient satisfaction.1,2
Higher life expectancies produce additional healthcare needs; a higher degree of socioeconomic development requires better quality of care and more information; and the increasing complexity of the diagnostic technologies and treatment resources causes sustainability problems within healthcare systems. Therefore, one of the priorities in healthcare is to identify the safest and most effective diagnostic procedures and treatments, and to ensure that such procedures and treatments do not cause harm or complications other than those from the underlying disease. At the very least, doctors must assess the risks and benefits of medical interventions in cases in which additional harm is expected and cannot be prevented.3–5 Although measuring the level of risk for a patient in contact with healthcare services is a difficult task, we must be well aware of the frequency and severity of medical incidents, accidents and errors, as has been recommended in recent years.6–10
Studies have been carried out at the international level to address patient safety within the healthcare environment. The study of reference was performed in 1984 in New York (Harvard Medical Practice Study, HMPS).11 This study estimated an ADE (adverse drug event) incidence rate of 3.7% in the 30121 medical records that were revised. Subsequent studies have shown AE (adverse event) rates ranging between 2.9%12 and 7% to 16%.13–16 The study by Healey et al.17 revealed the highest AE rates: in a sample of 4743 patients monitored prospectively, 31.5% of the patients experienced AEs. In 2002, a French pilot study coordinated by the Comité de Coordination de l’Évaluation Clinique et de la Qualité en Aquitaine was completed in order to establish the basis for France's national study (ENEIS).18 In 2004, Proyecto IDEA was published in the Region of Valencia (Spain) and subsequently used as a pilot project for ENEAS, Spain's national study, in 2005.19 In this study, researchers found that the incidence of patients with AEs directly related to hospital care was 8.4%.20 Completed in 2008, the APEAS study is the most recent national-level study carried out in Spain, and it specifically addresses patient safety at the primary care level. Of the total recorded AEs, 47.8% of the cases were drug-related, and 5.1% were drug-induced neurological alterations.21
We did not find any Spanish studies that addressed the specific problem of AEs presenting with neurological symptoms. The main purpose of this study is to determine the frequency and types of drug-induced neurological syndromes. Its secondary objectives are to determine the association between AE and comorbidities, polytherapy, sex, age, and pharmacological group; and to assess preventable AEs, the time elapsed before the syndrome resolves, and the levels of care from which the drugs were prescribed.
Subjects and methodsWe designed an observational study of a prospective cohort of patients included between December 2008 and January 2010. Patients were monitored until June 2010.
We analysed all subjects older than 14 years referred to the neurology department by primary care doctors or specialists by way of the emergency department, other hospital departments, or outpatient clinics. During the inclusion period, a total of 17896 patients were assessed. There were 3936 initial consultations, 13167 follow-up visits, and 793 hospital admissions. From the total, we selected those patients seeking a neurological consult for a reason shown by the initial assessment to be potentially drug-related. We also selected patients with known neurological conditions who made appointments due to unrelated symptoms or exacerbation of the underlying disease that could be drug-related (n=133). All patients in the initial selection were monitored for 6 months. Inclusion was definitive for patients whose clinical check-ups and subsequent complementary tests ruled out other aetiologies to confirm a pharmacological cause. This aetiology was supported by the complete resolution of all neurological symptoms the patients suffered; statistical studies were carried out for those symptoms (n=105). We excluded those patients whose neurological symptoms were due to neurological disease and not caused by medication (as determined at the time of the initial evaluation or after receiving the complementary test results). We also excluded patients who left the study and those whose results were not available at the end of the study period because definitive complementary tests were pending. We excluded patients whose medications suspected of provoking ADEs could not be suspended due to ethical reasons (e.g. patients with heart disease requiring antiarrhythmic agents) or treatment dependence (e.g. psychiatric patients requiring additional time to confirm pharmacological origin of symptoms following a change in neuroleptics or anxiolytic drugs), and any patients still presenting neurological symptoms at the end of the follow-up period. This may have resulted in the exclusion of cases in which syndromes required a longer washout period or were not reversible, such as tardive dyskinesias.
We did not study the adverse effects of procedures such as lumbar puncture.
We obtained the approval of the ethics committee and the research unit pertaining to the centre where the study was conducted.
The study was performed in a hospital whose neurology department included 16 beds, 4 hospital outpatient clinics and on-call neurologists. This centre, a secondary hospital in Castile-La Mancha (Ciudad Real, Spain), provides care to an area with 223669 inhabitants. In order to calculate the sample size, we used the information collected by the APEAS study21 regarding patient safety in primary care.
Three professional categories participated in the initial selection of subjects: neurology specialists, neurologists, and a neurology resident who had received specific training in clinical pharmacology for neurologists. Participating professionals received the appropriate instructions so as to prevent biases.
During the initial evaluation, the participants initially selected a total of 133 candidates for the study. They recorded data identifying the patients, the suspicious drug, and the neurological syndrome by filling in a specific form that was modified from the APEAS study questionnaire (Fig. 1).21 This form was drawn up according to consensus and based on a preliminary study with a list of conditions similar to that used in the New York,13 Utah, and Colorado studies.14 The drug was discontinued and patients were given appointments for check-ups at a later date. They were reassessed at that time and complementary laboratory and imaging tests were ordered where necessary. Changes during the follow-up period are shown in the patients’ medical histories. Data were delivered to two reviewers (a specialist and a supervised resident) who analysed all the medical histories before definitively selecting the 105 patients. Using this questionnaire, we created the database for statistical analysis.
We employed the universal taxonomy developed by organisations such as the World Health Organization or the Joint Commission on Accreditation of Health Care Organizations (JCAHO).22
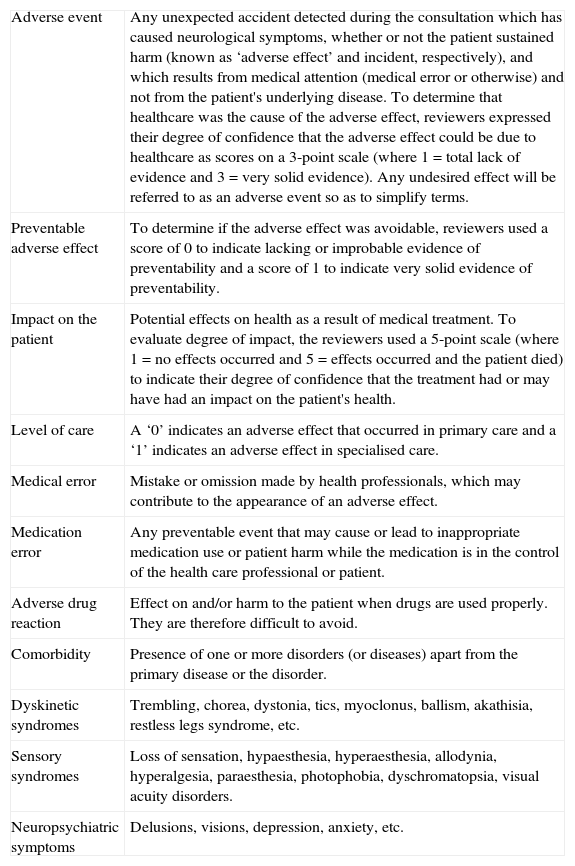
We studied the following variables: neurological syndrome (as an adverse effect), preventability, and time for the symptoms to resolve. Independent variables in the analysis were as follows: pharmacological group, level of care, comorbidities, age, sex, and polytherapy. Table 1 shows the operational definitions for the study.
Operational definitions.
| Adverse event | Any unexpected accident detected during the consultation which has caused neurological symptoms, whether or not the patient sustained harm (known as ‘adverse effect’ and incident, respectively), and which results from medical attention (medical error or otherwise) and not from the patient's underlying disease. To determine that healthcare was the cause of the adverse effect, reviewers expressed their degree of confidence that the adverse effect could be due to healthcare as scores on a 3-point scale (where 1=total lack of evidence and 3=very solid evidence). Any undesired effect will be referred to as an adverse event so as to simplify terms. |
| Preventable adverse effect | To determine if the adverse effect was avoidable, reviewers used a score of 0 to indicate lacking or improbable evidence of preventability and a score of 1 to indicate very solid evidence of preventability. |
| Impact on the patient | Potential effects on health as a result of medical treatment. To evaluate degree of impact, the reviewers used a 5-point scale (where 1=no effects occurred and 5=effects occurred and the patient died) to indicate their degree of confidence that the treatment had or may have had an impact on the patient's health. |
| Level of care | A ‘0’ indicates an adverse effect that occurred in primary care and a ‘1’ indicates an adverse effect in specialised care. |
| Medical error | Mistake or omission made by health professionals, which may contribute to the appearance of an adverse effect. |
| Medication error | Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional or patient. |
| Adverse drug reaction | Effect on and/or harm to the patient when drugs are used properly. They are therefore difficult to avoid. |
| Comorbidity | Presence of one or more disorders (or diseases) apart from the primary disease or the disorder. |
| Dyskinetic syndromes | Trembling, chorea, dystonia, tics, myoclonus, ballism, akathisia, restless legs syndrome, etc. |
| Sensory syndromes | Loss of sensation, hypaesthesia, hyperaesthesia, allodynia, hyperalgesia, paraesthesia, photophobia, dyschromatopsia, visual acuity disorders. |
| Neuropsychiatric symptoms | Delusions, visions, depression, anxiety, etc. |
Data were processed using PASW Statistics 18. In the data analysis, we used descriptive statistics for qualitative and quantitative variables. We calculated statistical associations among the qualitative variables by using the chi-square test or the Fisher exact test. Differences among groups for the quantitative and continuous variable ‘age’ were assessed using the Mann–Whitney U test; rank differences for ordinal variables were measured with the Kruskal–Wallis test.
Patient confidentiality was maintained.
ResultsThirteen neurology professionals contributed to the study and 17896 patients were treated during the study period. Based on the initial evaluation, we identified 133 cases of possible neurological syndromes caused by ADE; 105 patients were included in the definitive study. Of the 28 patients who were excluded, 4 had a very high probability of ADE with effects persisting at the end of the study (tardive dyskinesias) and 6 were excluded due to the difficulty of exchanging the harmful drug for another in view of comorbidities and the difficulties of interdisciplinary management (e.g. amiodarone in patients with cardiac arrhythmia). Another 11 had an underlying disease, which was discovered in some cases because of medication (Parkinson's disease and dopamine depletion secondary to neuroleptics). We were unable to confirm diagnosis in 7 patients since they were lost to follow-up or because the study period ended. Of the total of included patients, 5 experienced 2 or more AEs. One patient with myasthenia gravis became critical as a result of 3 myasthenic crises; another patient presented 2 demyelinating episodes with no known neurological history; the remaining 3 patients, all diagnosed with Parkinson's disease, each experienced exacerbations on 2 different occasions.
Of the patient total, 21% of the cases were admitted to hospital (consults requested by other departments are included in this percentage), 19% were referred by the emergency department, and 59% were referred by neurology outpatient clinics.
Males included in the study accounted for 29.5% of the total number of patients (95% CI, 20.7%–38.2%), with women comprising the remaining 70.5% (95% CI, 61.7%–79.2%). The mean age was 62.12 years. Men had a mean age of 57.32 years (95% CI, 47.89–66.75; SD=25.7) and women had a mean age of 64.14 years (95% CI, 59.42–68.85; SD=20.34).
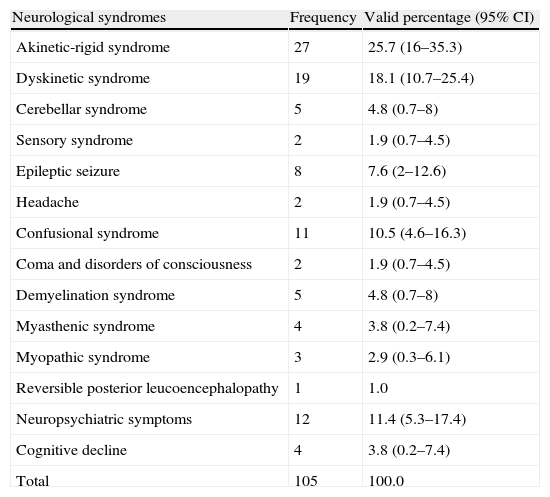
Tables 2 and 3 show the observed frequencies for neurological syndromes and the causative agents, respectively. Table 4 shows all the neurological syndromes observed in association with different pharmacological groups.
Percentages of observed neurological syndromes.
| Neurological syndromes | Frequency | Valid percentage (95% CI) |
| Akinetic-rigid syndrome | 27 | 25.7 (16–35.3) |
| Dyskinetic syndrome | 19 | 18.1 (10.7–25.4) |
| Cerebellar syndrome | 5 | 4.8 (0.7–8) |
| Sensory syndrome | 2 | 1.9 (0.7–4.5) |
| Epileptic seizure | 8 | 7.6 (2–12.6) |
| Headache | 2 | 1.9 (0.7–4.5) |
| Confusional syndrome | 11 | 10.5 (4.6–16.3) |
| Coma and disorders of consciousness | 2 | 1.9 (0.7–4.5) |
| Demyelination syndrome | 5 | 4.8 (0.7–8) |
| Myasthenic syndrome | 4 | 3.8 (0.2–7.4) |
| Myopathic syndrome | 3 | 2.9 (0.3–6.1) |
| Reversible posterior leucoencephalopathy | 1 | 1.0 |
| Neuropsychiatric symptoms | 12 | 11.4 (5.3–17.4) |
| Cognitive decline | 4 | 3.8 (0.2–7.4) |
| Total | 105 | 100.0 |
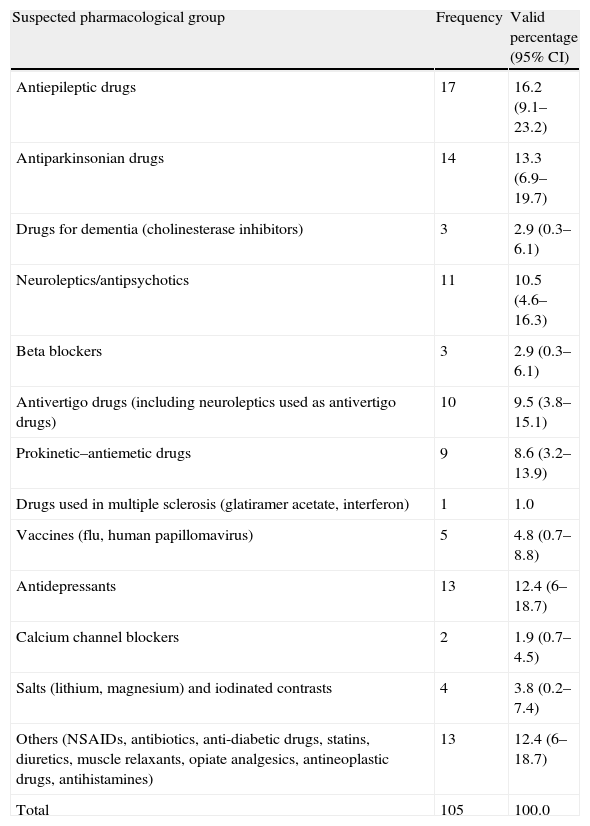
Observed frequencies of suspected causative agents.
| Suspected pharmacological group | Frequency | Valid percentage (95% CI) |
| Antiepileptic drugs | 17 | 16.2 (9.1–23.2) |
| Antiparkinsonian drugs | 14 | 13.3 (6.9–19.7) |
| Drugs for dementia (cholinesterase inhibitors) | 3 | 2.9 (0.3–6.1) |
| Neuroleptics/antipsychotics | 11 | 10.5 (4.6–16.3) |
| Beta blockers | 3 | 2.9 (0.3–6.1) |
| Antivertigo drugs (including neuroleptics used as antivertigo drugs) | 10 | 9.5 (3.8–15.1) |
| Prokinetic–antiemetic drugs | 9 | 8.6 (3.2–13.9) |
| Drugs used in multiple sclerosis (glatiramer acetate, interferon) | 1 | 1.0 |
| Vaccines (flu, human papillomavirus) | 5 | 4.8 (0.7–8.8) |
| Antidepressants | 13 | 12.4 (6–18.7) |
| Calcium channel blockers | 2 | 1.9 (0.7–4.5) |
| Salts (lithium, magnesium) and iodinated contrasts | 4 | 3.8 (0.2–7.4) |
| Others (NSAIDs, antibiotics, anti-diabetic drugs, statins, diuretics, muscle relaxants, opiate analgesics, antineoplastic drugs, antihistamines) | 13 | 12.4 (6–18.7) |
| Total | 105 | 100.0 |
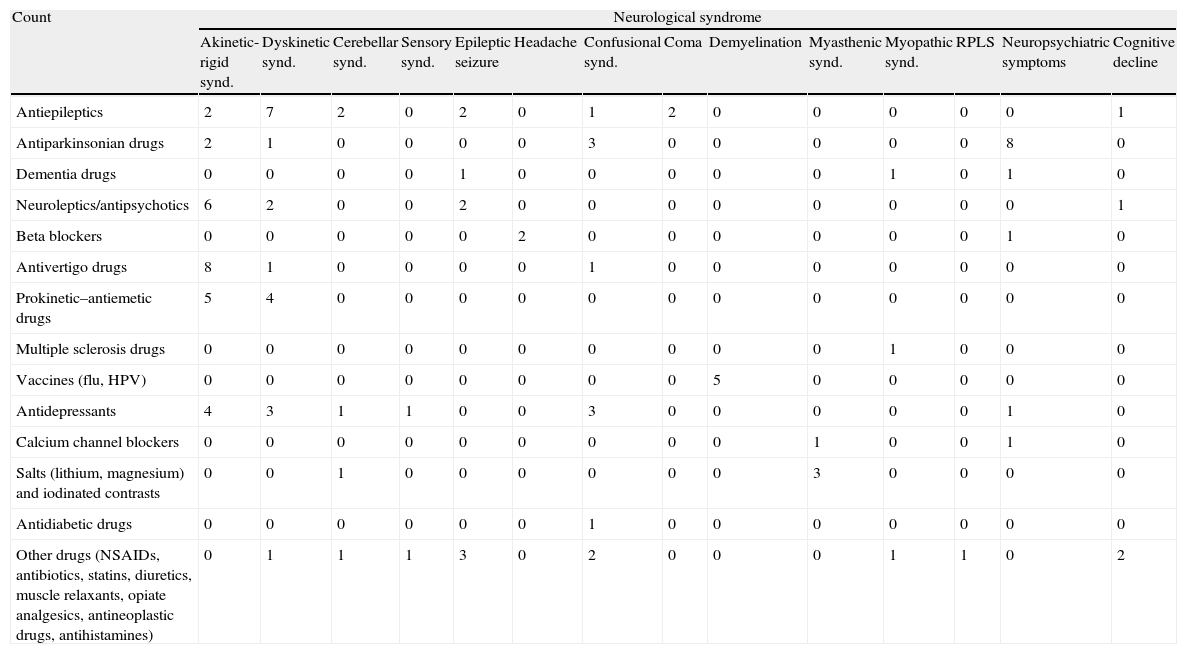
Counts for different neurological syndromes and pharmacological groups in sample.
| Count | Neurological syndrome | |||||||||||||
| Akinetic-rigid synd. | Dyskinetic synd. | Cerebellar synd. | Sensory synd. | Epileptic seizure | Headache | Confusional synd. | Coma | Demyelination | Myasthenic synd. | Myopathic synd. | RPLS | Neuropsychiatric symptoms | Cognitive decline | |
| Antiepileptics | 2 | 7 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| Antiparkinsonian drugs | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 8 | 0 |
| Dementia drugs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Neuroleptics/antipsychotics | 6 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Beta blockers | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Antivertigo drugs | 8 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prokinetic–antiemetic drugs | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Multiple sclerosis drugs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Vaccines (flu, HPV) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| Antidepressants | 4 | 3 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Calcium channel blockers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Salts (lithium, magnesium) and iodinated contrasts | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Antidiabetic drugs | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other drugs (NSAIDs, antibiotics, statins, diuretics, muscle relaxants, opiate analgesics, antineoplastic drugs, antihistamines) | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
RPLS: reversible posterior leucoencephalopathy; HPV: human papillomavirus vaccine.
We found most of the akinetic-rigid syndromes in patients undergoing prolonged treatment (some of which lasted years) with antivertigo, prokinetic and antiemetic drugs. Other patients suffered these syndromes due to an idiosyncratic reaction. Most of the neuropsychiatric symptoms occurred in subjects with long-term neurodegenerative disease receiving treatment with dopaminergic drugs. Among patients showing dyskinetic syndrome, trembling was more common in young subjects receiving antiepileptic treatment (valproic acid).
An interesting result is that there were 4 cases with demyelinating symptoms (5 demyelinating episodes) and a recent history of vaccination (human papillomavirus vaccine in 4 cases and 1 case of influenza vaccine). Only the influenza vaccine case had a history of multiple sclerosis. Concerning the others, 2 met the criteria for multiple sclerosis at a later date and the last subject is currently disease-free.23 These findings were deduced from our observations, since the statistical analysis did not show this association.
We found significant associations between antiepileptic drugs and dyskinetic syndrome (P=.013); dopaminergic drugs and neuropsychiatric symptoms (P=.000); antipsychotics/neuroleptics and akinetic-rigid syndrome (P=.031); and antivertigo–prokinetic–antiemetic drugs and akinetic-rigid syndrome (P=.000).
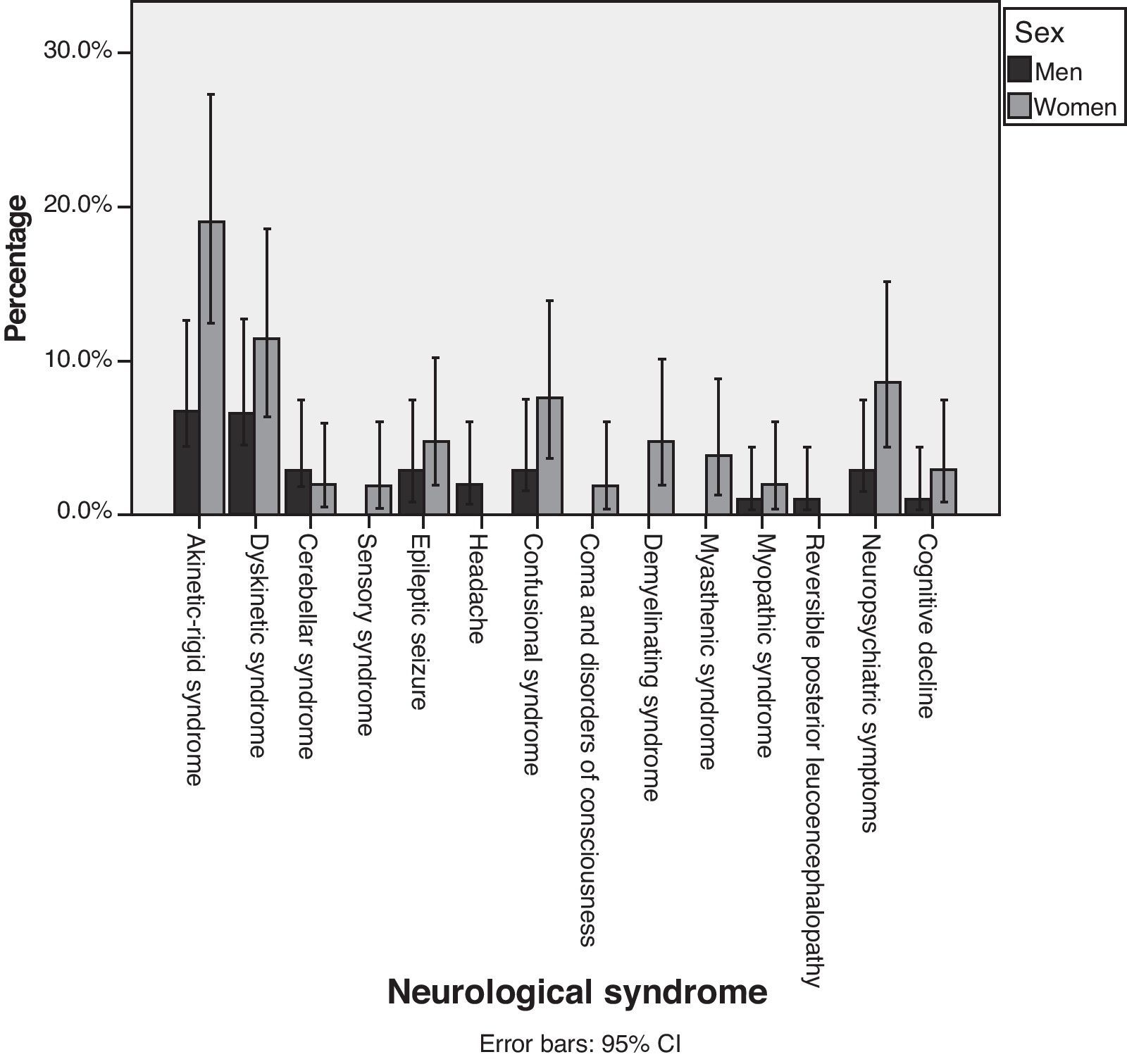
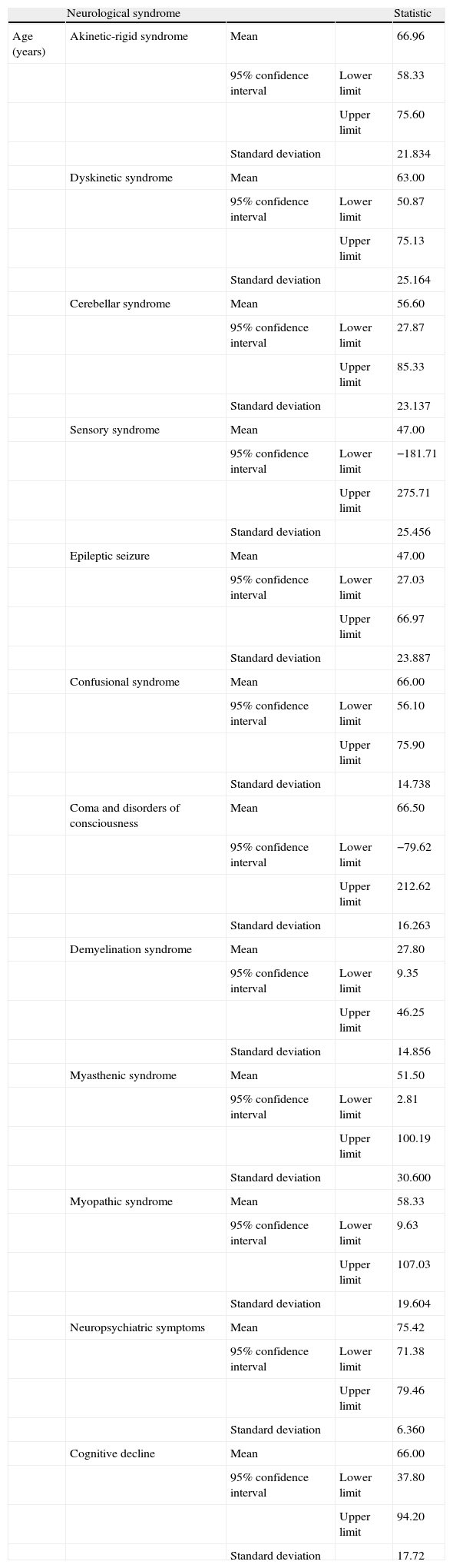
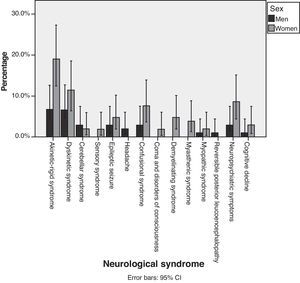
Fig. 2 shows percentages, broken down by sex, of each of the different syndromes observed. Although we observed that more women than men suffered from each of the different neurological syndromes, statistical analysis did not show that AEs were more common in women than in men (P=.848). Table 5 shows the mean ages for each neurological syndrome. We did not find any statistical differences among the main neurological symptoms.
Neurological syndromes and ages in sample.
| Neurological syndrome | Statistic | |||
| Age (years) | Akinetic-rigid syndrome | Mean | 66.96 | |
| 95% confidence interval | Lower limit | 58.33 | ||
| Upper limit | 75.60 | |||
| Standard deviation | 21.834 | |||
| Dyskinetic syndrome | Mean | 63.00 | ||
| 95% confidence interval | Lower limit | 50.87 | ||
| Upper limit | 75.13 | |||
| Standard deviation | 25.164 | |||
| Cerebellar syndrome | Mean | 56.60 | ||
| 95% confidence interval | Lower limit | 27.87 | ||
| Upper limit | 85.33 | |||
| Standard deviation | 23.137 | |||
| Sensory syndrome | Mean | 47.00 | ||
| 95% confidence interval | Lower limit | −181.71 | ||
| Upper limit | 275.71 | |||
| Standard deviation | 25.456 | |||
| Epileptic seizure | Mean | 47.00 | ||
| 95% confidence interval | Lower limit | 27.03 | ||
| Upper limit | 66.97 | |||
| Standard deviation | 23.887 | |||
| Confusional syndrome | Mean | 66.00 | ||
| 95% confidence interval | Lower limit | 56.10 | ||
| Upper limit | 75.90 | |||
| Standard deviation | 14.738 | |||
| Coma and disorders of consciousness | Mean | 66.50 | ||
| 95% confidence interval | Lower limit | −79.62 | ||
| Upper limit | 212.62 | |||
| Standard deviation | 16.263 | |||
| Demyelination syndrome | Mean | 27.80 | ||
| 95% confidence interval | Lower limit | 9.35 | ||
| Upper limit | 46.25 | |||
| Standard deviation | 14.856 | |||
| Myasthenic syndrome | Mean | 51.50 | ||
| 95% confidence interval | Lower limit | 2.81 | ||
| Upper limit | 100.19 | |||
| Standard deviation | 30.600 | |||
| Myopathic syndrome | Mean | 58.33 | ||
| 95% confidence interval | Lower limit | 9.63 | ||
| Upper limit | 107.03 | |||
| Standard deviation | 19.604 | |||
| Neuropsychiatric symptoms | Mean | 75.42 | ||
| 95% confidence interval | Lower limit | 71.38 | ||
| Upper limit | 79.46 | |||
| Standard deviation | 6.360 | |||
| Cognitive decline | Mean | 66.00 | ||
| 95% confidence interval | Lower limit | 37.80 | ||
| Upper limit | 94.20 | |||
| Standard deviation | 17.72 |
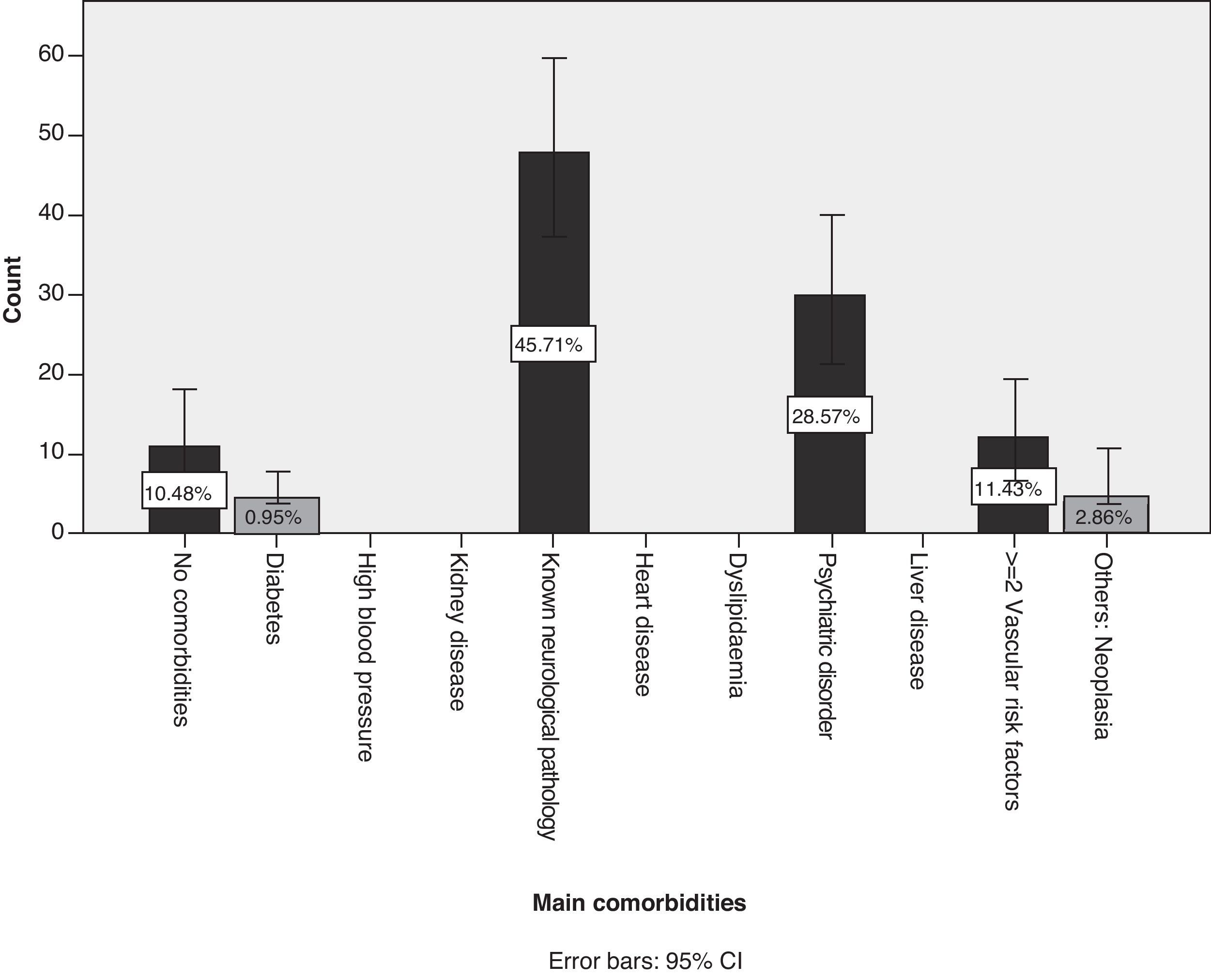
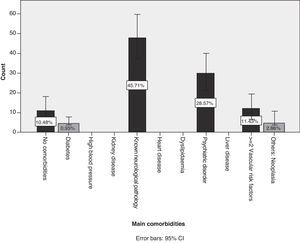
Of the patient total, 75.2% of the cases (95% CI, 66.9%–83.4%) were treated in polytherapy vs 24.8% (95% CI, 16.5%–33%) treated in monotherapy. We did not find any statistical associations between polytherapy and the presence of certain neurological syndromes. Of the patients with identified AEs, 89.5% presented comorbidities while 10.5% did not (Fig. 3). The most frequent comorbidity, accounting for 45.7% of the cases, was prior neurological disease. Comorbidities were as follows, in descending order of frequency: Parkinson's disease, epilepsy, headache, essential tremor, cognitive decline, cerebrovascular disease, demyelinating disease, myasthenia gravis, and brain tumour. Non-neurological comorbidities were as follows: history of psychiatric disorder in 28.6% and 2 or more cardiovascular risk factors in 11.4%.
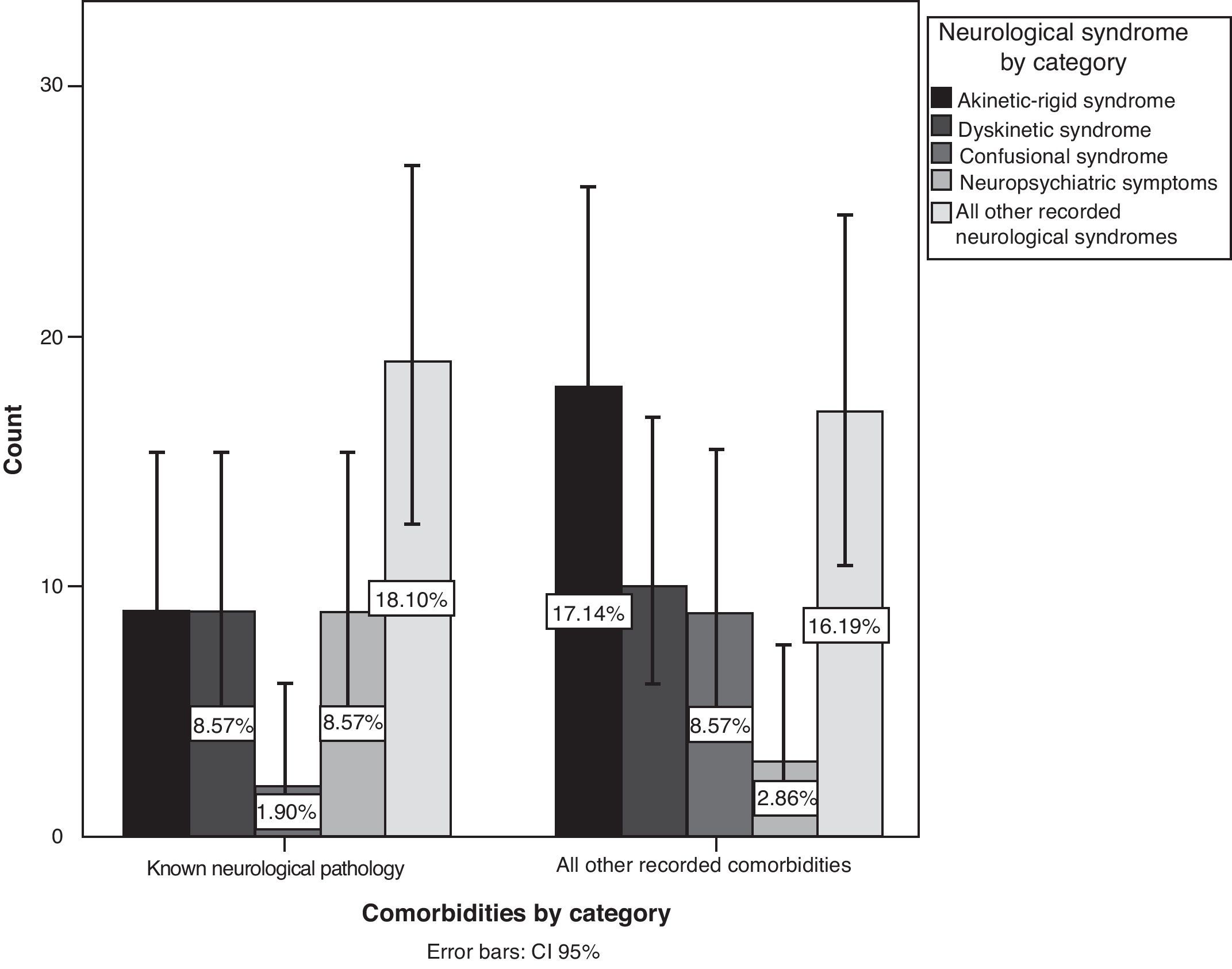
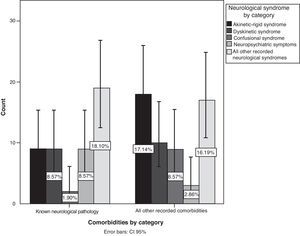
After recategorising symptoms into specific neurological syndromes, we found more AEs in patients with prior neurological disease (P=.042; 95% CI, 36.1%–55.2%), mainly neuropsychiatric symptoms, followed by akinetic-rigid syndrome, dyskinetic syndrome, and confusional syndrome (Fig. 4). On the other hand, we did not find any association with a history of psychiatric disorder (P=.742).
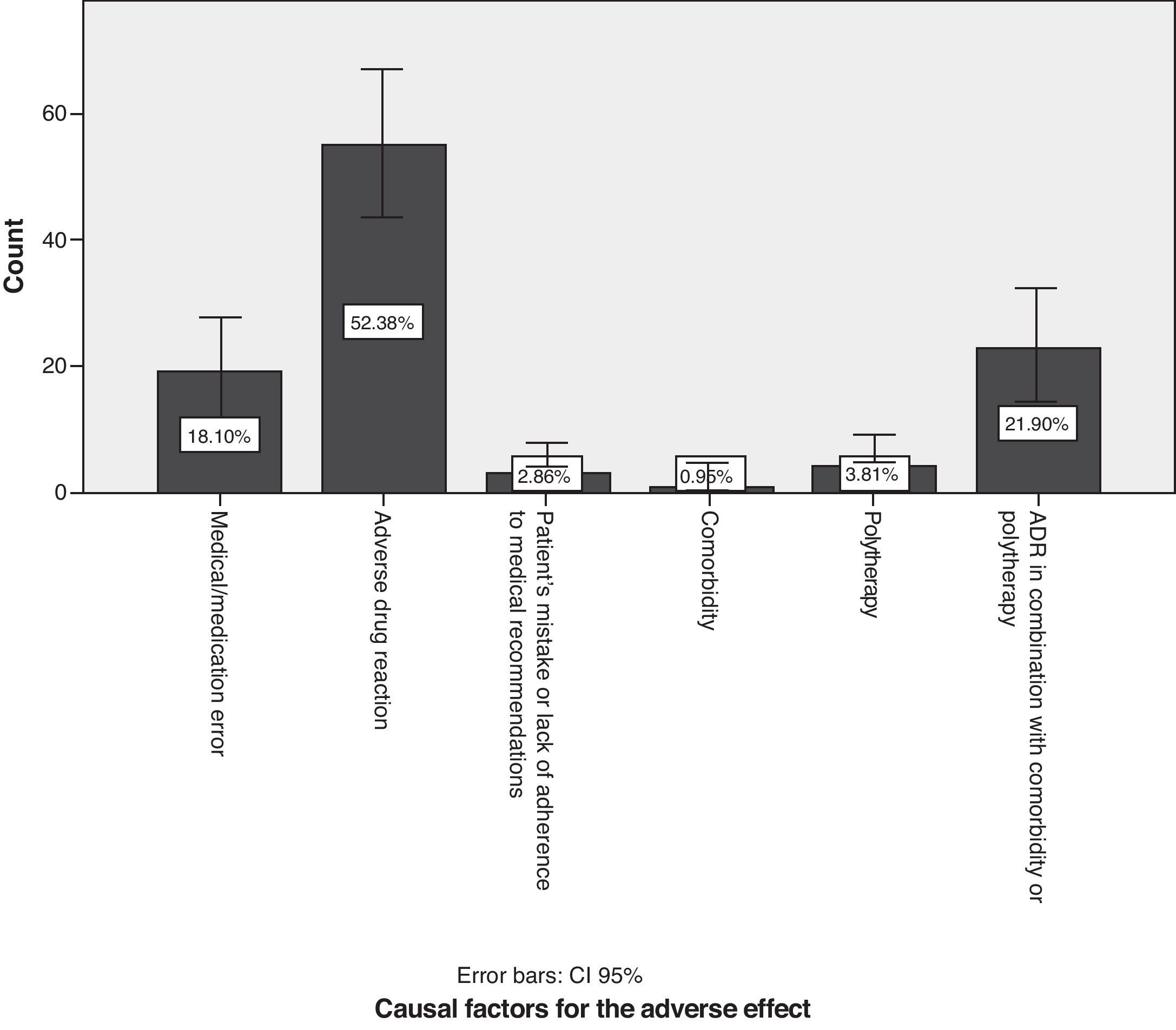
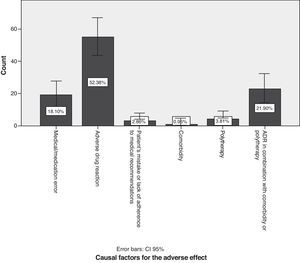
With regard to causal factors (Fig. 5), 18.1% of the cases with neurological symptoms arose due to medical or pharmacological error, e.g. prescription of sulpiride or its derivatives to patients with known Parkinson's disease; prolonged use (for years) of antivertigo, antiemetic and prokinetic drugs; prescribing calcium channel blockers and drugs containing magnesium to patients with myasthenia gravis; prescribing drugs without considering comorbidities such as kidney and liver failure; overdoses due to failure to consider interactions with antiepileptic drugs listed on the information leaflet (valproic acid and lamotrigine); initial antidepressants, muscle relaxants, and opioid analgesics, and any of their combinations given in quantities other than the recommended starting doses. A statistically significant association was found between medical error as a causal factor and antivertigo/prokinetic drugs (P=.042), 95% confidence). Of the total medical errors, 6.67% of the cases had to do with prescribing antivertigo or prokinetic drugs.
Adverse drug reactions (ADRs) accounted for 52.4% of the total (95% CI, 42.8%–61.9%); preventing such reactions is difficult. The highest percentage of patients with ADR was found in the group with a history of psychiatric disorders, followed by patients with a history of neurological disease and patients with cardiovascular risk factors (P=.025). In 21.9% of the cases (95% CI, 13.9%–29.8%), effects were caused by a combination of ADR, comorbidities, and possibly also polytherapy. Many subjects in the polytherapy group were known long-term sufferers of a neurological disease. As a result, there were more patients treated with antiparkinsonian drugs than with any other drug type (P=.000).
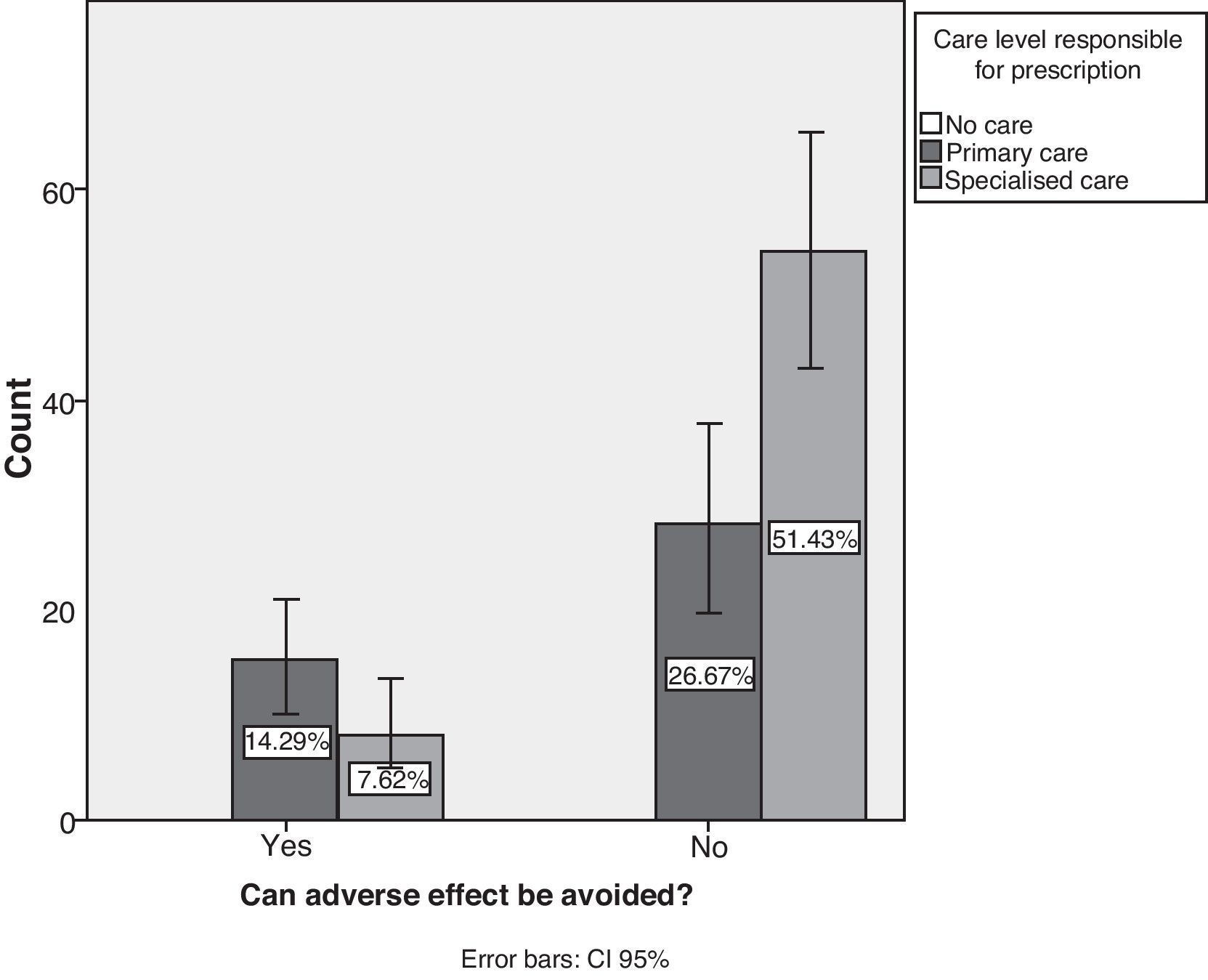
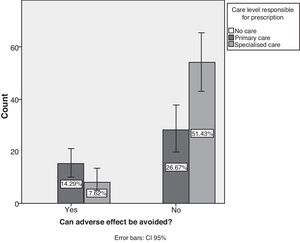
Studying the source of AEs reveals that treatment was prescribed in primary attention centres in 41% of the cases (95% CI, 31.5%–50.4%) and in specialist clinics in 59% of the cases. Only 21.9% of the cases of AE were considered preventable (95% CI, 14%–29.8%), with the remaining 78.1% being unpreventable (95% CI, 70–86%). The contingency analysis between preventability and the prescribing care level shows that primary care had the highest percentage of preventable AEs (P=.007) (Fig. 6).
Regarding the impact of AE, 91.4% of the patients (95% CI, 86%–96.7%) experienced a mild transient effect vs 8.6% of patients (95% CI, 3.2%–13.9%) who suffered a critical effect. These latter were patients with myasthenia gravis and drug-induced seizures due to drugs containing calcium channel blockers and magnesium; patients in status epilepticus treated with neuroleptic drugs; and semi-comatose patients treated with sedatives, analgesics, and antiepileptic drugs.
Of the patient total, 93.3% of the cases (95% CI, 88.6%–98%) required some type of care, ranging from suspension of the drug and monitoring to complementary tests, starting another treatment with a series of check-ups, or intensive care. Only 6.7% of the cases (95% CI, 1.9%–11.5%) did not require additional care. We found that the most serious adverse events occurred at the primary care level. However, the statistics did not show a link between the impact of AEs on patients and the care level (P=.058).
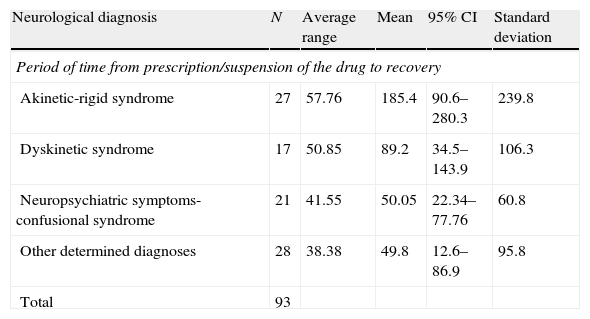
In order to evaluate the differences in the mean time to recovery from the most common entities, we performed the Kruskal–Wallis test. We found statistically significant differences among the groups (P=.038) (Table 6).
Kruskal–Wallis table. Mean resolution time for each neurological diagnosis.
| Neurological diagnosis | N | Average range | Mean | 95% CI | Standard deviation |
| Period of time from prescription/suspension of the drug to recovery | |||||
| Akinetic-rigid syndrome | 27 | 57.76 | 185.4 | 90.6–280.3 | 239.8 |
| Dyskinetic syndrome | 17 | 50.85 | 89.2 | 34.5–143.9 | 106.3 |
| Neuropsychiatric symptoms-confusional syndrome | 21 | 41.55 | 50.05 | 22.34–77.76 | 60.8 |
| Other determined diagnoses | 28 | 38.38 | 49.8 | 12.6–86.9 | 95.8 |
| Total | 93 | ||||
This is the first study to measure the frequency of exclusively neurological AEs and link them to certain drugs, comorbidities, and care levels. The overall objective is to show drains on healthcare resources arising from medical decisions and to what extent these drains can be prevented. This subject has recently been reviewed in our area.6,10
According to the results of the study, the most typical characteristics in our sample were as follows: female sex, approximate age of 62 years, presence of long-term and mainly neurological comorbidities, and polypharmacy. Although the design of our study does not allow us to identify the above characteristics as risk factors, it does suggest that these subjects were more susceptible to AEs. It may therefore be advisable to use extreme precaution in such cases. Polytherapy and old age have already been described as known risk factors for ADR in general.10 The presence of akinetic-rigid, dyskinetic, neuropsychiatric, or confusional syndrome should alert doctors to the possibility of a pharmacological aetiology. This is more crucial in elderly subjects, whose mean symptom resolution time is longer and who therefore have a higher risk of chronicity. On the other hand, younger subjects experienced more idiosyncratic reactions, such as dyskinesia (mainly trembling), in conjunction with antiepileptic drugs. As the literature already indicates, doctors should take down complete and exhaustive medical histories to determine all prescriptions and their administration times.24 Certain pharmacological groups should be under special supervision, given the following commonly-appearing associations: dyskinetic syndromes and antiepileptic drugs, neuropsychiatric syndrome and antiparkinsonian drugs, and akinetic-rigid syndrome and neuroleptic drugs (including antivertigo–prokinetic–antiemetic drugs). Patients with long-term neurological diseases and narrow therapeutic ranges require added precautions. This fact obliges doctors to assume the risk of higher ADR frequency when prescribing drugs. Our data support frequent revision of regular medications, cancellation of unnecessary or over-the-counter drugs, limiting administration time to the recommended period, and selecting any new prescriptions according to the type of neurological disease in this population. Based on the above, some drugs may represent a vital risk for some patients, such as those with myasthenia. In other cases, long-term treatment increases the risk of chronic and difficult to treat syndromes, such as tardive dyskinesia.
According to our observations in the group in question, the prevalence of neurological AEs was 0.586% compared to that of the initial total sample. Of this percentage, 0.12% of the cases were preventable and 0.457% were unpreventable. Given that 59% of the cases of AE were detected in outpatient clinics, and based on the total volume of patients attended, 0.36% of the cases referred to these clinics had AE. These figures seem to reflect a lower impact than that cited by prior studies, which indicated rates of neurological ADR as high as 5.1% (APEAS),21 with ADR accounting for 5.3% of all hospital admissions in general.10 On the other hand, 21.9% of the AE cases were detected in hospitals. Therefore, 2.89% of the patients admitted to hospitals during the study period had ADEs. These figures are similar to the ones previously described in the study which define ADR as the cause of 2.7% of all admissions to a hospital neurology department.25
The low prevalence here compared to other prior articles may be due to the study design and its possible biases. As this was a hospital-level study, we only recorded data from subjects referred directly to the neurology department without including patients assessed in primary care who were not considered for referral, or subjects treated by other hospital departments (internal medicine, ICU). In all these cases, the level of suspicion of AE may have been low, especially in the presence of less-common neurological syndromes. Other possibilities to be considered are the pharmacological cause being detected and treated at the primary care level, any deaths that may have occurred, or the resolution of symptoms prior to consultation with a neurologist.
Another factor that may have contributed to the low prevalence is exclusion of patients who still presented symptoms at the end of follow-up, although the cause of the symptoms may have been drug-related. Such would be the case with tardive dyskinesias, psychotropic drug dependency, or antiarrhythmic agent consumption, as stated before.
On the other hand, our series presented a very low frequency of common neurological syndromes such as dyskinesias secondary to dopaminergic treatment in patients with parkinsonian syndromes. We think that the study design may provide another explanation. Since the above AEs are well-known to neurologists, they may have not been considered in patients already diagnosed with Parkinson's disease since there were no new motives for consultation. As a result, many of these patients would not have been selected for the study. Moreover, as the sample had a very narrow therapeutic margin, a follow-up period of 1 month might not be long enough to observe improvement after a change in treatment.
Although there were proportionally fewer cases of AE at the primary care level, these were the most critical cases. These findings make us especially mindful of the care of patients with chronic diseases and a high risk of presenting serious and potentially preventable AEs (myasthenia gravis, epilepsy, and Parkinson's disease). Concerning specialised care, most of the cases were mild, predictable, and unpreventable, but we were surprised by how difficult it was to obtain interdisciplinary agreement and identify a suspected cause of symptoms.
Lastly, our results are valuable because they enhance current knowledge and raise awareness about the possibility of neurological symptoms being associated with a specific drug. This should be considered after taking a detailed medical history and performing a thorough clinical examination. As a consequence, fewer patients would be erroneously diagnosed with a disease and doctors would not need to order complementary tests or start unnecessary treatments with their accompanying risks and consumption of resources. Our study may only show the tip of the iceberg. Other studies that compensate for our study's limitations and involve cooperation between care levels are needed in order to increase our knowledge of epidemiology, propose a specific educational approach,26,27 identify the safest measures, and lastly, reduce needs for care, without forgetting the impact of the diagnosis on the patient.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Álvarez Soria MJ, et al. Síndromes neurológicos asociados al uso de medicamentos. Frecuencia y caracterización. Neurología. 2012;27:547–59.
Preliminary results from this study were presented in poster format at the 62nd annual meeting of the Spanish Society of Neurology.