Cardioembolic stroke is associated with poorer outcomes. Prevention is based on oral anticoagulant (OAC) therapy. Haemorrhage is the main complication of OACs, which are sometimes ineffective.
Patients and methodsWe retrospectively reviewed 1014 consecutive patients who suffered an ischaemic stroke between 2011 and 2013, analysing those who were receiving OAC treatment at stroke onset (107 patients in total) with special attention to aetiology, outcomes, and INR value in the acute phase.
ResultsThe mean age (SD) was 71.9 (10) years. Patients had been treated with OACs for 5.9 (5.5) years; 98.1% of them were being treated for heart disease. INR was <2 in 77 patients (72%), and 30 patients (28%) had an INR≥2. Nine patients (8.4%) had INR values within the therapeutic range. According to TOAST classification criteria, 88.8% of strokes were cardioembolic and 1.9% were atherothrombotic.
Anticoagulation therapy was discontinued in 48 patients (44.9%) due to haemorrhagic transformation (24 patients), extensive infarction (23), or endarterectomy (1). Therapy was resumed in 24 patients (50%) after a mean lapse of 36 days. This was not possible in the remaining patients because of death or severe sequelae.
New OACs (NOACs) were prescribed to 9 patients (18.7% of all potential candidates). At 3 months, patients with INR>1.7 in the acute phase exhibited better outcomes than patients with INR≤1.7 (mRS 0-2 in 62% vs 30.8%; death in 10% vs 38.4%; P=.0004).
ConclusionsSome patients taking OACs suffer ischaemic strokes that are usually cardioembolic, especially if INR is below the therapeutic range. OACs can be resumed without complications, and NOACs are still underused. Despite cases in which treatment is ineffective, outcomes are better when INR is above 1.7 at stroke onset.
Los ictus cardioembólicos tienen peor pronóstico. Su prevención se basa en el tratamiento anticoagulante por vía oral (ACO), cuya principal complicación es el sangrado y, en ocasiones, su ineficacia.
Pacientes y métodosSe ha revisado retrospectivamente a 1.014 pacientes consecutivos que presentaron ictus isquémico entre 2011 y 2013. De ellos, hemos analizado a los 107 pacientes en tratamiento con ACO en el momento del ictus, con especial atención a su etiología, evolución y valour del INR en la fase aguda.
ResultadosLa edad media±desviación estándar fue de 71,9±10 años. Tomaba ACO desde hacía 5,9±5,5 años el 98,1% por cardiopatía. Setenta y siete pacientes (72%) tenían INR<2 y 30 (28%) un INR≥2. Se encontraban en rango terapéutico 9 pacientes (8,4%). La etiología según TOAST fue cardioembólica en el 88,8% y aterotrombótica en el 1,9%.
Se suspendió la anticoagulación en 48 pacientes (44,9%): 24 por transformación hemorrágica, 23 por infarto extenso y uno por endarterectomía. Se reintrodujo en 24 de ellos (50%), a los 36 días de media; en los restantes, no fue posible por fallecimiento o secuelas severas.
En 9 pacientes (18,7% de potenciales candidatos) se iniciaron nuevos ACO (NACO). La evolución a los 3 meses fue mejor si el INR en la fase aguda>1,7 respecto al INR≤1,7 (mRS 0-2: 62% vs. 30,8% y fallecimiento 10% vs. 38.4%; p=0,0004).
ConclusionesAlgunos pacientes con ACO presentan ictus isquémicos, en general cardioembólicos, especialmente si el INR es infraterapéutico. Se pueden reintroducir ACO sin complicaciones, siendo el uso de NACO todavía escaso. A pesar de su ineficacia, la evolución es mejor si INR>1,7 al inicio del ictus.
Cardioembolic strokes, the most frequent type of ischaemic stroke in some epidemiological studies, account for 20% to 30% of the total.1,2 They are associated with greater neurological impairment, higher rates of early mortality, and poorer functional outcome at discharge. As a result, this type of stroke has a great socio-economic impact.3
Prevention is based on oral anticoagulants (OAC), as shown in several clinical trials and meta-analyses; these agents reduce the risk of stroke by >60%,4 even in patients aged over 75.5,6
However, traditional OACs (warfarin, acenocoumarol) have some limitations. Regular follow-up visits are necessary to maintain the international normalised ratio (INR) within the therapeutic range, which is only achieved in 60% to 70% of cases according to population-based studies.7 Due to lack of adherence to treatment and drug-drug and food-drug interactions, patients taking OACs usually have below therapeutic levels7 and are, as a result, at a greater risk of stroke.8 In addition, OACs cause a number of complications, mainly haemorrhaging at different sites. As a result, the practice of prescribing these agents has been questioned.9 Several tools have been developed to assess the risk of haemorrhage and the risks and benefits of OAC treatment. One example is the HAS-BLED score,10 which has been shown to be the most useful, especially for assessing the risk of intracranial haemorrhages.11,12
The introduction of new oral anticoagulants (NOAC), which require no regular follow-up visits and are associated with a lower rate of interactions, may help optimise cardioembolic stroke prevention. Several clinical trials have shown that NOACs are as effective as OACs for preventing ischaemic events, besides which they cause fewer haemorrhagic complications.13–16 However, their higher cost and the lack of specific antidotes make clinicians hesitate to prescribe these agents.17
Our purpose was to analyse stroke aetiology, treatment effectiveness, and progression (especially in terms of INR values during the acute phase) of a cohort of patients experiencing an ischaemic stroke despite OAC treatment.
Patients and methodsWe retrospectively reviewed the records of 1014 patients admitted to our neurology department with a diagnosis of ischaemic stroke during a 3-year period (2011–2013). Of these, we analysed the patients who were being treated with OACs at stroke onset (107 patients, 10.6%). All these patients showed good treatment compliance according to their relatives.
For each patient, we gathered the following epidemiological variables: sex; age; cardiovascular risk factors (CVRF: smoking, arterial hypertension, diabetes mellitus, dyslipidaemia); presence of emboligenic heart disease; scores on the CHADS2,18 CHA2DS2-VASc,19 and HAS-BLED scales; duration of OAC treatment; INR during the acute phase; aetiology (TOAST20 criteria); and topographical location (Oxfordshire Community Stroke Project classification21). We also recorded treatment continuation or discontinuation and the eventual resumption in the latter case, and use of NOACs. Prognosis was evaluated using the modified Rankin Scale (mRS) scores at baseline, at discharge, and at 3 months.
Patients were considered to be within the therapeutic range for anticoagulation when their INR value was 2-3 for isolated atrial fibrillation (AF) and 2.5-3.5 in patients with a metallic heart valve.
The aetiology of stroke was determined with a vascular study (echo-Doppler study of the supra-aortic trunks and CT angiography or MRI angiography of the circle of Willis) and transthoracic echocardiogram. Patients were telemetrically monitored during their stay in the stroke unit. When necessary, patients also underwent a transoesophageal echocardiogram (TEE), 24-hour Holter monitoring, and an autoimmune profile. The echocardiogram (either transthoracic or transoesophageal) detected the presence, if any, of a right-to-left shunt.
The neurological examinations to calculate mRS and NIHSS scores at admission and at discharge were performed by neurologists in our stroke unit.
These variables were analysed using StatView statistical software version 10.0, which performed univariate and multivariate analyses with a significance level of α=0.05, the chi-square test for categorical variables, and the non-parametric Mann–Whitney U test for intervals.
In the regression analysis, the variables related to progression were adjusted for age, CVRF, NIHSS score, baseline mRS score, and whether patients had received thrombolytic therapy. An INR of 1.7 is the cut-off value for considering a patient to be under anticoagulant effects and eligible for primary or secondary rescue treatments. ROC curve analysis showed INR values close to that point.
ResultsWe collected data from 107 patients with a male/female ratio of 1.33:1. Mean hospitalisation time was 14.7 days (range, 2-107). Mean age (±SD) at diagnosis was 71.93±10.14 years; no significant differences were found between sexes.
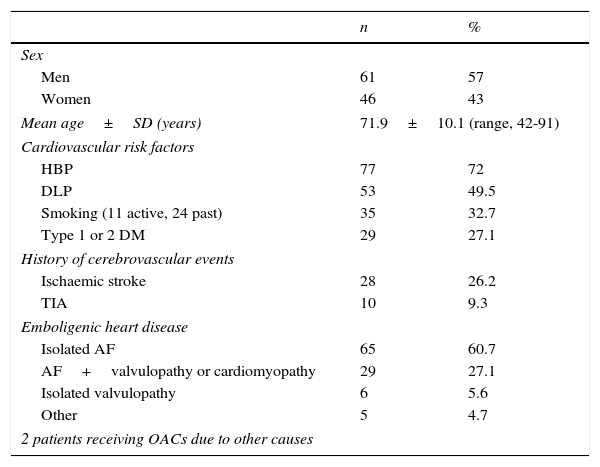
Nearly all the patients (98.1%) were receiving OACs due to emboligenic heart disease; most of them had AF (87.8%), whether isolated (n=65) or in association with other heart diseases (n=29; patients with mitral and/or aortic heart valve prostheses, metallic in most cases, or with a low ejection fraction). Eleven patients (10.3%) had one of the heart diseases mentioned previously or had experienced an acute myocardial infarction recently. Finally, 2 patients (1.9%) were being treated with OACs for other reasons (pulmonary thromboembolism or recurrent stroke with critical stenosis of the internal carotid artery). Only 2 patients were also receiving antiplatelet therapy with acetylsalicylic acid. Regarding CVRF, nearly 75% of the patients had hypertension and 50% had dyslipidaemia. Approximately one third were smokers (of these 31.4% were still actively smoking) and a fourth had type 1 or type 2 diabetes mellitus. Likewise, about a fourth had a history of ischaemic stroke (none of them had experienced more than one stroke previously or had a history of haemorrhagic stroke), and 9% had a history of transient ischaemic attack (TIA) (Table 1).
Demographic data and presence of cardiovascular risk factors/embolic heart disease in 107 patients with ischaemic stroke receiving OACs.
| n | % | |
|---|---|---|
| Sex | ||
| Men | 61 | 57 |
| Women | 46 | 43 |
| Mean age±SD (years) | 71.9±10.1 (range, 42-91) | |
| Cardiovascular risk factors | ||
| HBP | 77 | 72 |
| DLP | 53 | 49.5 |
| Smoking (11 active, 24 past) | 35 | 32.7 |
| Type 1 or 2 DM | 29 | 27.1 |
| History of cerebrovascular events | ||
| Ischaemic stroke | 28 | 26.2 |
| TIA | 10 | 9.3 |
| Emboligenic heart disease | ||
| Isolated AF | 65 | 60.7 |
| AF+valvulopathy or cardiomyopathy | 29 | 27.1 |
| Isolated valvulopathy | 6 | 5.6 |
| Other | 5 | 4.7 |
| 2 patients receiving OACs due to other causes | ||
TIA: transient ischaemic attack; DLP: dyslipidaemia; AF: atrial fibrillation; HBP: high blood pressure; n: number of patients.
The median score on the CHADS2 and CHA2DS2-VASc scales was 3 and 4, respectively. In fact, 93.5% of the patients scored≥2 (high risk of stroke) on the CHA2DS2-VASc scale. Regarding risk of bleeding, the median score on the HAS-BLED scale was 2.
At time of stroke, the patients had been receiving OACs for a mean of 5.86±5.51 years (no data available for 5 of the patients).
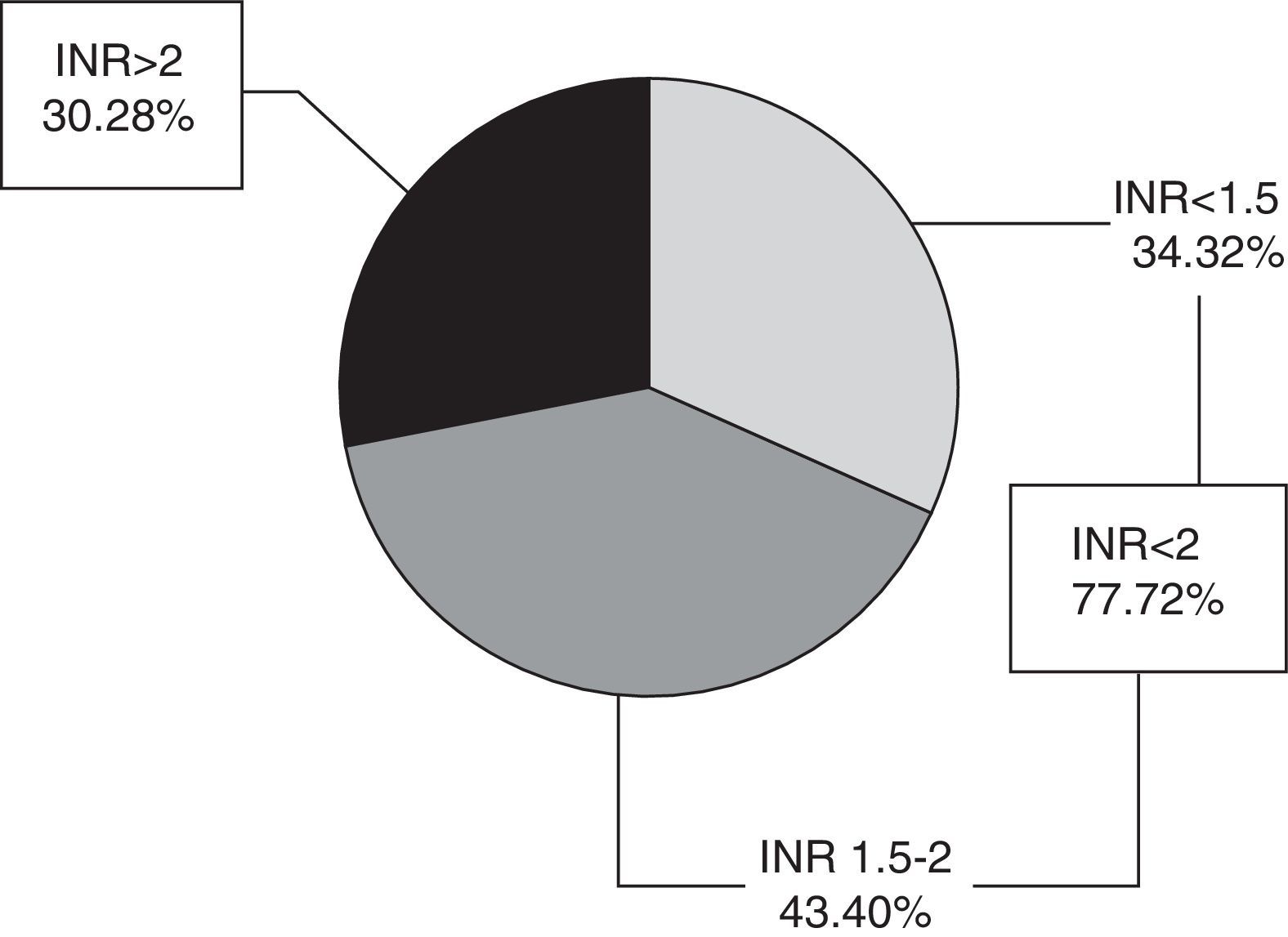
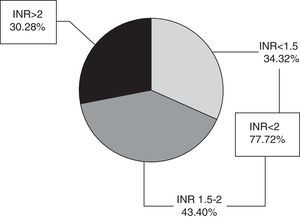
The mean INR during the acute phase was 1.94±0.94. Seventy-seven patients (72%) had an INR<2 (of whom 34 had an INR<1.5 and 43 an INR between 1.5 and 2) and 30 patients (28%) an INR≥2 (range, 2-5.36) (Fig. 1). Only 9 patients (8.4%) were within the therapeutic range at the time of stroke.
The median baseline NIHSS score was 14. Regarding the affected territory, the stroke affected the anterior circulation in 94 patients (87.9%) and the posterior circulation in 13 (12.1%).
Intravenous thrombolysis was indicated for 32 patients (INR≤1.7), 10 underwent rescue surgery, and 19 underwent surgery as primary treatment for stroke.
According to the TOAST criteria, strokes were cardioembolic in 95 patients (88.8%), and of undetermined aetiology in 9 (5 due to 2 possible causes, 4 due to an incomplete evaluation), all of which were outside the therapeutic range, atherothrombotic in 2 patients, and of undetermined cause in one (antiphospholipid syndrome). Fourteen percent of the patients underwent a TEE, which revealed in some cases a thrombus of the heart valve or in the left atrial appendage. None of the patients displayed significant plaque on the aortic arch or the right-to-left shunt. From a topographical viewpoint, most of the strokes (n=68) were classified as TACI (63.5%), 26 as PACI (24.3%), and 13 as POCI (12.1%).
The main complication was haemorrhagic transformation (31 patients, 29%); only 7 patients had PH1 or PH2 and 2 had subarachnoid haemorrhage. In 14 of the cases, haemorrhage occurred in the context of intravenous and/or mechanical thrombolysis and only 6 of the cases had an INR≥2.
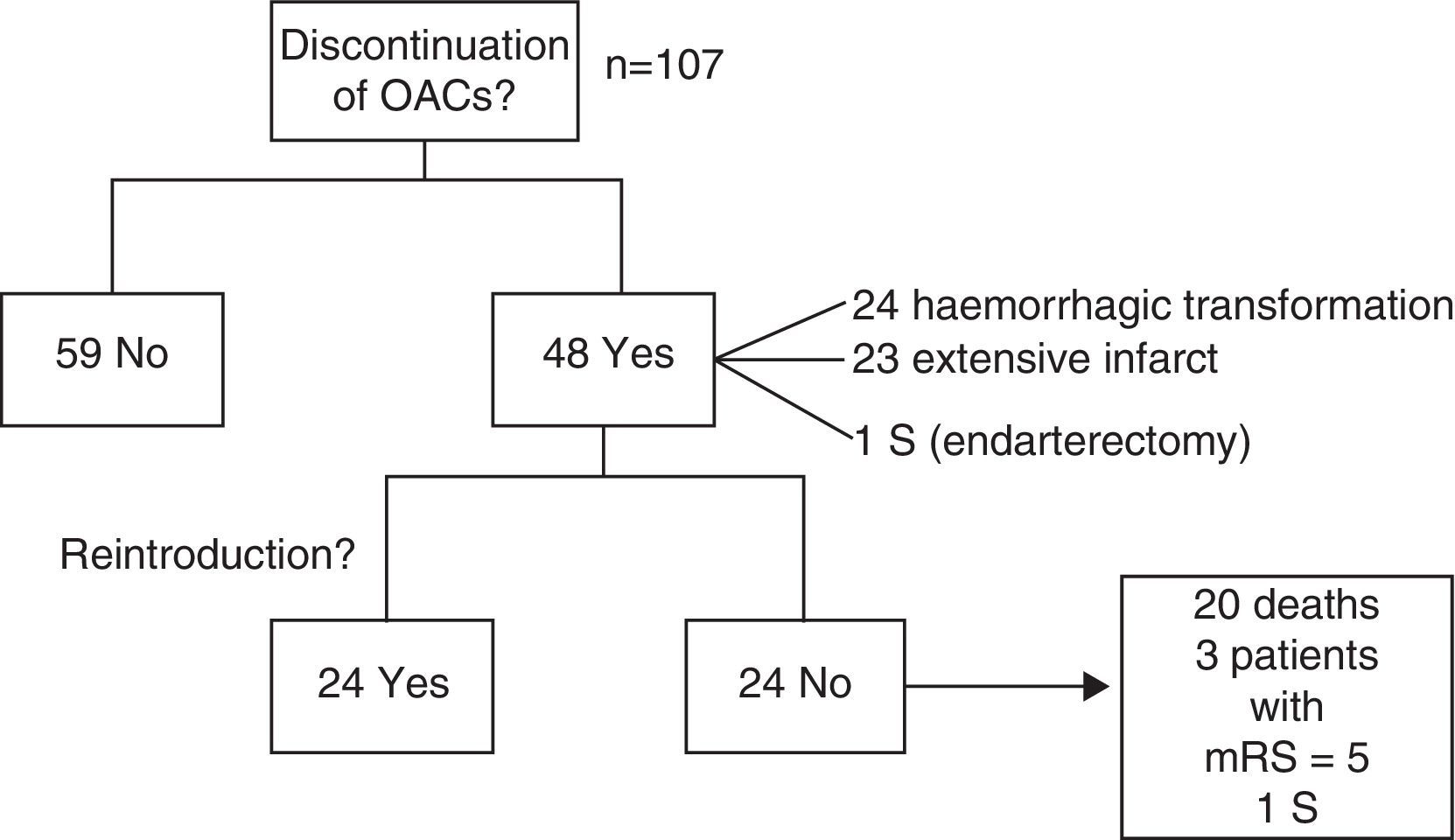
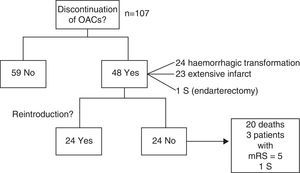
OAC treatment was maintained in 59 patients (55.1%) in the acute phase and discontinued in 48 (44.9%) due to haemorrhagic transformation (24 patients), extensive infarction (23), or surgery (1).
Twenty-four (50%) of the patients discontinuing OAC use resumed treatment without complications after a mean of 36 days (range, 5-180). In the remaining patients, OAC therapy was not resumed due to: death (20 patients, 41.7%; secondary to stroke in 12 patients), a mRS score=5 during follow-up (3 patients, 6.2%), and surgery (endarterectomy, one patient); in the latter case, stroke was considered to be atherothrombotic (Fig. 2).
Treatment was modified by introducing a NOAC (dabigatran in 6, rivaroxaban in 2, apixaban in one) to 9 of the 48 stroke survivors with non-valvular AF who were eligible (18.7%).
In addition, we evaluated the level of dependence at discharge and at 3 months and classified the patients according to their scores on the mRS as able to carry out daily living activities (mRS=0-2) or functionally dependent (mRS=3-5).
At discharge, 49 patients (45.8%) were functionally dependent; 13 (12.1%) had died during hospitalisation.
Eighty-nine patients were followed up after discharge during the 3 months following stroke (5 patients were lost to follow-up). During that period, 12 patients died (3 as a result of stroke and 9 due to other causes). Thirty of the remaining patients (28% of the initial series) remained functionally dependent.
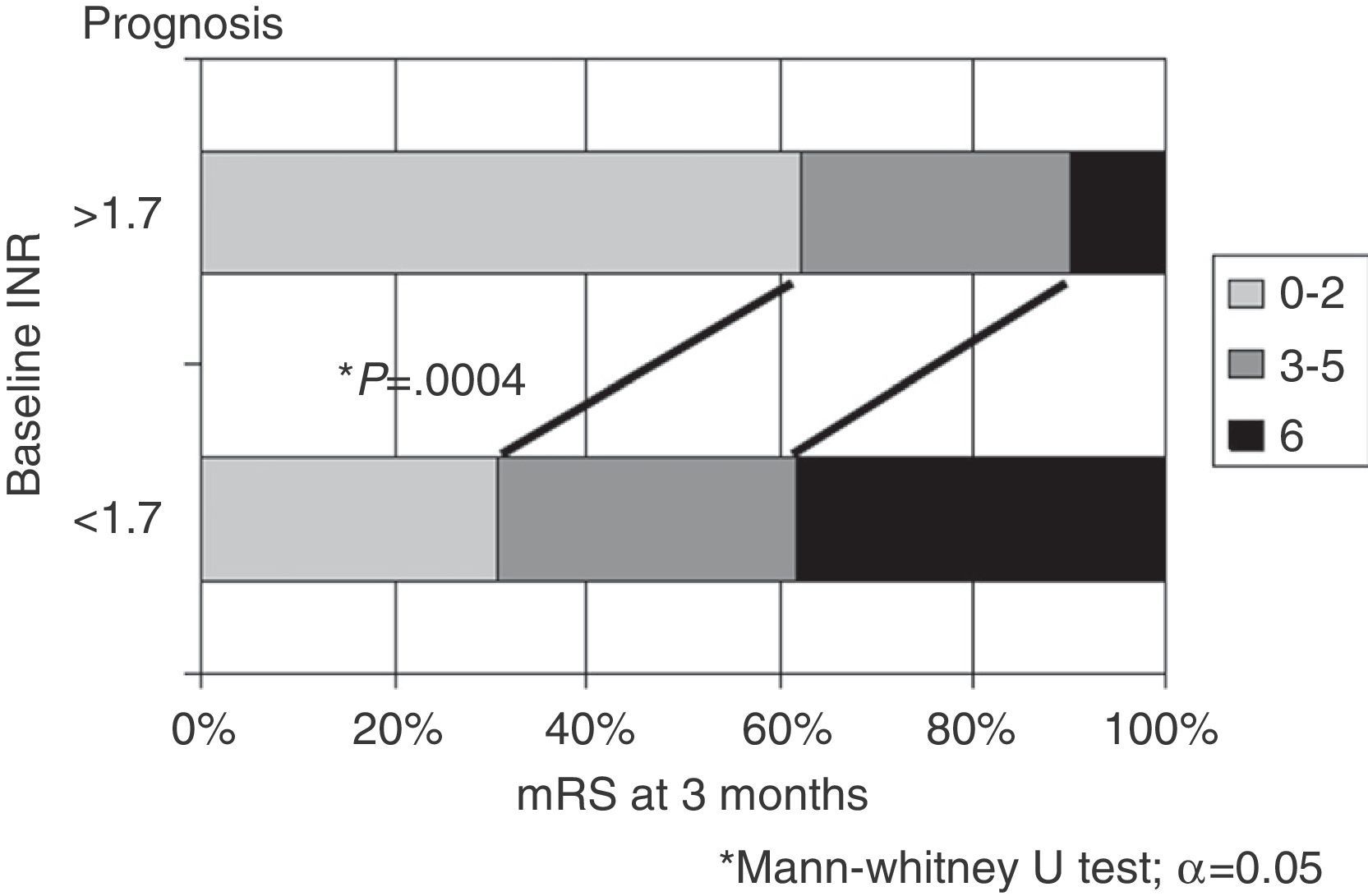
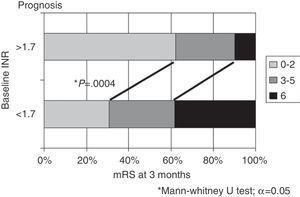
Considering INR values at time of stroke, greater differences are found when setting the cut-off point at 1.7. Outcomes were significantly better in patients with an INR≥1.7 than in those with an INR<1.7, in terms of both the percentage of patients with functional independence (62% vs 30.8%) and mortality rates (10% vs 38.4%; P=.0004) (Fig. 3). Analysing by topographical subtype, distribution by INR (<1.7 vs ≥1.7) showed no significant differences (TACI, 35 vs 33; PACI, 12 vs 14; POCI, 7 vs 6).
Considering a cut-off value of 2, which is the threshold for considering a patient within the therapeutic range in most cases, we found significant differences in outcomes, with better outcomes in the patients with an INR≥2 (P=.007).
During the follow-up period, none of the patients experienced another ischaemic event.
DiscussionAt time of stroke, nearly 75% of our patients showed INR values below the therapeutic range. Although all patients were reported to show good treatment compliance, it may not have been true in some cases. In any case, it is a well-known fact that it is difficult to maintain an adequate INR despite appropriate dosage of the OAC.7 We lack data on each patient's therapeutic range, which would have been a more objective measure.
The remaining 25% showed an INR≥2. In all these patients, except for one, we found no other cause for stroke. A considerable percentage of the patients had one or more CVRF; however, this did not translate into any findings that may indicate either atherothrombotic aetiology or undetermined aetiology for 2 possible causes. Therefore, and despite being within therapeutic levels, these patients had a cardioembolic stroke due to underlying heart disease. This contradicts the results of previously published studies in which a high percentage of the events were due to other aetiologies.22
We should highlight that few patients displayed thrombi on an artificial heart valve or in the left atrial appendage, and none of the patients showed significant plaque on the aortic arch or the right-to-left shunt. However, the number of patients undergoing TEE was low.
Most of the patients received OACs due to AF, especially non-valvular. OAC prescription was justified in nearly all of them considering their scores on the CHADS2 and CHA2DS2-VASc scales.
OAC treatment was maintained in slightly more than half of the patients due to the high risk of embolism as a result of the patients’ underlying heart diseases. OAC treatment was discontinued in patients with haemorrhagic transformation or extensive infarctions but was resumed without complications in less than 6 months, except for the patients who died or were left with very severe sequelae. These drugs should be started within 1-2 weeks after stroke, except for cases of extensive infarctions or other risk factors for bleeding; in such situations, administration of OACs should be delayed until clinical and radiological progression is seen.23 Before deciding to either maintain or resume OAC treatment, we evaluated CHA2DS2-VASc and HAS-BLED scores to identify patients with a higher risk of ischaemic events than of haemorrhagic events.
However, only 18.7% of the stroke survivors eligible for NOACs were prescribed these agents, despite OAC therapeutic failure in many cases and the recent studies supporting NOAC use. In our centre, the prescription of NOACs is discussed with the haematology department. Different NOACs have been approved for in-hospital protocols in the past years. As a result, these agents are prescribed increasingly frequently, a trend that is expected to continue.
Regarding outcomes, approximately a fourth of the patients died during the acute phase or the 3-month follow-up period, and an additional fourth were left functionally dependent at 3 months. These results agree with other studies reporting poorer prognosis in patients with cardioembolic stroke.3
A baseline INR>1.7 was associated with better functional and vital prognosis. According to a recent study, an INR≥2 is associated with favourable clinical outcomes.24 In our study, patients with an INR≥2 had a better prognosis; however, the difference was even more significant for a cut-off point of 1.7. Interestingly, many patients with an INR≤1.7 were treated with intravenous thrombolysis but showed no significant improvement compared to patients who did not receive this treatment.
According to the literature, patients taking OACs present smaller infarcts than those not receiving this treatment. Furthermore, an inverse correlation between the INR at admission and lesion volume in brain MR images has been described.25 We cannot corroborate this hypothesis since we did not measure lesion volume in our sample. We analysed the amount of TACI, PACI, and POCI for INR < or ≥1.7 and found that they were distributed homogeneously.
Likewise, OACs have been found to accelerate thrombolysis by inhibiting thrombotic activity and favouring fibrinolytic activity.26 This results in smaller and weaker thrombi which can be destroyed more easily.
Invasive procedures and complications in the form of haemorrhagic transformation, which can also affect progression, were distributed homogeneously between the 2 groups.
The limitations of our study are those inherent to retrospective studies. However, it was conducted in a single centre, which decreases the number of deviations from protocol and delivers a more homogeneous application of treatments and evaluations. In addition, as we only considered admitted patients, we did not study patients with TIA or stroke with low NIHSS scores, who are usually cared for in outpatient clinics or transferred to other centres. This may explain why none of our patients had a LACI and/or a lacunar stroke.
In conclusion, some patients experience an ischaemic stroke despite OAC treatment, especially when their INR values are below the therapeutic range; these strokes are cardioembolic, which supports studies reporting OAC failure.
OAC treatment may be resumed without complications in some cases, and NOAC use is still scarce. Despite the ineffectiveness of OACs, patients treated with these drugs show better progression when their INR>1.7 at stroke onset.
FundingThe authors have received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cano LM, Cardona P, Quesada H, Lara B, Rubio F. Ictus isquémico en pacientes en tratamiento anticoagulante por vía oral. Neurología. 2016;31:395–400.
Part of this study was presented orally at the 65th Annual Meeting of the Spanish Society of Neurology.