Psychiatric comorbidities are common in epileptic patients, and evaluating the impact of antiepileptic drugs on patients’ moods is therefore essential. The aim of this study is to assess the effects of lacosamide on behaviour and quality of life in people with epilepsy.
MethodsWe conducted a multicentre prospective observational study of poorly-controlled epileptic patients who received lacosamide as an adjuvant treatment. Patients were evaluated on 4 occasions during a 12-month period. The impact of lacosamide on patients’ mood and quality of life was assessed with the Quality of Life in Epilepsy Inventory-10 (QOLIE-10), the Hospital Anxiety and Depression Scale (HADS), and the Barratt Impulsiveness Scale (BIS-11). As a secondary objective, we evaluated the effectiveness and safety of lacosamide.
ResultsWe included 55 patients with a mean age of 47.1±18.4 years. At baseline, 34.5% of the patients had psychiatric comorbidities; the mean number of crises in the previous month was 3.6±4.3. The QOLIE-10 and HADS scales revealed statistically significant improvements in patients with a poor baseline condition (anxiety, depression, and/or poor quality of life). The BIS-11 scale detected no impulsive behaviour during follow-up. After 12 months of treatment, 51.9% of the patients were seizure-free and 77.8% experienced a reduction of at least 50% in seizure frequency. Adverse effects were mild in most cases; lacosamide was discontinued in 10 patients (18.2%).
ConclusionsLacosamide is a safe and effective treatment option for patients with epilepsy and psychiatric comorbidities.
La comorbilidad psiquiátrica es común en epilepsia, de ahí la importancia de considerar en qué medida los fármacos antiepilépticos pueden influir en el estado de ánimo. El objetivo de este trabajo es analizar el efecto de lacosamida en la calidad de vida y en la conducta del paciente epiléptico en la práctica clínica.
MétodosEstudio multicéntrico, observacional y prospectivo en pacientes diagnosticados de epilepsia, mal controlados que recibieron tratamiento adyuvante con lacosamida. Mediante 4 visitas durante 12 meses se valoró el impacto del fármaco en la calidad de vida y el estado de ánimo utilizando el cuestionario de calidad de vida QOLIE-10, la escala hospitalaria de ansiedad y depresión (HADS) y la escala de impulsividad de Barratt (BIS-11), además se determinó su eficacia y seguridad.
ResultadosSe incluyeron 55 pacientes, edad media 47,1±18,4 años; porcentaje inicial de comorbilidad psiquiátrica 34,5% y número medio de crisis/mes previo 3,6±4,3. Las escalas QOLIE-10 y HADS reflejaron mejoras estadísticamente significativas en pacientes que partían de una situación basal desfavorable (ansiedad, depresión y/o baja calidad de vida). La escala BIS-11 no detectó la aparición de conductas impulsivas durante el seguimiento. Tras 12 meses de tratamiento el 51,9% de los pacientes estuvo sin crisis, y un 77,8% presentó una reducción≥50%. La mayoría de efectos adversos fueron leves, obligando a retirar el fármaco en 10 casos (18,2%).
ConclusionesLacosamida ofrece un perfil de eficacia y seguridad favorable, y podría constituir una opción terapéutica útil en pacientes con epilepsia y comorbilidad psiquiátrica.
Epilepsy is a chronic disease with considerable social impact: prevalence in Spain is 14.87 cases per 1000 population1; worldwide incidence is approximately 50 cases per 100000 person-years.2 The stigma associated with the disease, adverse drug reactions (ADR), and the unpredictability and consequences of seizures affect patients’ quality of life. Patients with epilepsy also have an increased risk of psychiatric disease: 20% to 40% of patients are affected by these conditions.3 Furthermore, psychiatric symptoms often do not present spontaneously, which makes it necessary to apply a range of scales for quantifying these symptoms and to consider the potential influence of antiepileptic drugs (AED).4
Lacosamide (LCM) is a third-generation AED used as an adjuvant therapy for focal epilepsy.5 Clinical trials have shown LCM to be safe from the perspective of behavioural disorders, although this conclusion should be confirmed by further observational clinical studies employing a wider range of doses and longer follow-up periods.4,6
The main aim of the present study is to evaluate the effect of adjuvant therapy with LCM on mood and quality of life in patients with epilepsy in everyday clinical practice at a series of hospitals in Alicante and Murcia. Patients were followed up for 12 months with the Quality of Life in Epilepsy Inventory-10 (QOLIE-10),7 the Hospital Anxiety and Depression Scale (HADS),8 and the Barratt Impulsiveness Scale (BIS-11).9 As a secondary objective, we analysed the drug's effectiveness and tolerability.
Patients and methodsWe performed a prospective, observational, multi-centre study over a period of one year at the following 11 hospitals in the provinces of Alicante and Murcia: Hospital General, Alicante; Hospital General, Elche; Hospital Vega Baja, Orihuela; Hospital Virgen de la Salud, Elda; Hospital Marina Baja, Villajoyosa; Hospital IMED-Levante, Benidorm; Hospital de Denia-Marina Salud, Denia; Hospital Virgen de la Arrixaca, Murcia; Hospital Comarcal del Noroeste, Caravaca de la Cruz; and Hospital Los Arcos del Mar Menor, San Javier. Patients were recruited between February 2013 and July 2014, were aged over 15, had been diagnosed with focal epilepsy, were receiving at least one AED, and required their treatment to be modified with the prescription of LCM, as per each centre's clinical protocols. At the baseline visit, data were gathered on the following epidemiological variables: sex, age, comorbidities, seizure type and location,10 disease progression, seizure frequency over the previous year, AEDs used at the time of study inclusion, AED dosage, other drugs, number of AEDs previously used, and initial LCM dose. At this initial visit, patients also completed the validated Spanish-language versions of the QOLIE-10, HADS, and BIS-11 questionnaires.7–9 At follow-up consultations at 3, 6, and 12 months, patients completed the same questionnaires, and data were collected on LCM dose, number of seizures, ADRs, and other AEDs used. The study complies with the standards of the research ethics committee; all patients gave written informed consent before receiving LCM. Statistical analysis was performed with SPSS version 19.0, with statistical significance set at P<.05. We performed a descriptive study, with qualitative variables expressed as absolute frequencies and percentages, and quantitative variables expressed as means and standard deviations (SD) or as medians with the 25th and 75th percentiles, and the number of valid cases. The last-observation-carried-forward method was used in cases where data were not available. Quality of life and mood trends were studied using the Friedmann test; initial and final values were compared using the Wilcoxon signed rank test. To analyse effectiveness, we identified seizure-free patients and responders (patients with a ≥50% reduction in the number of seizures) and studied trends in QOLIE-10, HADS, and BIS-11 scores in these groups using the Friedmann test and the Wilcoxon signed rank test; intergroup comparisons were made using the Mann–Whitney U test. We created tables identifying patients’ ADRs, their percentages of occurrence, and frequencies, and studied trends in quality of life and mood as a function of presence of ADRs using the Friedman test and the Wilcoxon signed rank test; the Mann–Whitney U test was used for intergroup comparisons.
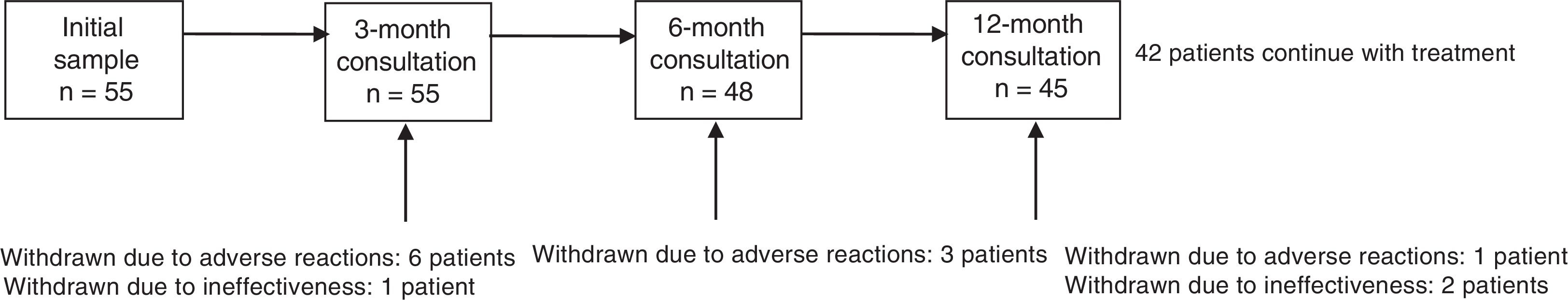
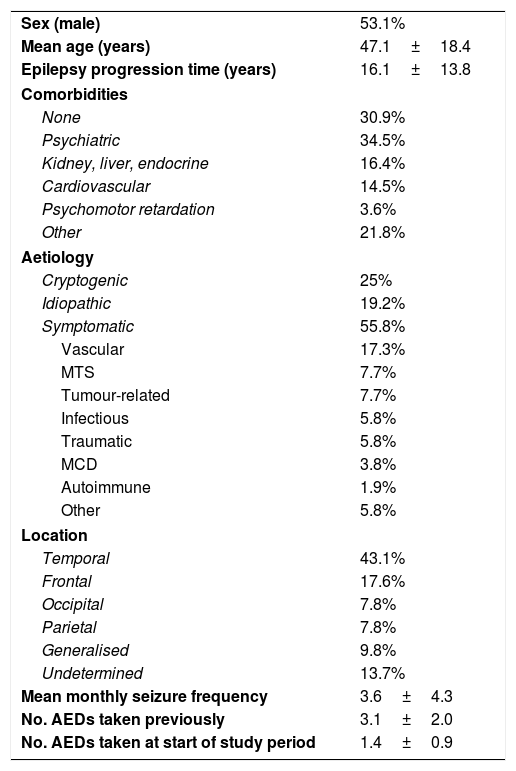
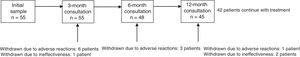
ResultsThe sample included 55 patients (mean age, 47.1±18.4 years; 53.1% men). Mean disease progression time was 16.1±13.8 years; 69.1% of patients had comorbidities, of which psychiatric disease was the most frequent (34.5%). By aetiology, symptomatic epilepsy was the most frequent (55.8%), followed by cryptogenic (25%) and idiopathic epilepsy (19.2%). The most frequent reason for study inclusion was poor seizure control (75%). Mean seizure frequency over the 12 months prior to onset of treatment with LCM was 3.6±4.3 seizures per month (median, 2). Patients had used a mean of 3.1±2 AEDs (median: 3) before starting LCM. At onset of treatment with LCM, 49.1% of patients were using AEDs; the most common drugs were levetiracetam (38.2%) and valproate (30.9%); 40% of patients were using at least one sodium channel blocking AED (Table 1). There was no significant difference in LCM dose over the study period (267.7±94.8mg at 12 months; range, 100-400mg). At the end of the study period, 42 patients (76.4%) continued taking LCM. Thirteen patients stopped using the drug: 10 (18.2%) due to ADRs and 3 (5.5%) due to ineffectiveness. The 12-month retention rate was 81.8% (95% confidence interval [CI], 70.7-92.2). Fig. 1 shows treatment adherence over the study period.
Clinical and demographic data on the sample.
| Sex (male) | 53.1% |
| Mean age (years) | 47.1±18.4 |
| Epilepsy progression time (years) | 16.1±13.8 |
| Comorbidities | |
| None | 30.9% |
| Psychiatric | 34.5% |
| Kidney, liver, endocrine | 16.4% |
| Cardiovascular | 14.5% |
| Psychomotor retardation | 3.6% |
| Other | 21.8% |
| Aetiology | |
| Cryptogenic | 25% |
| Idiopathic | 19.2% |
| Symptomatic | 55.8% |
| Vascular | 17.3% |
| MTS | 7.7% |
| Tumour-related | 7.7% |
| Infectious | 5.8% |
| Traumatic | 5.8% |
| MCD | 3.8% |
| Autoimmune | 1.9% |
| Other | 5.8% |
| Location | |
| Temporal | 43.1% |
| Frontal | 17.6% |
| Occipital | 7.8% |
| Parietal | 7.8% |
| Generalised | 9.8% |
| Undetermined | 13.7% |
| Mean monthly seizure frequency | 3.6±4.3 |
| No. AEDs taken previously | 3.1±2.0 |
| No. AEDs taken at start of study period | 1.4±0.9 |
AED: antiepileptic drug; MCD: malformation of cortical development; MTS: mesial temporal sclerosis.
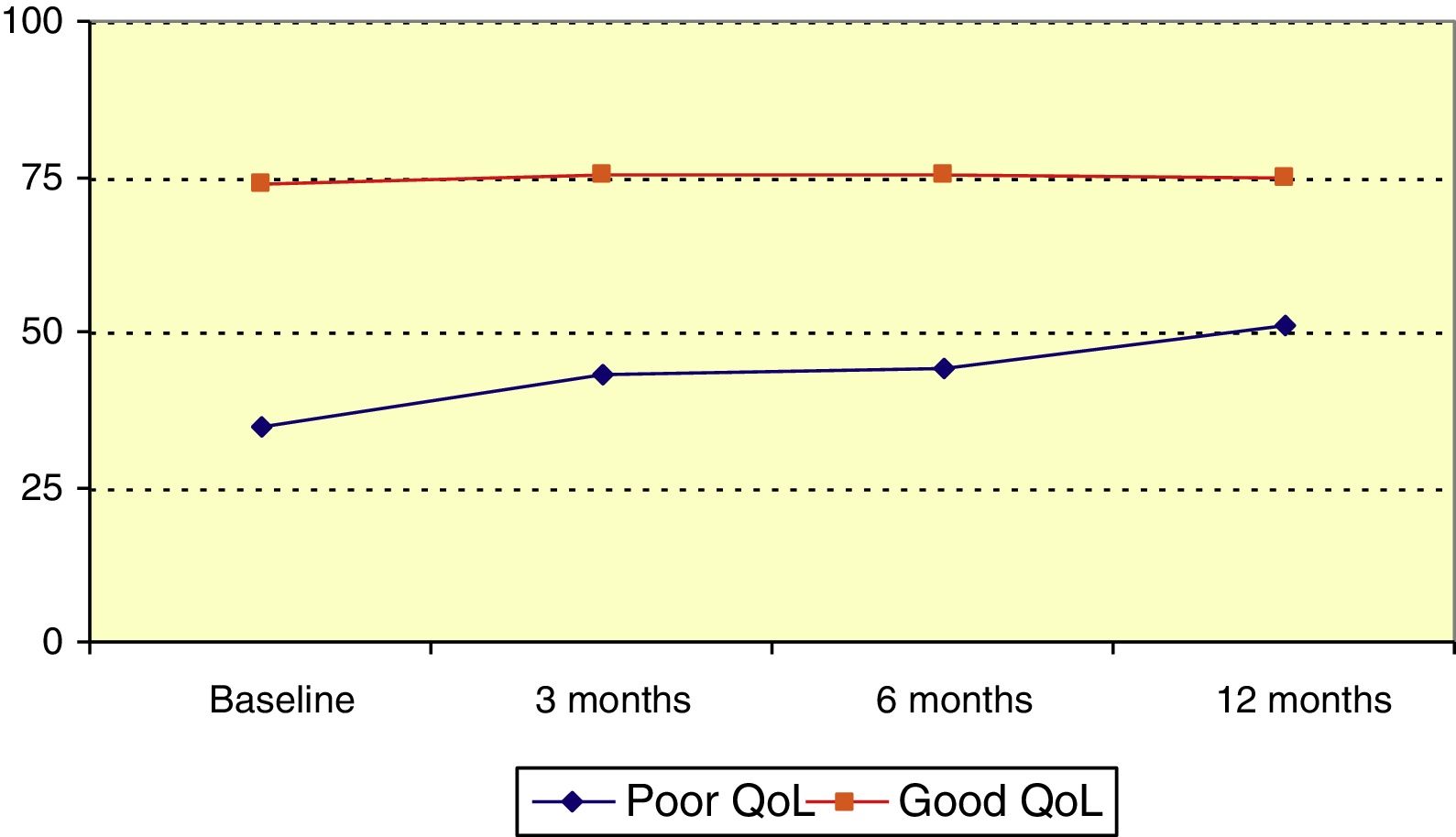
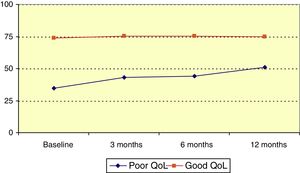
Mean QOLIE-10 score at baseline was 60.3 (median, 65), and rose over the follow-up period (P=.023, Friedman test); this increase was statistically significant with respect to the baseline score (P<.001, Wilcoxon signed rank test). Patients were divided into 2 groups according to QOLIE-10 score: poor quality of life (0-50) and good quality of life (51-100). The poor quality of life group accounted for 34.5% of patients before onset of LCM treatment; QOLIE-10 score increased significantly among members of this group over the follow-up period (P=.021, Friedman test), as illustrated in Fig. 2.
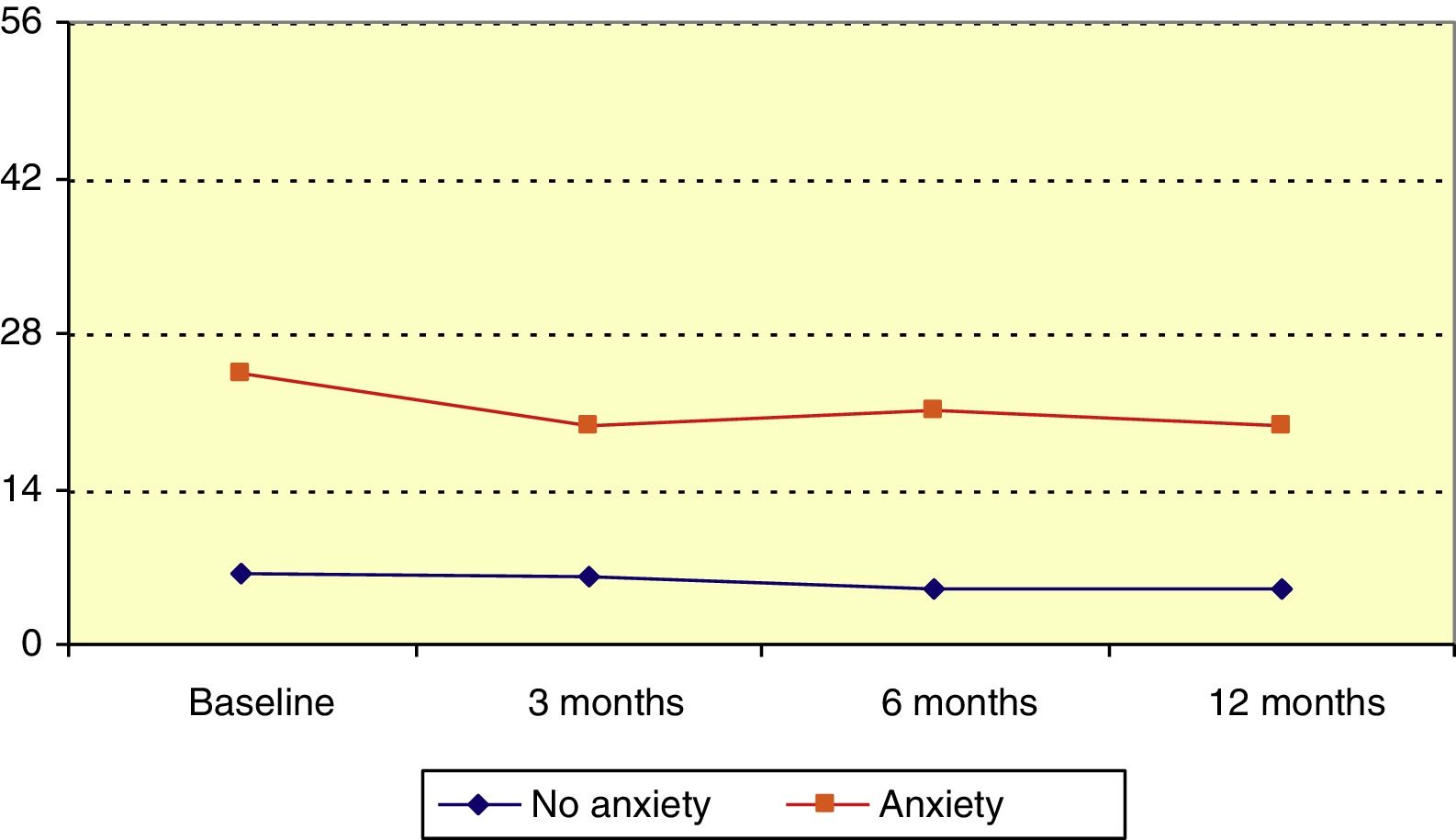
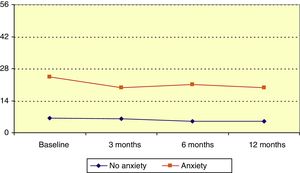
Hospital Anxiety and Depression Scale: anxietyMean HADS score for anxiety at baseline was 12.3 (median, 9). Patients’ scores decreased significantly over the follow-up period (P=.006, Friedman test); this change was statistically significant with respect to the baseline score at 3, 6, and 12 months (P=.045, P=.014, P=.020, respectively; Wilcoxon signed ranks test). Patients were divided into 3 groups according to HADS anxiety score: no anxiety (0-7), probable anxiety (8-10), and anxiety (11-21). At the beginning of the study, 32.7% of patients were in the latter category. In the anxiety group, HADS anxiety score reduced significantly over the follow-up period (P=.045, Friedman test), as illustrated in Fig. 3.
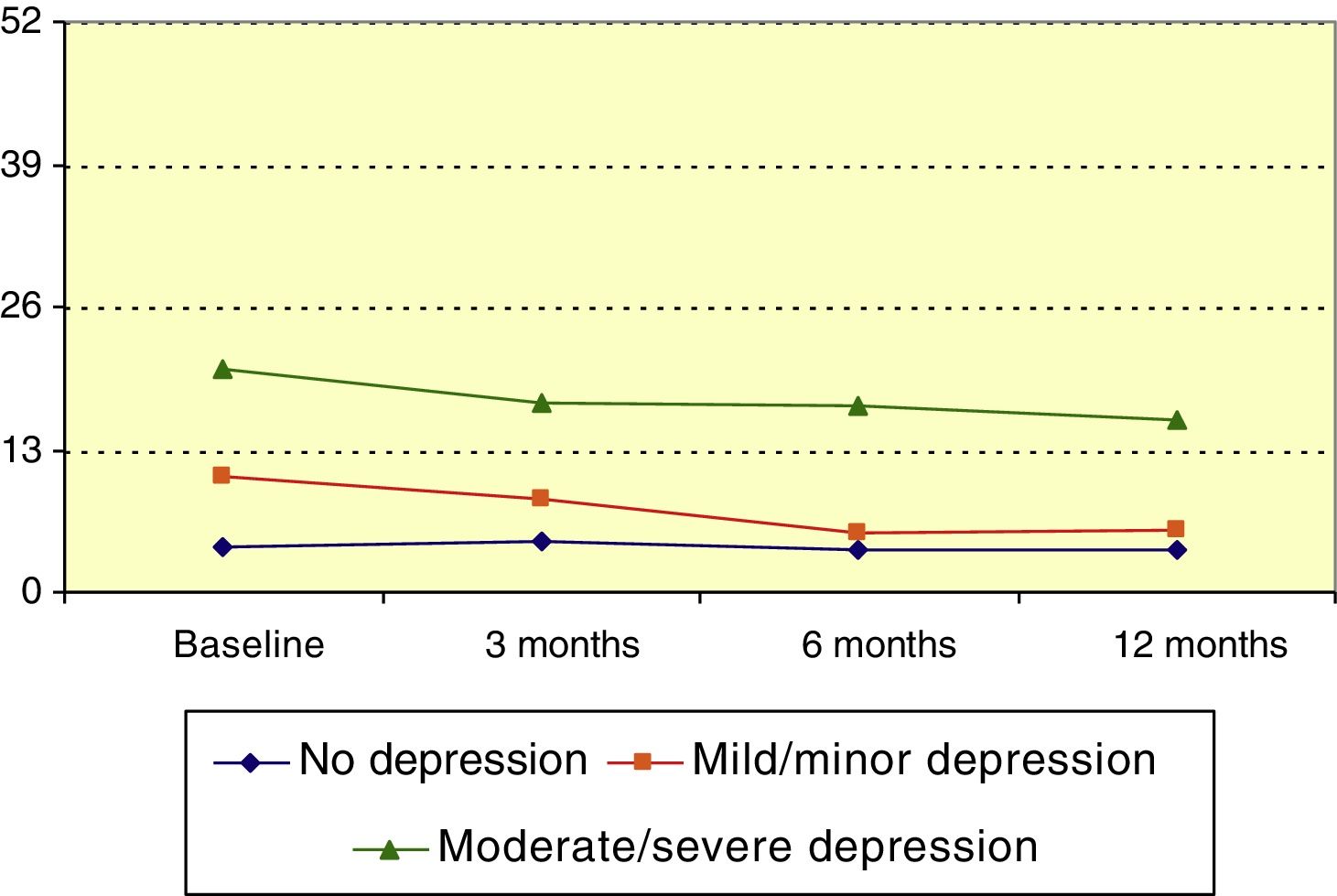
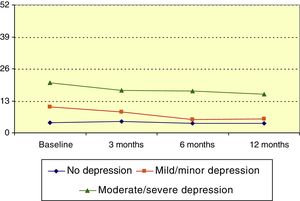
Hospital Anxiety and Depression Scale: depressionMean HADS score for depression at baseline was 9.1 (median, 7). HADS depression score significantly decreased over the course of the follow-up period (P<.001, Friedman test); this change was statistically significant with respect to the baseline score at 3, 6, and 12 months (P=.023, P<.001, P<.001, respectively; Wilcoxon signed ranks test). Patients were divided into 3 groups according to HADS depression score: no depression (0-7), mild depression (8-13), and moderate to severe depression (14-21). The mean score decreased over the study period among members of the mild depression and moderate to severe depression groups (P<.01 and P=.031, respectively; Friedman test), as shown in Fig. 4.
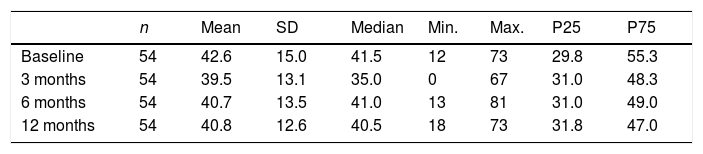
Barratt Impulsiveness Scale-11No significant difference was observed in BIS-11 score over the follow up period (P=.303, Friedman test), as shown in Table 2.
BIS-11 scores for impulsiveness over the study period.
| n | Mean | SD | Median | Min. | Max. | P25 | P75 | |
|---|---|---|---|---|---|---|---|---|
| Baseline | 54 | 42.6 | 15.0 | 41.5 | 12 | 73 | 29.8 | 55.3 |
| 3 months | 54 | 39.5 | 13.1 | 35.0 | 0 | 67 | 31.0 | 48.3 |
| 6 months | 54 | 40.7 | 13.5 | 41.0 | 13 | 81 | 31.0 | 49.0 |
| 12 months | 54 | 40.8 | 12.6 | 40.5 | 18 | 73 | 31.8 | 47.0 |
P: percentile; SD: standard deviation.
The effectiveness of the treatment was assessed using the percentage of seizure-free patients and responders (≥50% reduction in number of seizures). The percentage of patients who were seizure-free increased over the follow-up period: 32.1% of patients were seizure-free at 3 months (95% CI, 18.6%-45.6%), 48.1% at 6 months (95% CI, 33.9%-62.4%), and 51.9% at 12 months (95% CI, 37.6%-66.1%). Significant improvements were observed among seizure-free patients in the QOLIE-10 (P=.016, Friedman test), HADS scores for anxiety and depression (P<.001, Friedman test), and BIS-11 (P=.002, Friedman test).
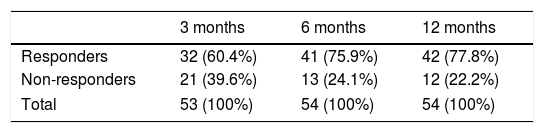
A total of 60.4% of patients were identified as responders at 3 months from treatment onset (95% CI, 46.3%-74.5%), increasing to 75.9% at 6 months (95% CI, 63.6%-88.3%) and 77.8% at 12 months (95% CI, 65.8%-78.8%), as shown in Table 3. Significant improvements were observed among responders in the QOLIE-10 (P=.004, Friedman test) and in the HADS scores for anxiety and depression (P=.002 and P<.001, respectively; Friedman test). No significant differences were observed in the BIS-11 (P=.102, Friedman test).
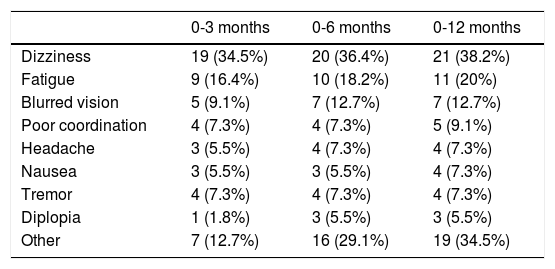
Table 4 lists the most common ADRs, with the most frequent being dizziness (38.2%), fatigue (20.0%), and blurred vision (12.7%). Most of these reactions were mild; the drug was withdrawn due to ADRs in 10 cases (18.2%). ADRs were not associated with significant differences in scores for any scale.
Classification of adverse reactions during follow-up.
| 0-3 months | 0-6 months | 0-12 months | |
|---|---|---|---|
| Dizziness | 19 (34.5%) | 20 (36.4%) | 21 (38.2%) |
| Fatigue | 9 (16.4%) | 10 (18.2%) | 11 (20%) |
| Blurred vision | 5 (9.1%) | 7 (12.7%) | 7 (12.7%) |
| Poor coordination | 4 (7.3%) | 4 (7.3%) | 5 (9.1%) |
| Headache | 3 (5.5%) | 4 (7.3%) | 4 (7.3%) |
| Nausea | 3 (5.5%) | 3 (5.5%) | 4 (7.3%) |
| Tremor | 4 (7.3%) | 4 (7.3%) | 4 (7.3%) |
| Diplopia | 1 (1.8%) | 3 (5.5%) | 3 (5.5%) |
| Other | 7 (12.7%) | 16 (29.1%) | 19 (34.5%) |
Our understanding of psychiatric disorders associated with epilepsy has expanded in recent years; however, it is difficult to demonstrate a causal relationship between an AED and changes in mood or quality of life.3,4,11 The major problem in assessing the effect of these drugs on behaviour is the fact that the majority of studies have been conducted in diverse populations, with polymedicated patients, wide variation in dosage and study duration, and short follow-up periods.4
The LAM study prospectively analyses the impact of LCM on mood and quality of life in a sample of 55 patients in everyday clinical practice, with an extended follow-up period. To that end, we employed widely-used, validated Spanish-language scales, which provide an objective assessment of patients’ symptoms. The QOLIE-10 questionnaire reliably measures the health-related quality of life of patients with epilepsy.7 The self-administered HADS has high validity and reliability for detecting anxiety and depression disorders,8 and the BIS-11 can identify patterns of impulsive behaviour in the long term.9 We observed considerable improvements in most of these scales over the follow-up period, particularly in patients with poorer initial well being and emotional status; these improvements appear to be correlated with seizure control. In view of this, we should emphasise the high percentage of patients receiving monotherapy with levetiracetam or valproate at the beginning of the study period. These broad-spectrum drugs are used to treat both focal and generalised epilepsy, are effective against several types of seizures, and have recognised psychoaffective effects.4 Our results appear to show that adjuvant therapy with LCM may counteract ADRs to levetiracetam and strengthen the effects of valproate as a mood stabiliser. The objective of AED treatment has traditionally been to reduce seizure frequency; mood disorders have been treated with less importance. However, this trend is changing and physicians are searching for effective drugs which also improve or at least do not exacerbate behavioural disorders associated with epilepsy.4,11 Various studies have reported a favourable cognitive side effect profile for adjuvant treatment with LCM, which shows fewer cognitive ADRs than topiramate, performing similarly to lamotrigine.12 The drug is safe and potentially beneficial for epileptic patients with mood disorders13: its anxiolytic properties improve symptoms of depression.14 Our study appears to support this, as we observed no exacerbation of anxiety or depression, nor appearance of such paradoxical reactions of stimulation as impulsive behaviour, despite 34.5% of patients having a psychiatric disorder before receiving LCM.
We also observed a high percentage of seizure-free patients (51.9%) and responders (77.8%), with 81.8% of patients adhering to the treatment. The high percentage of responders may be due to our patients being less refractory than those included in other studies. A total of 36.9% of our patients had previously used one or 2 AEDs, and the mean number of seizures per month prior to the introduction of LCM was 3.6±4.3 (median, 2); this rate is very similar to those reported in the GALACO,15 REALLY,16 and VITOBA17 studies, but lower than those reported in the RELACOVA18 and LACO-EXP19 studies (18.7 and 10.5, respectively). However, our study did include patients with highly refractory epilepsy (10.9% of patients were unresponsive to 6 AEDs), our results are therefore significant in terms of effectiveness and freedom from seizures. LCM also displayed a good safety profile, with patients displaying only mild ADRs, similar to those reported in other studies.11,15–19 One limitation of the LAM study may be its design: this was a prospective, observational study, in which the researchers were not blinded during evaluation; it also lacked a control group. These issues may have introduced biases.11,15
In conclusion, we have observed that LCM has a positive effect on epilepsy-associated behavioural disorders, improving symptoms of anxiety and depression, as well as patients’ quality of life. Patients with a poorer initial status showed more marked improvements. The drug has a good safety and tolerability profile. In everyday clinical practice, we not only need AEDs for seizure control, but also approaches that treat the condition from a broader perspective, significantly improving quality of life for patients with epilepsy.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Patricia Santagueda, Laura Betancourt, and Vanessa Llorca for their technical assistance in performing this study.
Please cite this article as: Alfaro A, Asensio M, García-Escrivá A, Medrano V, Salom JM, Tortosa D, et al. Estudio LAM: conducta y calidad de vida en pacientes diagnosticados de epilepsia tratados con lacosamida. Neurología. 2019;34:1–6.