Neoplastic meningitis (NM) is a relatively frequent metastatic complication of cancer associated with high levels of neurological morbidity and generally poor prognosis. It appears in 5%-15% of patients with solid tumours, the most frequent being breast and lung cancer and melanoma. Symptoms are caused by involvement of the cerebral hemispheres, cranial nerves, spinal cord, and nerve roots, and are often multifocal or present with signs and symptoms of intracranial hypertension. The main diagnostic tools are the neurological examination, brain and spinal cord contrast-enhanced magnetic resonance imaging, and cerebrospinal fluid analysis including cytology, although studies have recently been conducted into the detection of tumour cells and DNA in the cerebrospinal fluid, which increases diagnostic sensitivity. With the currently available therapies, treatment aims not to cure the disease, but to delay and ameliorate the symptoms and to preserve quality of life. Treatment of NM involves a multimodal approach that may include radiotherapy, intrathecal and/or systemic chemotherapy, and surgery. Treatment should be individualised, and is based mainly on clinical practice guidelines and expert opinion. Promising clinical trials are currently being conducted to evaluate drugs with molecular and immunotherapeutic targets. This article is an updated review of NM epidemiology, clinical presentation, diagnosis, prognosis, management, and treatment; it is aimed at general neurologists and particularly at neurologists practicing in hospital settings with oncological patients.
La meningitis neoplásica (MN) es una complicación metastásica relativamente frecuente en pacientes con cáncer, con alta morbilidad neurológica y en general pobre pronóstico. La prevalencia está en torno al 5-15% de los tumores sólidos, y los más frecuentes son el cáncer de mama, el de pulmón y el melanoma. La clínica se produce por afectación de hemisferios cerebrales, nervios craneales, médula y raíces nerviosas, siendo en muchos casos multifocal, y presenta a menudo síntomas y signos de hipertensión intracraneal. Las principales herramientas diagnósticas son la exploración neurológica, la resonancia magnética cerebral y medular con contraste, y el análisis y la citología del líquido cefalorraquídeo (LCR), aunque recientemente se están investigando técnicas como la detección de células tumorales y ADN circulante en el LCR, que aumentan la sensibilidad diagnóstica. Con las terapias disponibles en la actualidad el objetivo del tratamiento no es curativo, sino retrasar y disminuir los síntomas y preservar la calidad de vida de los pacientes, e implica un enfoque multimodal que puede incluir radioterapia, quimioterapia intratecal y/o sistémica y cirugía. El tratamiento debe ser individualizado y se basa principalmente en guías y opiniones de expertos. Actualmente se están llevando a cabo ensayos clínicos prometedores de fármacos contra dianas moleculares e inmunoterápicos. Este artículo es una revisión actualizada de la MN, e incluye epidemiologia, presentación clínica, diagnóstico, pronóstico, manejo y opciones terapéuticas; se dirige al neurólogo general, y en particular al neurólogo que ejerce su práctica en centros con pacientes oncológicos.
Neoplastic meningitis (NM) is a common metastatic complication in patients with cancer; it is associated with considerable neurological morbidity and generally (although with some exceptions) with short survival times, even when managed with intensive treatment. Early diagnosis and onset of treatment and/or palliative care, depending on the case, is extremely important. For these reasons, we consider it beneficial to present an updated review of the diagnosis, prognosis, and treatment of NM, which we hope may be valuable for general neurologists, particularly those practising in hospitals with oncology departments.
NM is defined as the metastatic spread of cells from solid tumours or haematologic malignancies to the meninges and subarachnoid space.1 It may occur in isolation or in association with intraparenchymal metastases in the brain and/or spinal cord. This complication, also known as leptomeningeal carcinomatosis, occurs in 4%-15% of cases of solid tumours1–3; this association is the main subject of this review. Autopsy series report even greater prevalence, up to 20% in symptomatic and 8% in asymptomatic patients. NM also occurs in 5%-15% of patients with haematologic malignancies1,3 and 1%-2% of those with primary tumours of the nervous system, particularly ependymomas, medulloblastomas, and primary central nervous system lymphomas, but also in gliomas and other tumours.1,4
Lung cancer (associated with NM prevalence of 9%-25%)1 and melanoma (6%-18%)1,5 are the solid tumours presenting the greatest likelihood of metastasis to the meninges. However, on account of its high incidence, breast cancer is the primary solid tumour most frequently associated with NM (27%-50% of all cases), followed by lung cancer (22%-36%) and melanoma (12%).1,3,6 Other solid tumours, including gastric,7 ovarian,8 prostate,9 and colon cancer,10 may also be associated with NM, albeit less frequently.
NM usually presents at advanced stages of disease progression (in more than 70% of cases), but may also appear after a disease-free interval (20%) or even as the first manifestation of the tumour (5%-10%)3,6; compatible symptoms should give rise to strong suspicion, even in the absence of active or known systemic neoplastic disease.
SymptomsClinical signs of NM are caused by focal or global involvement of the brain hemispheres (15%), cranial nerves (35%), and/or spinal cord and nerve roots (60%)2,6; multifocal involvement3,6,11,12 and signs of intracranial hypertension (26%) are also frequent.1 Onset is usually subacute, with a progressive course over a period of several weeks; progression may last days or months in rarer cases (Table 1).
Clinical characteristics of neoplastic meningitis.
| Cerebral hemispheres (15%) |
|---|
| Headache |
| Confusion |
| Hemiparesis |
| Impaired consciousness |
| Focal seizures with or without secondary generalisation |
| Cranial nerves (35%) |
| Diplopia (the sixth cranial nerve is most frequently affected, followed by the third and fourth cranial nerves) |
| Motor or sensory alterations of the fifth cranial nerve |
| Hypoacusia due to eighth cranial nerve involvement |
| Spinal cord and/or nerve roots (60%) |
| Weakness |
| Segmental sensory disturbance |
| Loss of sphincter control |
| Radicular or back pain |
| Hydrocephalus (26%) (communicating or obstructive) |
| Symptoms of intracranial hypertension (nausea, vomiting, headache, impaired consciousness) |
| Episodes of transient neurological dysfunction (plateau waves) |
The most frequent manifestations of hemispheric involvement due to meningeal irritation are headache, confusion, hemiparesis, and seizures. Common signs and symptoms secondary to cranial nerve involvement include diplopia (the sixth cranial nerve is most frequently affected, followed by the third and fourth cranial nerves), motor or sensory alterations of the fifth cranial nerve, and hypoacusia due to involvement of the eighth cranial nerve. Finally, spinal cord and nerve root involvement may cause weakness (mainly in the lower limbs), segmental sensory disturbance, loss of sphincter control, perineal anaesthesia, and back or radicular pain.3,6
Intracranial hypertension typically manifests as headache, nausea, vomiting, and impaired consciousness; examination reveals papilloedema.1 Intracranial hypertension is caused by altered cerebrospinal fluid (CSF) flow, and may present with communicating or obstructive hydrocephalus.13 Some patients with NM present transient episodes of neurological dysfunction attributable to plateau waves, which are defined as episodic increases in intracranial pressure, normally induced by changes of posture, and cause decreased cerebral perfusion. They manifest as impaired consciousness, tonic limb posturing, vomiting, and incontinence; differential diagnosis should seek to rule out seizures.14
DiagnosisIn the event of clinical suspicion of NM, the 2 main diagnostic tools are MRI and CSF analysis (Table 2).
Diagnostic tests and sensitivity.
| Brain MRI | Brain and spinal cord, 3 segments with contrast | Sensitivity 70%-80% |
| CSF analysis, general parameters | Elevated opening pressure (> 20 cm H2O) | 50% |
| Lymphocytic pleocytosis (> 4 leukocytes/mm3) | 25%-64% | |
| Low glucose level (< 60 mg/dL) | 50% | |
| Elevated protein level (> 50 mg/dL) | 60%-90% | |
| CSF cytology (gold standard) | Increased diagnostic sensitivity if: | Sensitivity 60%-90% |
| Specificity 95% | |
| ||
| ||
|
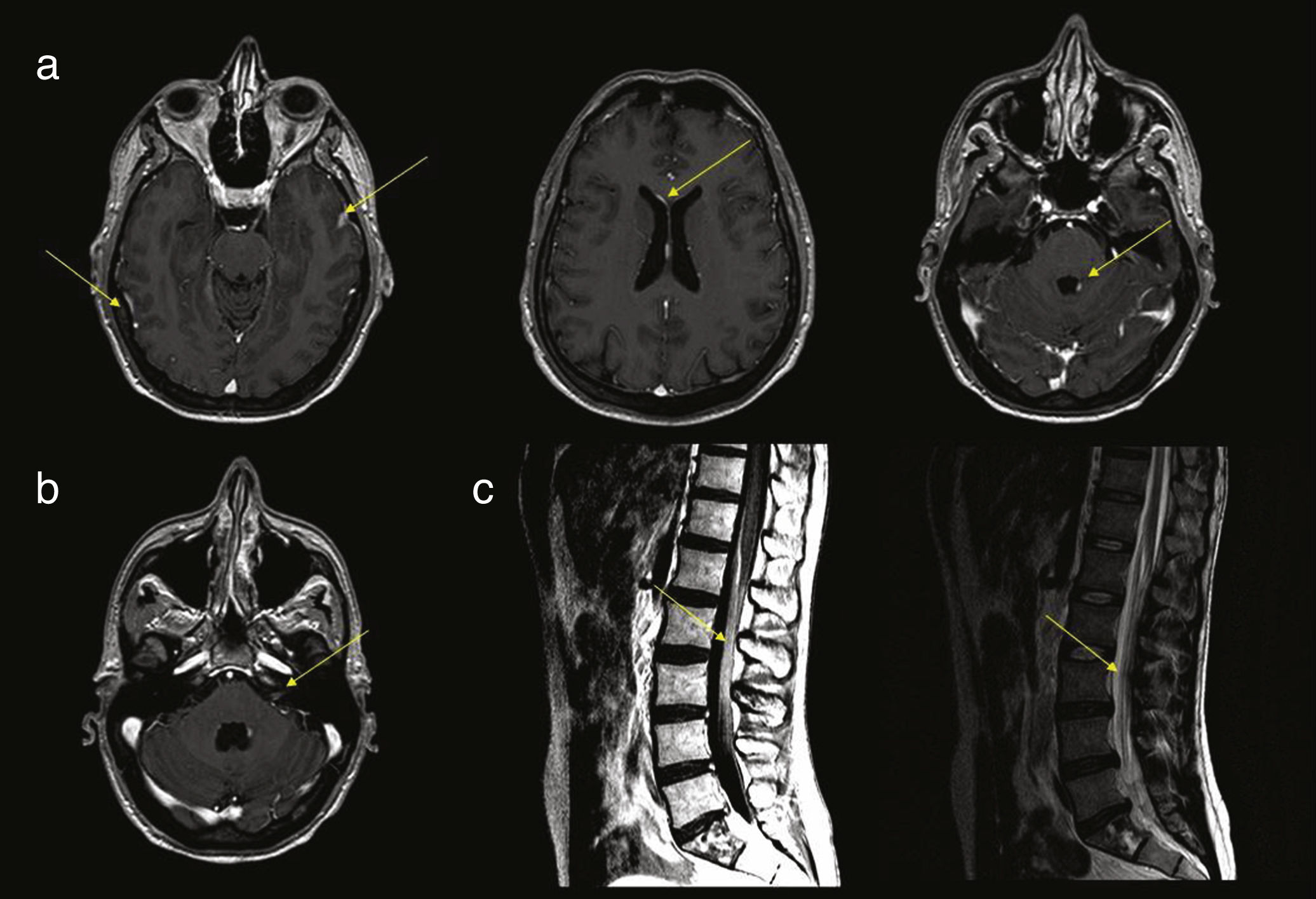
Gadolinium-enhanced MRI of the brain and spinal cord is the neuroimaging technique of choice in patients with suspected NM; this technique presents 70%-80% specificity,2,3,15,16 compared to 30% for head CT. The most frequent neuroimaging signs compatible with diagnosis of NM are focal or distal enhancement of the meninges, subarachnoid or intraventricular nodules, and focal enhancement in such structures as the ependyma, cranial nerves, and nerve roots.15,17,18 It is advisable to perform neuroimaging studies prior to lumbar puncture, as the latter technique may cause transient meningeal enhancement, giving rise to false-positive results (Fig. 1).
(a) Several axial slices from a contrast T1-weighted MRI sequence, showing enhancement and thickening of the dura mater and leptomeninges, periventricular enhancement, and nodular enhancement of the fourth ventricle. (b) Contrast T1-weighted brain MRI scan showing enhancement of the left acoustic-facial bundle. (c) Contrast T1- and T2-weighted sagittal MRI scan of the lumbar spine, showing thickening and enhancement of the roots of the cauda equina.
Suggestive, though nonspecific, CSF analysis findings compatible with NM include elevated opening pressure (> 20 cm H2O; observed in 30%-57% of cases), lymphocytic pleocytosis (> 4 leukocytes/mm3; 33%-79% of cases),7 low glucose level (< 60 mg/dL; 24%-62% of cases), and elevated protein level (> 50 mg/dL; 63%-90% of cases).13 Protein level determination presents the greatest sensitivity but not the best specificity.19–21
Currently, the best test for diagnosing NM in patients with solid tumours is CSF cytology, which demonstrates the presence of tumour cells with sensitivity of 60%-90% and specificity of 95%.17,22 Sensitivity increases if 2 lumbar punctures are performed. However, performing 3 or more lumbar punctures does not significantly improve diagnostic sensitivity. Other measures that increase the likelihood of detecting malignant cells include obtaining 2 samples, sample volume of at least 10.5 mL, processing samples immediately, and performing the puncture close to an area with clinical or radiological signs of disease.1–3,17
Several studies, such as that by Subirá et al.,23 have analysed the value of flow cytometry in the diagnosis of NM in patients with solid tumours, finding no clear benefit compared to cytology; therefore, this test is not routinely performed in CSF analysis. However, multiple studies evaluating flow cytometry in patients with haematologic malignancies have demonstrated clear advantages over cytology studies in terms of sensitivity for detecting tumour cells in the CSF, and recommend performing this study.17,24–26
Another technique under study in CSF analysis is the detection of circulating tumour cells using the modified CellSearch system. This system has been used to count malignant cells in the peripheral blood (identified as a prognostic factor) in patients with such solid tumours as breast and colon cancer. Studies using the technique for CSF cell counts report high sensitivity and a strong correlation with cytology findings. Some studies suggest the potential value of this technique as a marker of early central nervous system involvement and treatment response.27,28 However, its use currently remains experimental. Finally, another experimental method for detecting central nervous system metastasis is the analysis of circulating DNA in the CSF. Several studies have found this technique to be more sensitive than cytology; furthermore, it may assist in the molecular characterisation of metastatic tumour cells.29,30
In the absence of compatible neuroimaging or CSF findings, diagnosis of NM may be based on clinical manifestations and history of cancer, after other causes are ruled out,31 although diagnosis should ideally be confirmed with positive cytology results and/or compatible MRI findings in order to start specific treatment.1 Although patients with negative results in the initial evaluation are unlikely to develop NM, all patients must undergo clinical follow-up, and MRI studies and CSF analysis should be repeated in the event of new or progressive symptoms.32
Prognosis and treatment indicationsWithout treatment, prognosis is generally poor, with a mean survival time of 4-6 weeks; this increases to 3-6 months with treatment.1,3,19,33 However, survival depends on the type of primary tumour,1 with some studies reporting survival times of 7-12 months in patients with breast cancer19,34 (11%-25% survival one year after diagnosis) and 4 months in patients with melanoma35 and lung cancer.36
Studies have addressed the influence of multiple factors on the prognosis of NM; the clearest factor is the patient’s functional status, with patients scoring below 70 on the Karnofsky Performance Status (KPS) scale presenting shorter survival times (Table 3).37,38
Karnofsky performance status scale.
| Score | State of health |
|---|---|
| 100 | Normal, no complaints or evidence of disease |
| 90 | Able to perform normal activity; minor signs and symptoms of disease |
| 80 | Able to perform normal activity with effort; some signs and symptoms of disease |
| 70 | Cares for self, unable to perform normal activity or to do active work |
| 60 | Requires occasional assistance but is able to care for most of own needs |
| 50 | Requires considerable assistance and frequent medical care |
| 40 | Requires special care and assistance; disabled |
| 30 | Hospitalisation indicated, although death not imminent; severely disabled |
| 20 | Hospitalisation necessary; active supportive treatment required, very sick |
| 10 | Fatal processes progressing rapidly; moribund |
| 0 | Dead |
Additional prognostic factors have been studied in the specific context of NM in patients with breast cancer, melanoma, and lung cancer. A retrospective study of 103 patients with NM associated with breast cancer found that hormone receptor status (oestrogen and progesterone receptors) and stage at diagnosis were the most significant factors influencing the time between initial diagnosis of the tumour and onset of NM. Advanced tumour stage and negativity for hormonal receptors and HER2 (triple-negative breast cancer) were associated with earlier development of NM. After diagnosis of NM, these factors are also related to survival time: prognosis is better in patients positive for hormonal receptors and poorer in patients with triple-negative breast cancer.32,39 Regarding the prognostic factors reported in patients with melanoma and NM, a retrospective study including 110 patients reports that intrathecal chemotherapy improved prognosis, but did not find poorer prognosis in patients with greater CNS tumour burden, contradicting the study’s primary hypothesis.40 In NM associated with lung cancer, a multivariate analysis in a retrospective study of 149 patients found that factors including low scores on the Eastern Cooperative Oncology Group performance status scale, low CSF protein level and cell count, and intrathecal chemotherapy were associated with poor prognosis, whereas radiotherapy (RT) and tyrosine kinase inhibitors targeting the epidermic growth factor receptor (EGFR) improved prognosis.41 Given the retrospective design of these studies, we cannot rule out the possibility that this improved prognosis was influenced by a selection bias (ie, the patients with the best prognosis may have been selected to receive treatment).
Besides the patient’s functional status, other factors associated with poor prognosis include age older than 50 years, diagnosis of NM less than 12 months after diagnosis of the primary tumour, multiple established neurological deficits, presence of encephalopathy, high burden of leptomeningeal disease, CSF flow obstruction, and advanced systemic disease with limited treatment options.1,3,15,42,43
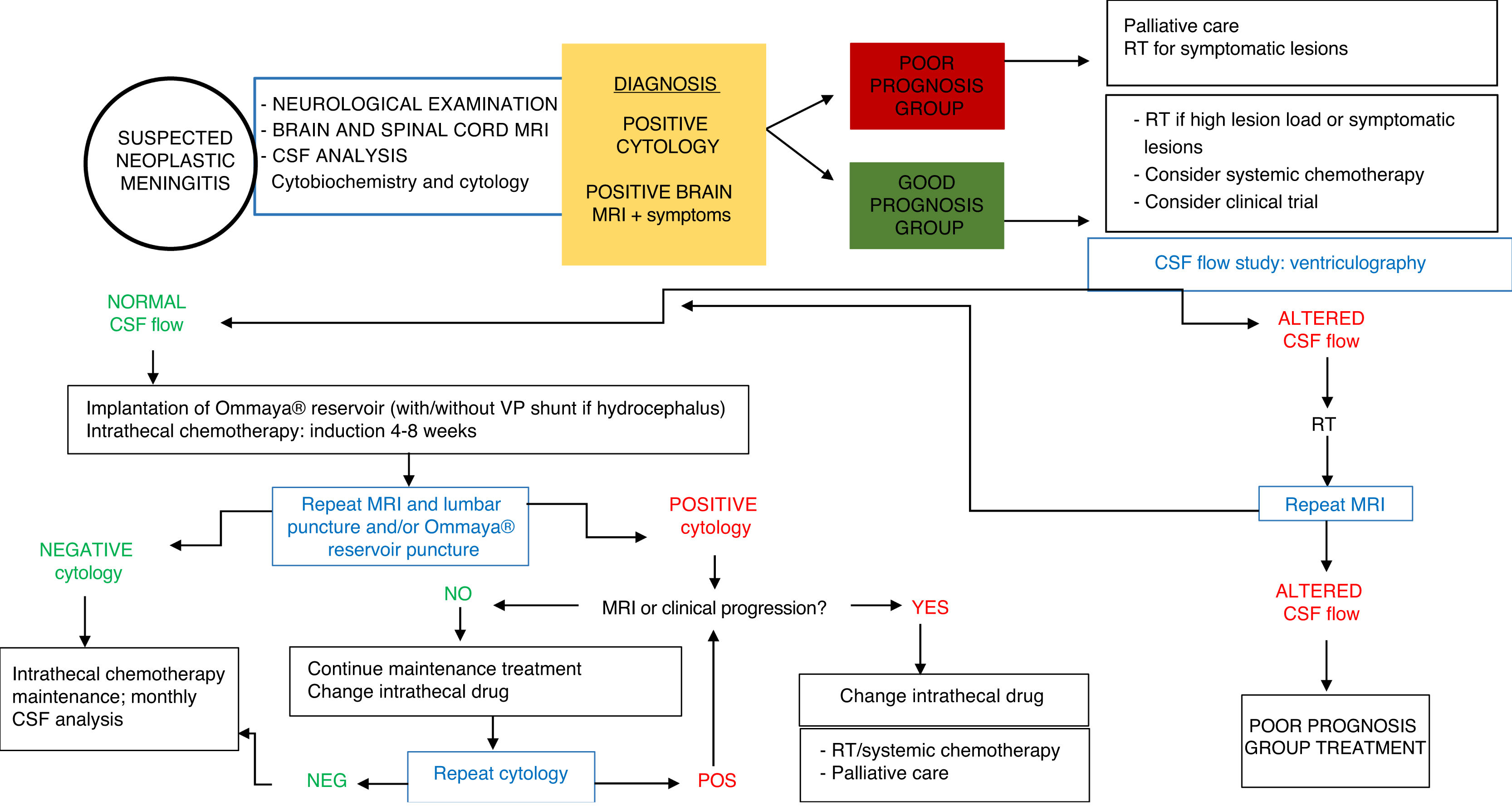
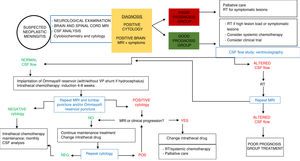
Based on these factors, patients with NM may be classified into 2 groups with different treatment indications (Table 4). The group of patients eligible for intensive treatment would include those with better expected outcomes and treatment response (KPS > 60; systemic disease under control or with appropriate treatment options, absence of major neurological deficits). In patients with poorer prognosis (KPS ≤ 60), uncontrolled systemic disease or lack of treatment options, and/or presence of significant neurological deficits, intensive treatment for NM is highly unlikely to be beneficial, with supportive or palliative care being more appropriate (Fig. 2).
Current management approaches aim not to treat, but rather to delay and to diminish as much as possible the neurological symptoms of the disease and to preserve patients’ quality of life. The best strategy is individualised treatment; in the majority of cases, this involves a combination of the different types of treatment available (RT; intrathecal or systemic chemotherapy) over the course of the disease, as well as supportive treatment appropriate to the patient’s situation. Treatment decisions are typically based on variables including lesion load, type of tumour, the availability of systemic treatments active against NM, and the speed of symptom progression. In intrathecal chemotherapy, the most appropriate drug will depend on such factors as adverse reactions, patient comfort, and the healthcare centre’s experience with the specific drug; randomised clinical trials have not shown any drug to be superior.
TreatmentSeveral forms of treatment may be used for NM, including intrathecal chemotherapy, RT, and systemic chemotherapy, as well as supportive or palliative care for the associated symptoms. Treatment decisions must be made on a case-by-case basis; ideally, the treatment plan should be designed by a multidisciplinary team (Fig. 2).
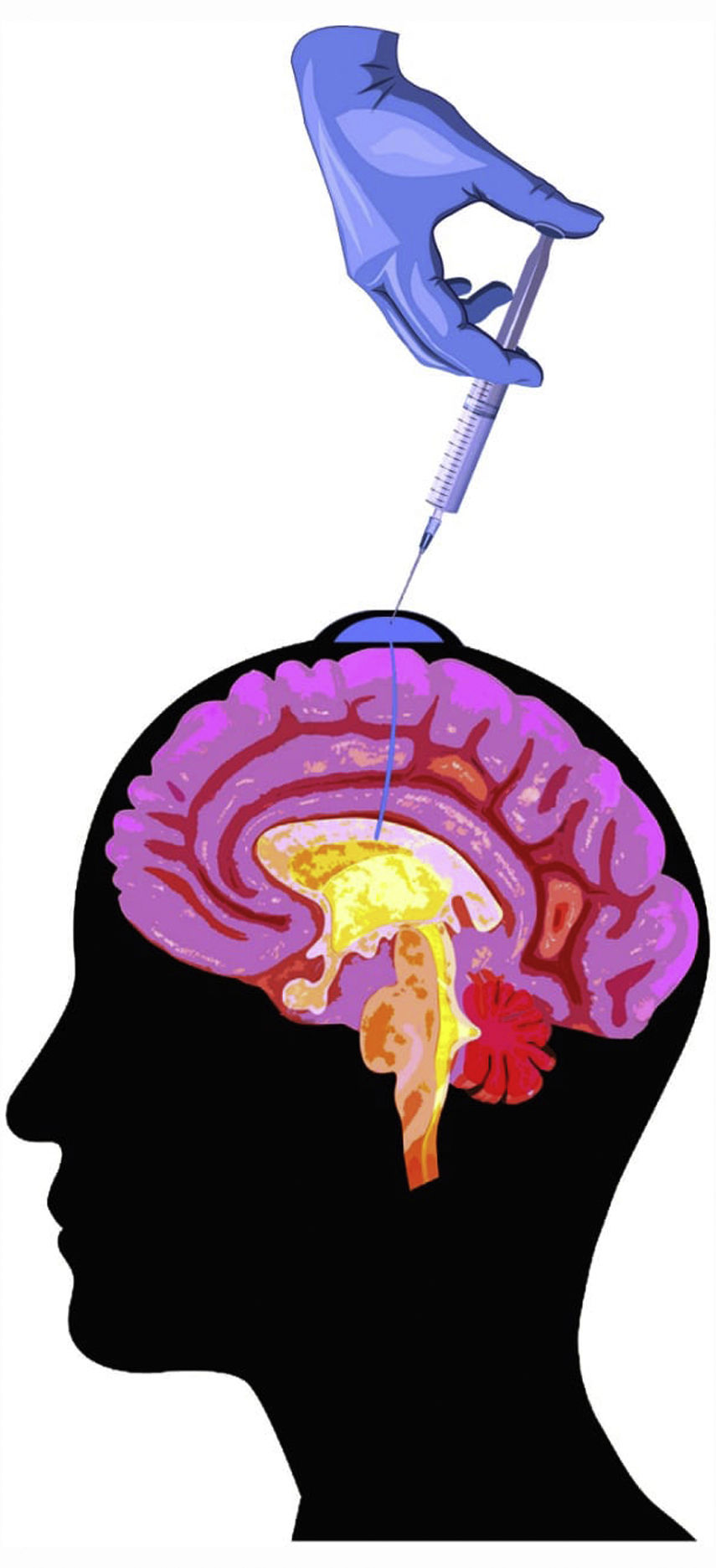
Intrathecal chemotherapyMeans of administrationIntrathecal chemotherapy consists in the administration of drugs directly to the subarachnoid space, and may use 2 different routes: repeated lumbar punctures or an intraventricular catheter connected to an Ommaya® reservoir implanted under the scalp (Fig. 3). This device must be implanted by a neurosurgeon, and a CT study should be performed after the procedure to verify correct placement before treatment is started, as the device may not be correctly positioned in 3%-12% of cases.3 The main advantages of Ommaya® reservoirs over administration via lumbar puncture are the better distribution of the drug in the intrathecal space, which is particularly important for drugs with very short half-lives,1,44 such as methotrexate (MTX) or cytarabine (Ara-C), as well as the greater ease of administering treatment and better patient comfort. The main disadvantage is the need for surgical intervention and the greater frequency of infections, compared to lumbar puncture.1
A CSF flow study is recommended prior to onset of intrathecal chemotherapy, as flow may be obstructed in 30% of patients with NM; the cerebral convexity, skull base, and spinal cord are the main regions affected.45,46 Obstructed CSF flow is associated with poor prognosis in these patients, possibly due to suboptimal distribution of the drug and the potential for accumulation in the area of obstruction, causing toxicity.45,47 The technique of choice for studying CSF flow is ventriculography with indium-111 or technetium-99.48 If an obstruction is detected, the patient may be treated with focal RT, which is able to restore flow in 30%-50% of cases, improving prognosis.45,47 Intrathecal chemotherapy is contraindicated if obstruction persists despite RT.
Drugs available for intrathecal administrationThe drugs most frequently used for NM are MTX, Ara-C and the slow-release form cytarabine liposome, thiotepa, and topotecan. Numerous studies have compared the effectiveness of these drugs or combinations of these drugs, finding no significant differences in terms of treatment response or global survival times.49–52 Combination therapy is rarely used in patients with haematologic malignancies, unlike in the case of patients with NM and solid tumours.1
- •
Methotrexate. MTX is a folic acid antagonist that inhibits DNA synthesis through the inhibition of dihydrofolate reductase. The drug has a half-life of 4-8 hours. The typical administration protocol consists of 10-15 mg administered twice weekly for 4 weeks for induction, once per week for 4 weeks for consolidation, and once per month for maintenance treatment (Table 5).1,2,53
Table 5.Intrathecal chemotherapy administration protocols.
Drug Induction Consolidation Maintenance Methotrexate 10-15 mg 2 times/week for 4 weeks 10-15 mg 1 time/week for 4 weeks 10-15 mg 1 time/month Cytarabine 25-100 mg 2 times/week for 4 weeks 25-100 mg 1 time/week for 4 weeks 25-100 mg 1 time/month Cytarabine liposome 50 mg 1 time/2 weeks for 8 weeks 50 mg 1 time every 4 weeks 50 mg 1 time every 4 weeks Thiotepa 10 mg 2 times/week for 4 weeks 10 mg 1 time/week for 4 weeks 10 mg 1 time/month Topotecan 0.4 mg 2 times/week for 6 weeks 0.4 mg 1 time/week for 6 weeks 0.4 mg 2 times/month for 4 months and subsequently 1 time/month - •
Cytarabine. Ara-C (cytosine arabinoside, cytarabine) is a pyrimidine analogue that is metabolised to the active metabolite ara-cytidine-5′-triphosphate (ara-CTP), which inhibits DNA polymerase. The drug has a half-life of 1-3 hours. The typical administration protocol consists of 25-100 mg administered twice weekly for 4 weeks for induction, once per week for 4 weeks for consolidation, and once per month for maintenance treatment (Table 5).2,52
- •
Cytarabine liposome is a slow-release compound that remains at cytotoxic levels in the CSF for 10-14 days (half-life of 100-263 hours); therefore, it may be administered at longer intervals. Glantz et al.54 conducted a clinical trial of patients with meningeal lymphomatosis, comparing the standard formulation of Ara-C against the slow-release compound, finding the latter to present a higher response rate (CSF cytology converting to negative and lack of neurological progression) but not longer survival times. Another clinical trial by the same research group compared the drug against MTX. Response rates were similar, but the group treated with cytarabine liposome presented longer delays to neurological worsening; however, the difference in survival time was not statistically significant.55 The typical administration protocol consists of 50 mg administered every 2 weeks for 8 weeks for induction and every 4 weeks for consolidation and maintenance treatment (Table 5).2,54
- •
Thiotepa. Thiotepa, an alkylating agent that does not depend on the cell cycle, is another drug available for intrathecal administration, although it is not widely used.1,2 Several clinical trials support its usefulness; it has a similar efficacy rate to the remaining treatments and a low level of toxicity.19,56,57 The drug has a half-life of 6 hours. The typical administration protocol consists of 10 mg administered twice weekly for 4 weeks for induction, once per week for 4 weeks for consolidation, and once per month for maintenance treatment (Table 5).2,56,57
In addition to the more common treatments, clinical trials have evaluated intrathecal administration of other drugs. These include:
- •
Topotecan, a topoisomerase type I inhibitor approved for systemic use in patients with ovarian and lung cancer. Clinical trials assessing its safety and effectiveness in patients with NM, as well as several retrospective studies, report response rates and survival times similar to those of other intrathecal drugs, and good tolerability.2,58,59 The drug has a half-life of 2-3 hours. The typical administration protocol consists of 0.4 mg administered twice weekly for 6 weeks for induction, once per week for 6 weeks for consolidation, and twice per month for maintenance treatment, followed by administration once per month if the disease does not progress (Table 5).58,59
- •
Trastuzumab, a monoclonal antibody targeting HER2, is used for systemic therapy in patients with metastatic breast cancer and HER2 overexpression; response is very good, except in cases of brain and leptomeningeal metastasis. Several case series report good response and tolerance to intrathecal administration in the treatment of patients with NM and HER2-positive breast cancer. Two clinical trials currently underway aim to establish the drug’s efficacy and safety and the most suitable dose.1,60–62 The intravenous form of trastuzumab is unsuitable for intrathecal administration as the reconstituted vials contain benzyl alcohol, which is neurotoxic.
- •
Interleukin 2 (IL-2) is an immune system cytokine that plays an important role in regulating T lymphocyte responses. A retrospective study of 43 patients with melanoma and NM found that intrathecal administration of IL-2 increased survival. The most frequent secondary effects are headache, chills, nausea, and fever. It is administered 4 times per week for 4 weeks for induction, followed by a maintenance dose every 1-3 months if an improvement is observed63.
- •
Infectious meningitis.3,64,65 This complication occurs in 2%-15% of patients, most frequently in those with Ommaya® reservoirs. Clinical manifestations include headache, fever, nausea and vomiting, impaired consciousness, and malfunctioning of the reservoir. Typical CSF findings include pleocytosis with predominance of polymorphonuclear leukocytes, increased protein level, and low glucose level. Gram-positive micro-organisms are most frequently isolated, particularly coagulase-negative Staphylococcus. Treatment requires intravenous antibiotics; intrathecal administration of antibiotics and early removal of the catheter should also be considered. In the event of asymptomatic colonisation, removal of the catheter is not always necessary, and antibiotic treatment should be considered.
- •
Aseptic meningitis.66,67 Aseptic meningitis presents as transient headache, meningism, and mild fever, presenting several hours after infusion and disappearing within 3 days. This is an idiosyncratic reaction and does not usually recur with subsequent doses of treatment. It presents in up to 10% of patients receiving MTX, and also in those receiving thiotepa and Ara-C; it is more frequent and more prone to recurrence in the case of cytarabine liposome. It responds to treatment with corticosteroids and non-steroidal anti-inflammatory drugs.
- •
Acute myelopathy.66,68,69 Acute myelopathy is a rare but devastating complication of intrathecal chemotherapy. It is usually associated with MTX treatment, but has also been reported in patients receiving cytarabine and thiotepa. It can appear days or months after administration of the drug, and presents as paraparesis, tetraparesis, or even locked-in syndrome. Spinal MRI studies display T2-hyperintense lesions to the posterior columns, although findings are normal in some cases. There is no established treatment, although a case has been published of a patient who presented a partial improvement with supplementation with high doses of folic acid, cyanocobalamin, methionine, and S-adenosyl methionine starting 3 days after symptom onset.43
Differential diagnosis between this entity and myelopathy secondary to tumour progression is complex. Determination of myelin basic protein in the CSF may be valuable, as levels of this protein increase if spinal cord damage is caused by demyelination but not in the event of tumour progression.69
- •
Progressive leukoencephalopathy.1,66,67 This chronic complication may present from 6 months after administration of MTX. Typical symptoms are cognitive and behavioural alterations, incontinence, seizures, and gait alterations. Incidence is 2% in patients receiving intrathecal MTX, but rises to 48% if the drug is administered after RT to the brain. Therefore, it is recommended that intrathecal MTX be administered 2-3 weeks after radiation, if this combination of treatments is needed.
- •
Myelosuppression.1,3 This complication is rare in patients receiving intrathecal chemotherapy; before treatment onset, blood testing should confirm the following values: haemoglobin level > 10 g/dL, platelet count > 100 000 platelets/μL, and granulocyte count > 1500 cells/μL. If MTX is to be administered, prophylactic folic acid is recommended at a dose of 10-15 mg/6 hours for 2 days.
- •
Arachnoiditis and conus medullaris syndrome. This is a common complication (affecting 17% of patients) of treatment with cytarabine liposome.39 Neurological complications decrease with preventive measures such as spacing intrathecal cytarabine liposome and systemic chemotherapy treatment, or the use of oral or intrathecal corticosteroids; however, the risk does not completely disappear.70
RT is one of the main treatments for NM. The different available forms of RT are holocranial, focal, and craniospinal irradiation.
Holocranial RT is administered to patients with concomitant cerebral metastases, high lesion load, large leptomeningeal deposits (which drugs are unable to fully penetrate), or severe or rapidly progressive symptoms.1,13 The typical dose is 30-36 Gy1 in fractions of 3 Gy per day, although doses may range from 20 to 40 Gy.13 While the treatment has not been shown to improve survival times in patients with NM associated with breast or lung cancer,71,72 it is useful in palliative care.
Focal RT is particularly useful in large or symptomatic spinal lesions due to its ability to quickly control symptoms,73 and in lesions causing alterations to CSF flow: focal RT restores CSF flow in 30%-50% of cases.47
Craniospinal irradiation is not generally used to treat NM associated with solid tumours as it is not a curative treatment and may cause bone marrow toxicity that can interfere with subsequent administration of systemic chemotherapy.13
Systemic chemotherapySystemic cytotoxic drugsSystemic chemotherapy generally presents limited efficacy in patients with NM associated with solid tumours due to the low concentrations that the drugs reach in the CSF and brain parenchyma and the lack of antitumour efficacy for some cancers. Despite these limitations, a retrospective study evaluating prognostic factors, which included 155 patients with NM and different types of tumour (73 solid tumours), found that systemic chemotherapy in isolation or in combination with other treatments was associated with better survival times than other treatment options that did not include systemic chemotherapy.43 Another retrospective study including 155 patients with NM reported similar results.74
Numerous drugs are able to cross the blood-brain barrier (e.g., temozolomide, capecitabine, nitrosoureas, methotrexate, and topotecan), but their efficacy in treating NM associated with solid tumours is very limited. A case series by Glantz et al.75 reports good results with longer survival times in 13 of the 16 patients treated with high-dose intravenous MTX, with a mean survival time of 14 months, although most of the primary tumours were gliomas and lymphomas. Several cases have been published of effective treatment of NM with temozolomide.13 However, a phase 2 clinical trial studying NM associated with solid tumours had to be terminated prematurely due to inefficacy of the treatment.76 Cases have been reported in which patients with NM secondary to breast cancer responded to capecitabine.77
Systemic drugs with molecular targetsIn breast cancer treatment, lapatinib, a dual HER1/HER2 tyrosine kinase inhibitor that is administered orally and is able to cross the blood-brain barrier, has shown good results in the treatment of brain metastasis, although no prospective studies have evaluated its usefulness in treating NM. Isolated cases have been reported of response to hormone therapy, although the current evidence is insufficient to support the use of these treatments for NM secondary to breast cancer.19,78
A retrospective study by Liao et al.79 demonstrated the safety of EGFR tyrosine kinase inhibitors (erlotinib, gefitinib, and afatinib) in treating NM in patients with non-small-cell lung cancer with EGFR mutations. The main issue with these drugs is that, while they cross the blood-brain barrier, they only reach very low levels in the CSF. One possible solution is to administer drugs at high doses via alternative routes. A phase 1 clinical trial of high-dose gefitinib reported an improvement in neurological symptoms in 57% of patients.80 Another retrospective study showed that erlotinib at high doses is safe and effective in the event of treatment failure with standard doses. In the case of ALK mutations, present in 5% of patients with lung cancer, several cases have been published of patients who responded to such second-generation ALK inhibitors as alectinib and ceritinib.78
Finally, several published cases of patients with NM associated with melanoma1,81 and the BRAF (V600E) mutation report response to vemurafenib and dabrafenib and such MEK inhibitors as trametinib, either alone or in combination,82 although no prospective clinical trials have addressed this issue. Some authors also report response to treatment with such immune checkpoint inhibitors as ipilimumab83; several clinical trials currently underway address the use of nivolumab and pembrolizumab to treat NM secondary to melanoma.
The role of surgerySurgery has a limited role in treating NM, with indications restricted to the placement of Ommaya® reservoirs, biopsy in the event of unknown primary tumours and inconclusive cytology findings, and ventriculoperitoneal shunt in patients with hydrocephalus. Patients with hydrocephalus may undergo implantation of a shunt with an on-off valve together with a reservoir, which enables intrathecal administration of chemotherapy drugs with the valve in the “off” position and acts as a shunt in the interval between treatments, with the valve in the “on” position. Another surgical option in these patients is endoscopic ventriculostomy.
Evaluation of treatment responseEvaluating treatment response in patients with NM is complex, and should include clinical and radiological assessment and CSF analysis (Fig. 2). According to the results for each parameter, the disease may be classified as responsive to treatment, stable, or progressive. Decisions about whether to continue treatment are based on these criteria. The Response Assessment in Neuro-Oncology (RANO) working group84 recently published an article summarising the current consensus on the assessment of treatment response in NM.
Neurological evaluation should include an assessment of symptoms and physical examination. Treatment response is defined as improvement or absence of clinically significant changes. The RANO group proposes an examination including 10 items, each of which should be assigned a level from 0 to 3; NM is defined as progressive if an item increases by 2 levels or reaches level 3.
Regarding CSF analysis, RANO proposes that cytology results should only be characterised as positive or negative. This analysis should be performed prior to treatment onset (baseline) and during treatment, once per week or twice per month during induction, then every one or 2 months during the maintenance period. Cytology results changing from positive to negative are considered to indicate treatment response. NM is considered progressive if cytology results change from negative to positive or if positive results persist after the treatment induction phase.
Brain and spinal cord MRI are useful in assessing treatment response, and should be performed before treatment onset and after induction. Progression is defined as increased size or extension of existing lesions in contrast-enhanced sequences. The RANO working group proposes a scale for scoring MRI results; this is yet to be validated.
Overall, disease progression is defined as worsening of neurological symptoms, increased size of MRI lesions, or cytology findings of persistent positivity or a change from negative to positive results (however, disease progression cannot be established based on cytology results alone).
ConclusionMultifocal, subacute, progressive neurological symptoms should give rise to strong suspicion of NM in patients with known solid tumours. In addition to the routine MRI and CSF cytology tests, we should also consider flow cytometry and screening for circulating tumour cells or DNA; the latter 2 tests are currently under research, although studies support their high sensitivity. Each patient should be treated on an individualised basis, depending on their characteristics (age, functional status, neurological symptoms) and the characteristics of the tumour (type of primary tumour, presence of systemic disease and treatment options, leptomeningeal lesion load, presence of CSF flow obstruction). Treatment is based on 3 fundamental pillars: RT, intrathecal chemotherapy, and systemic chemotherapy, as well as supportive care to control symptoms. Most current research is focused on drugs with molecular targets; immunotherapy is also beginning to gain prominence in clinical trials, especially in the context of melanoma.
This article presents an updated review of today’s clinical practice and possible future directions; furthermore, we propose a treatment algorithm that may serve as a guideline for the management of NM.
The treatment (and therefore also the prognosis) of these patients may improve in the future with the validation of techniques for the early diagnosis of NM and for monitoring treatment response; further multicentre clinical trials specifically designed to study NM associated with specific tumour types or tumours with different origins, but sharing the same genetic/molecular characteristics; multidisciplinary management; and national and international consensus guidelines.
FundingThis study has received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
This article was drafted while Marta Penas-Prado was working at the MD Anderson Cancer Center at the University of Texas. The opinions expressed in this article are the author’s, and do not reflect the views of the National Institutes of Health, the United States Department of Health and Human Services, or the United States government.
Please cite this article as: García Molina E, Penas-Prado M. Meningitis neoplásica en tumores sólidos: revisión actualizada de diagnóstico, pronóstico, manejo terapéutico y direcciones futuras. Neurología. 2022;37:794–805.