The management of antithrombotic therapy after intracerebral haemorrhage (ICH) in anticoagulated patients is not well defined. We analysed the risks and benefits of antiplatelet therapy (AG) against the resumption of anticoagulation with vitamin K antagonists (AVK) in a series of patients.
Material and methodsRetrospective study of ICH in anticoagulated patients was done. We registered demographic data, history of hypertension (HT), time of follow-up and new cerebral vascular events (ICH, stroke [IC]).
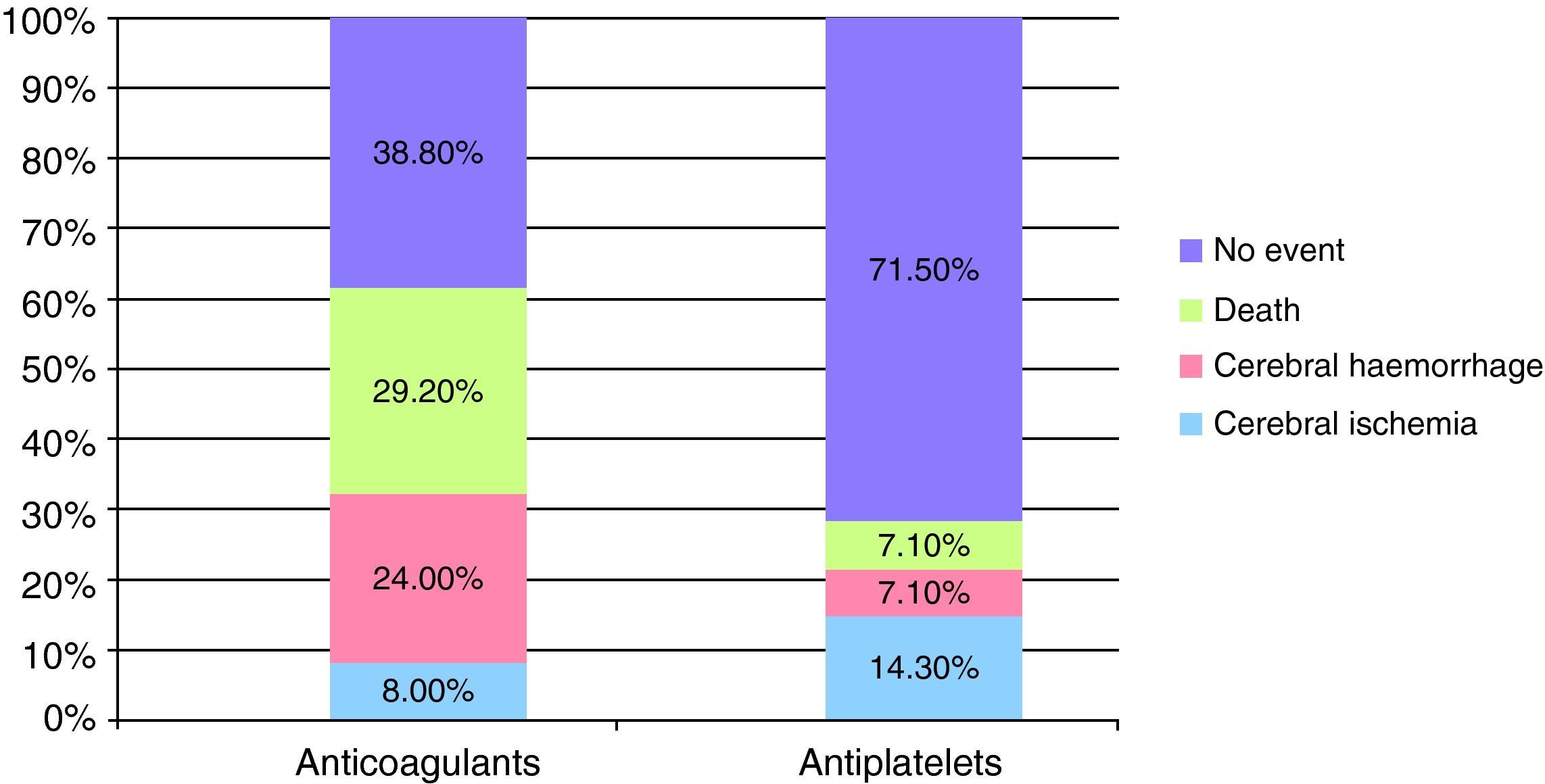
ResultsWe evaluated 88 patients, mean age 69±9 years, 50% men, 73% hypertensive. During the acute phase 18 patients died and the follow-up was lost in 31. Of the remaining (n=39), AVKs were resumed in 25 and changed to AG in 14. Comparing the characteristics of both groups, the anticoagulated group was younger (P=.005) and the embolic sources were more often of higher risk (P=.003). After an average follow-up of 54±31 months, the distribution of events was: IC (AVKs 8%, AG 14.3%, P=.6), ICH (AVKs 24%, AG 7.1%, P=.38), IC or ICH (AVKs 32%, AG 21.4%, P=.48) and death (AVKs 29%, AG 7.1%, P=.21). This trend of increased risk of new events in patients with AVKs was confirmed by Kaplan–Meier curves, although without statistical differences.
ConclusionsRestarting AVK treatment after ICH in anticoagulated patients could increase the risk of new bleeding events and mortality. Prospective studies are needed to define a better and appropriate antithrombotic therapy after ICH related with anticoagulation.
El manejo del tratamiento antitrombótico tras una hemorragia intracerebral (HIC) en pacientes anticoagulados no está bien definido. Analizamos los riesgos y beneficios de la antiagregación (AG) frente al reinicio de la anticoagulación con antagonistas de la vitamina K (AVK) en una serie de pacientes.
Material-métodosEstudio retrospectivo de HIC en pacientes anticoagulados. Se registraron datos demográficos, antecedentes de hipertensión arterial, tiempo de seguimiento y nuevo evento vascular cerebral (HIC, infarto cerebral [IC]).
ResultadosSe evaluó a 88 pacientes, de edad media 69±9 años, 50% varones, 73% hipertensos. Durante la fase aguda fallecieron 18 pacientes y el seguimiento se perdió en 31. De los restantes (n=39), se reinició AVK en 25 y se cambió a AG en 14. Comparando las características de ambos grupos, el grupo anticoagulado era de menor edad (p=0,005) y las fuentes cardioembólicas eran con mayor frecuencia de alto riesgo (p=0,003). Tras un seguimiento promedio de 54±31 meses, la distribución de eventos fue: IC (grupo AVK 8%, grupo AG 14,3%, p=0,6); HIC (AVK 24%, AG 7.1%, p=0,38); IC o HIC (AVK 32%, AG 21,4%, p=0,48); muerte (AVK 29%, AG 7,1%, p=0,21). Esta tendencia de mayor riesgo de nuevos eventos en pacientes con AVK se confirmó mediante curvas de Kaplan-Meier, aunque sin significación estadística.
ConclusionesEl reinicio del tratamiento con AVK tras una HIC en pacientes anticoagulados podría aumentar el riesgo de nuevos eventos hemorrágicos y la mortalidad. Son necesarios estudios prospectivos, para definir mejor el tratamiento antitrombótico idóneo tras una HIC relacionada con la anticoagulación.
The use of vitamin K antagonists (VKA)1 as preventive treatment of cardioembolic ischaemic stroke has increased following the publication of clinical trials which demonstrated their efficacy. Moreover, the increased prevalence of atrial fibrillation (AF), probably associated with ageing in the population, has led to an increase in the use of anticoagulant treatment.2
Despite its unquestionable benefits in preventing stroke or systemic embolism, anticoagulant therapy also carries risks, the most prominent of which, due to its morbidity and mortality, is intracerebral haemorrhage (ICH). This is the most frequent occurrence and, when it takes place, is also more severe than in non-anticoagulated patients.3–6
Once the acute phase has been overcome, there is often a therapeutic dilemma about whether or not to restart anticoagulation or switch to an antiplatelet drug (AP). Both antithrombotic treatments have proven effective in reducing cerebral ischaemic events in patients with potentially cardioembolic diseases. However, anticoagulant therapy is more effective than antiplatelet treatment in the prevention of cardioembolic stroke (relative risk reduction of about 40%).1 Despite this increased protection, anticoagulant therapy also carries an increased risk of spontaneous or traumatic ICH recurrence.
In the present study we performed a retrospective analysis of the risks and benefits of antiplatelet therapy versus the restart of anticoagulation in a series of patients with ICH, related to treatment with VKA.
Material and methodsWe conducted a retrospective study of patients who were treated with VKAs and who suffered an ICH in the period between 1997 and 2007. Patients were identified from the database of the Thrombosis and Haemostasis Unit at Hospital de la Santa Creu i Sant Pau.
Follow-up data were obtained by reviewing the medical records of those patients who were monitored at the hospital.
Follow-up time was calculated in months, from the month of the ICH until the month in which the patient suffered a new cerebrovascular event, died, or the last clinical annotation was made. We excluded those patients for whom no hospital follow-up was available after the first haemorrhagic event.
For each patient we collected the clinical and demographic data following the ICH episode: age, sex, vascular risk factors (arterial hypertension [AHT], diabetes mellitus, dyslipidaemia [DLP] and history of smoking); history of previous embolism (systemic or cerebral [stroke]); heart pathology which lead to VKA treatment (atrial fibrillation, mechanical valve replacement or other); time elapsed from the start of anticoagulation until the ICH (months); international normalised ratio (INR) at the time of the intracerebral haemorrhagic event and location of the ICH (lobar vs deep-basal ganglia, brainstem or cerebellum).
Patients were divided into those who resumed VKA therapy and those who switched to AP treatment (acetylsalicylic acid, clopidogrel, the combination of both or other antiplatelet agents). Those patients who were prescribed VKA again, either during hospitalisation or during outpatient follow-up, were included in the restart group.
The final events assessed in the study were the recurrence of a new ICH episode and the onset of a cerebral stroke (CS) or transient ischaemic attack (TIA), both demonstrated by a cerebral CT or MRI scan, or death by either cause.
In the statistical analysis comparing the 2 groups we used the Student t test to compare means from independent samples for continuous variables. We dichotomised the remaining variables and used contingency tables with the chi squared test, defining P<.05 as a statistically significant difference. We also employed the Kaplan–Meier survival curves to study the distribution of events in the different groups during follow-up.
ResultsBetween 1997 and 2007, 88 patients who were following treatment with VKA suffered an ICH. The mean age of the sample was 69±9 years and 50% of patients were male. The distribution of vascular risk factors was as follows: 73% of patients were hypertensive, 36.5% were diabetic, 28.4% were dyslipidaemic and 9.5% were smokers.
A total of 18 patients died during the acute phase (20.4%) and 31 were lost to follow-up, so the present study examined a cohort of 39 patients. Baseline characteristics were similar between the group that was lost during follow-up and that which was finally analysed, with the only highlight being a greater proportion of diabetic patients in the group lost during follow-up (48.7% vs 22.9%; P=.008).
Regarding the indications for VKA treatment, 23 patients (59%) were taking it as a primary preventive treatment. Of these, 11 patients (47.8%) presented AF and 9 (39.1%) had a mechanical valve. The remaining 16 patients (41%) were undergoing treatment as secondary prevention. Of these, 13 had suffered a cerebral embolism (CS or TIA) and 3 had suffered systemic embolism. Most patients who received VKA as secondary prevention suffered AF (13 patients) and only 3 had a mechanical valve.
The time elapsed from the onset of VKA treatment until the haemorrhagic event was 48.64±53.8 months.
Once the acute phase was over, VKA treatment was resumed by 25 patients (64.1%) and changed to AP in 14 patients (35.9%). The treatment was chosen based on the decision of the responsible physician, with input from the family and the patient, but it was not a random or protocolised decision.
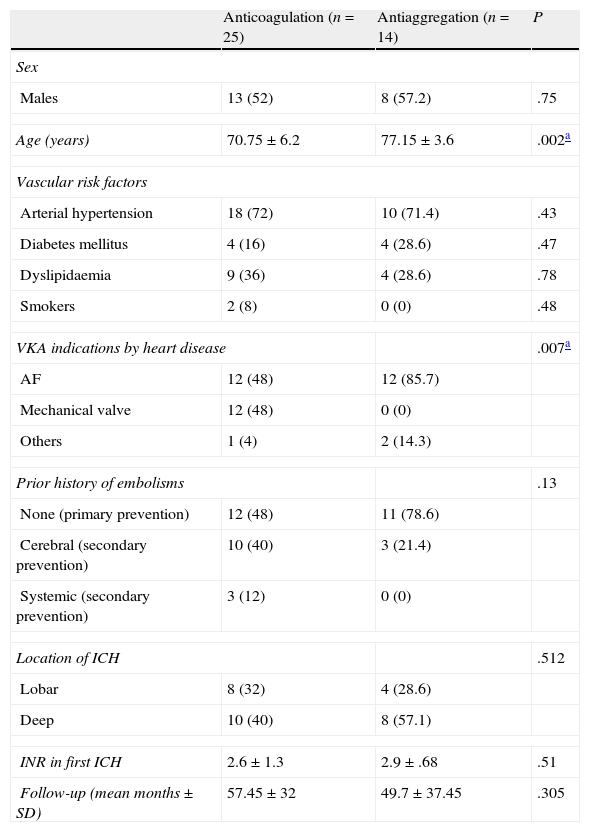
As seen in Table 1, patients who restarted VKA therapy were younger and the indication for VKA treatment (prior to the ICH) was mainly due to having a high level of risk of embolism (48% of patients used prosthetic heart valves), or selected as secondary prevention due to having experienced some form of systemic embolism in 52% of cases.
Clinical characteristics of the groups: restart of anticoagulation versus change to antiaggregation.
| Anticoagulation (n=25) | Antiaggregation (n=14) | P | |
| Sex | |||
| Males | 13 (52) | 8 (57.2) | .75 |
| Age (years) | 70.75±6.2 | 77.15±3.6 | .002a |
| Vascular risk factors | |||
| Arterial hypertension | 18 (72) | 10 (71.4) | .43 |
| Diabetes mellitus | 4 (16) | 4 (28.6) | .47 |
| Dyslipidaemia | 9 (36) | 4 (28.6) | .78 |
| Smokers | 2 (8) | 0 (0) | .48 |
| VKA indications by heart disease | .007a | ||
| AF | 12 (48) | 12 (85.7) | |
| Mechanical valve | 12 (48) | 0 (0) | |
| Others | 1 (4) | 2 (14.3) | |
| Prior history of embolisms | .13 | ||
| None (primary prevention) | 12 (48) | 11 (78.6) | |
| Cerebral (secondary prevention) | 10 (40) | 3 (21.4) | |
| Systemic (secondary prevention) | 3 (12) | 0 (0) | |
| Location of ICH | .512 | ||
| Lobar | 8 (32) | 4 (28.6) | |
| Deep | 10 (40) | 8 (57.1) | |
| INR in first ICH | 2.6±1.3 | 2.9±.68 | .51 |
| Follow-up (mean months±SD) | 57.45±32 | 49.7±37.45 | .305 |
AF, atrial fibrillation; ICH, intracerebral haemorrhage; INR, international normalised ratio; SD, standard deviation; VKA, vitamin K antagonists.
Data expressed as absolute number (percentage) or as mean±standard deviation.
There were no differences in the distribution of vascular risk factors or the location of the intracerebral haemorrhage between both groups.
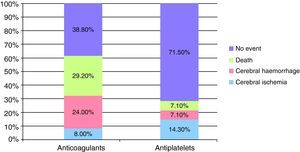
After a mean follow-up of 53.8 months (range 4–131 months) for both groups, 2 of the 25 patients (8%) who continued VKA treatment and 2 of the 14 patients (14.3%) who switched to AP treatment suffered a cerebral ischaemic event. As for new episodes of ICH, these were suffered by only 1/14 patients (7.1%) in the AP group compared to 6/25 patients (24%) in the VKA group. The mean INR value at the time of cerebral rebleeding was 3.5±1.7.
During follow-up, more patients died in the group which continued VKA treatment (7/25 patients [29.2%] versus 1/14 [7.1%] patients in the AP group). The causes of death in the group that restarted anticoagulant therapy were as follows: 2 patients died during the acute phase of a new cerebrovascular event (1 due to a new cerebral haemorrhage and the other due to cerebral ischaemia), the remaining 5 died due to other causes, not related to antithrombotic therapy. The death of the patient who had switched to antiplatelet therapy took place during the acute phase of a CS episode.
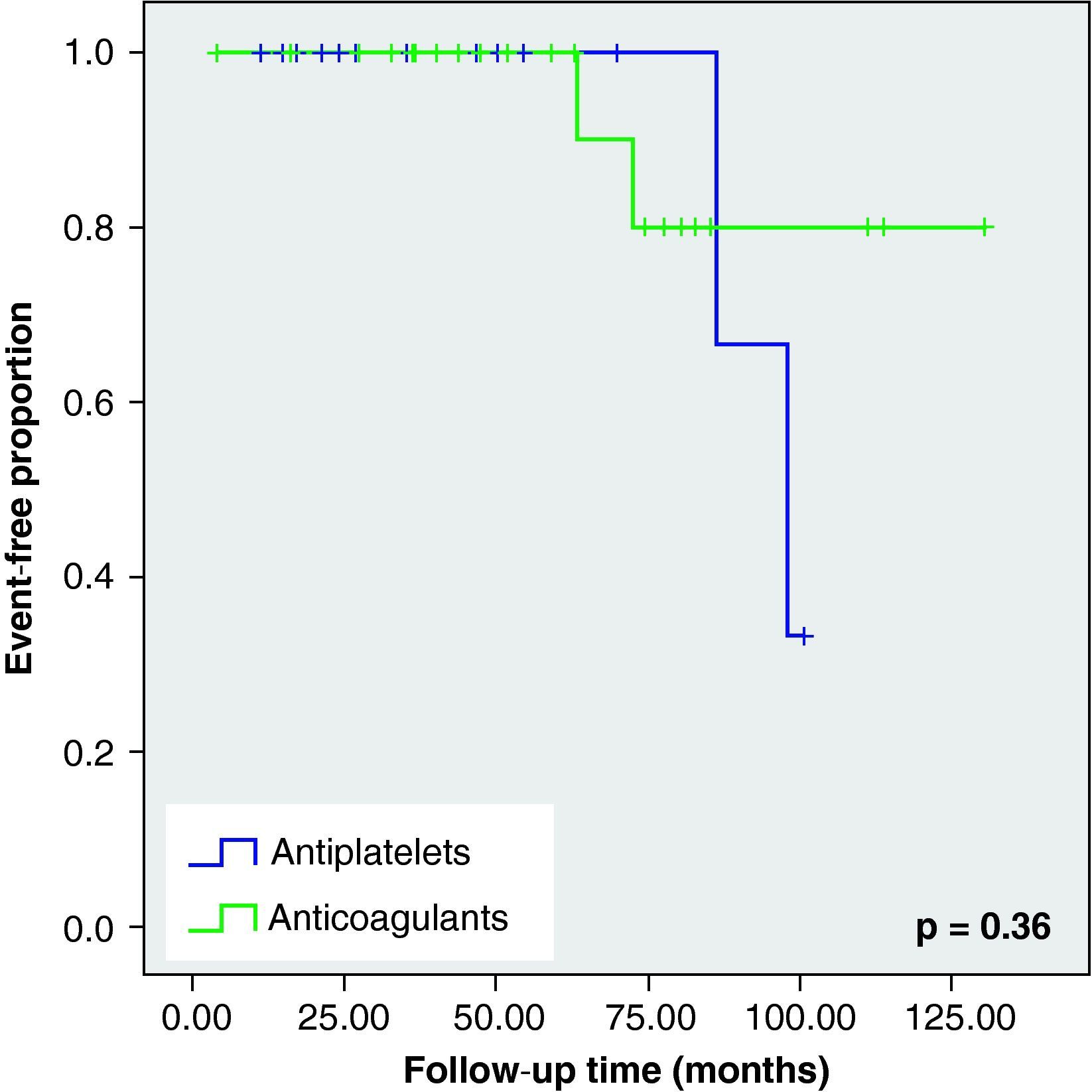
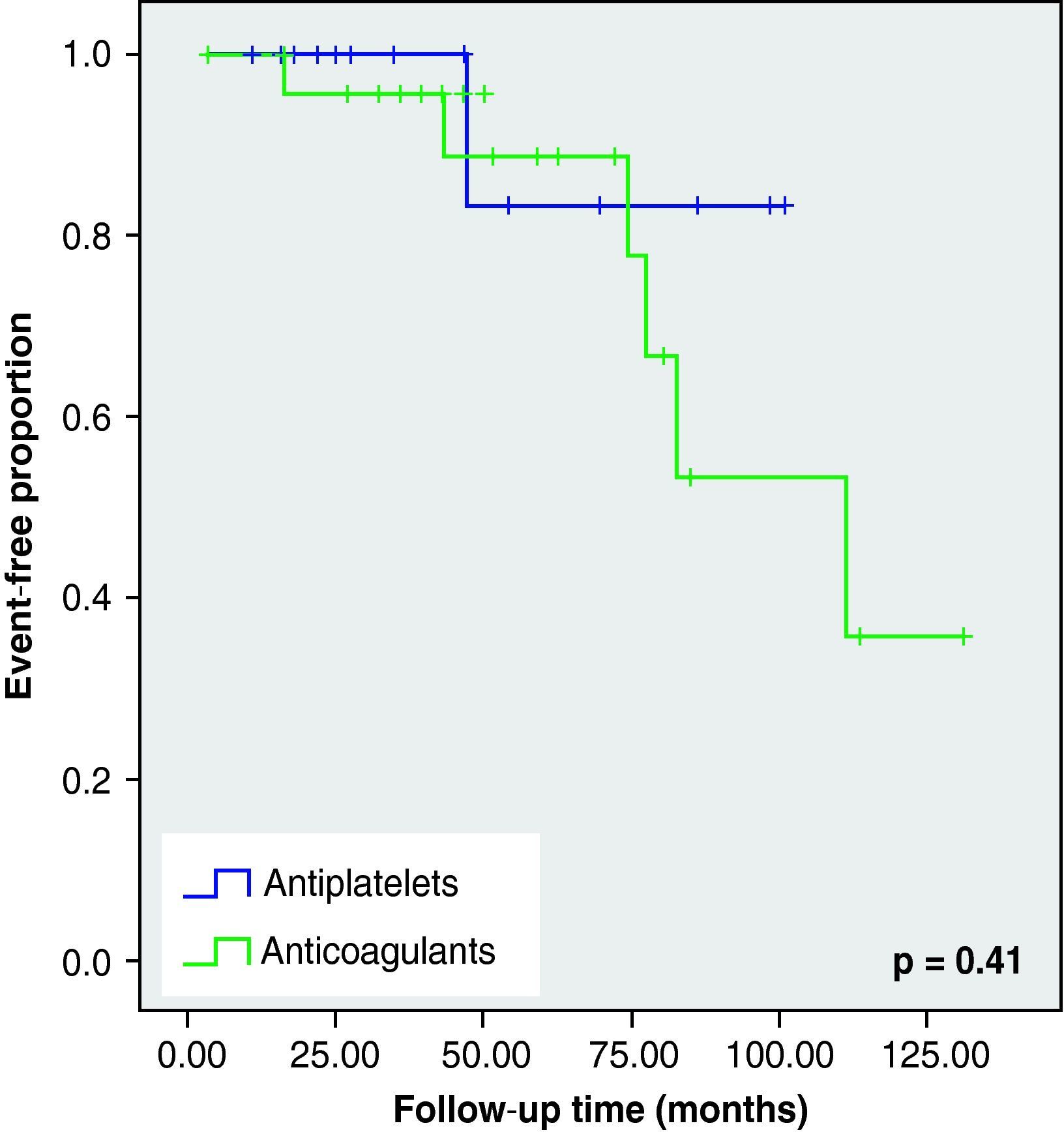
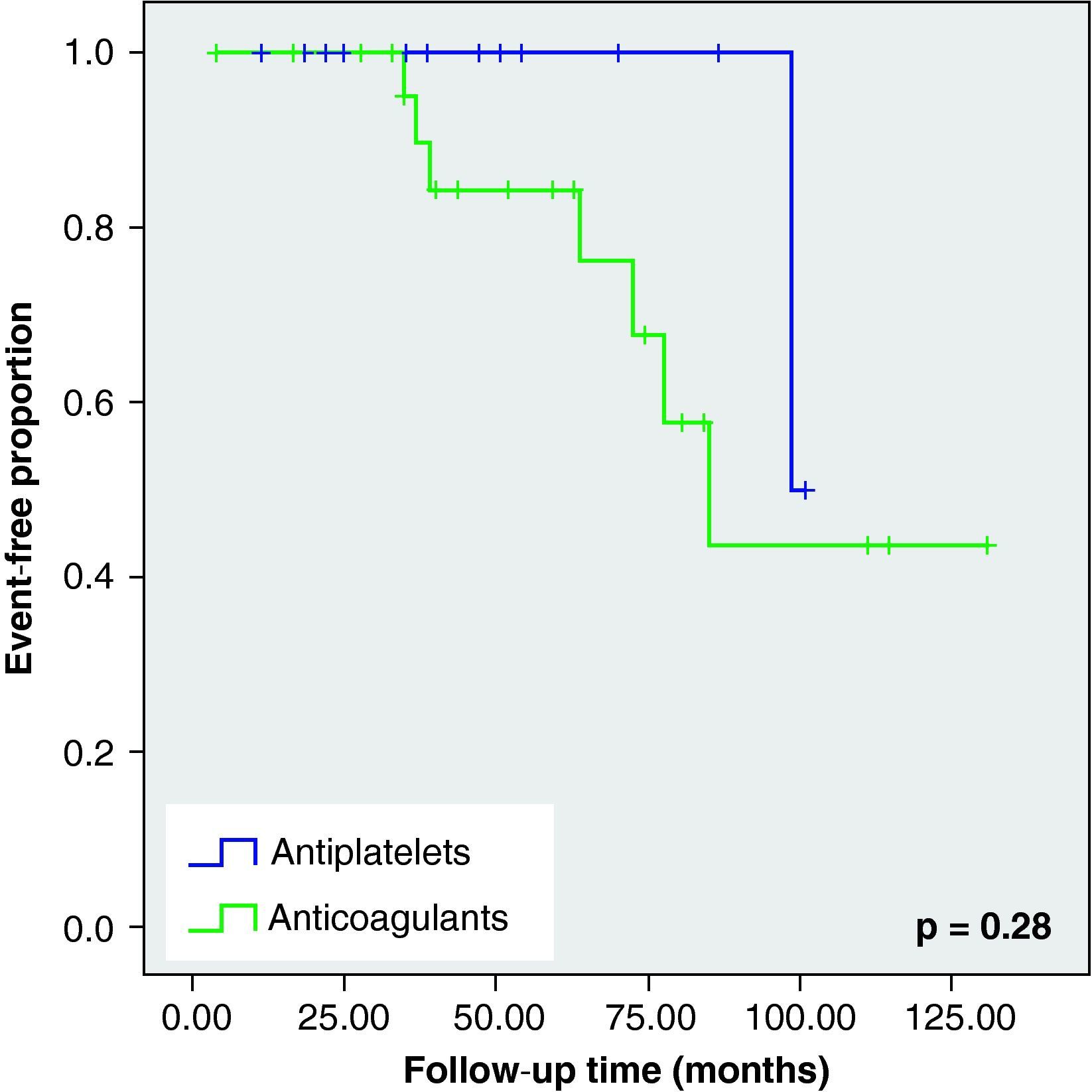
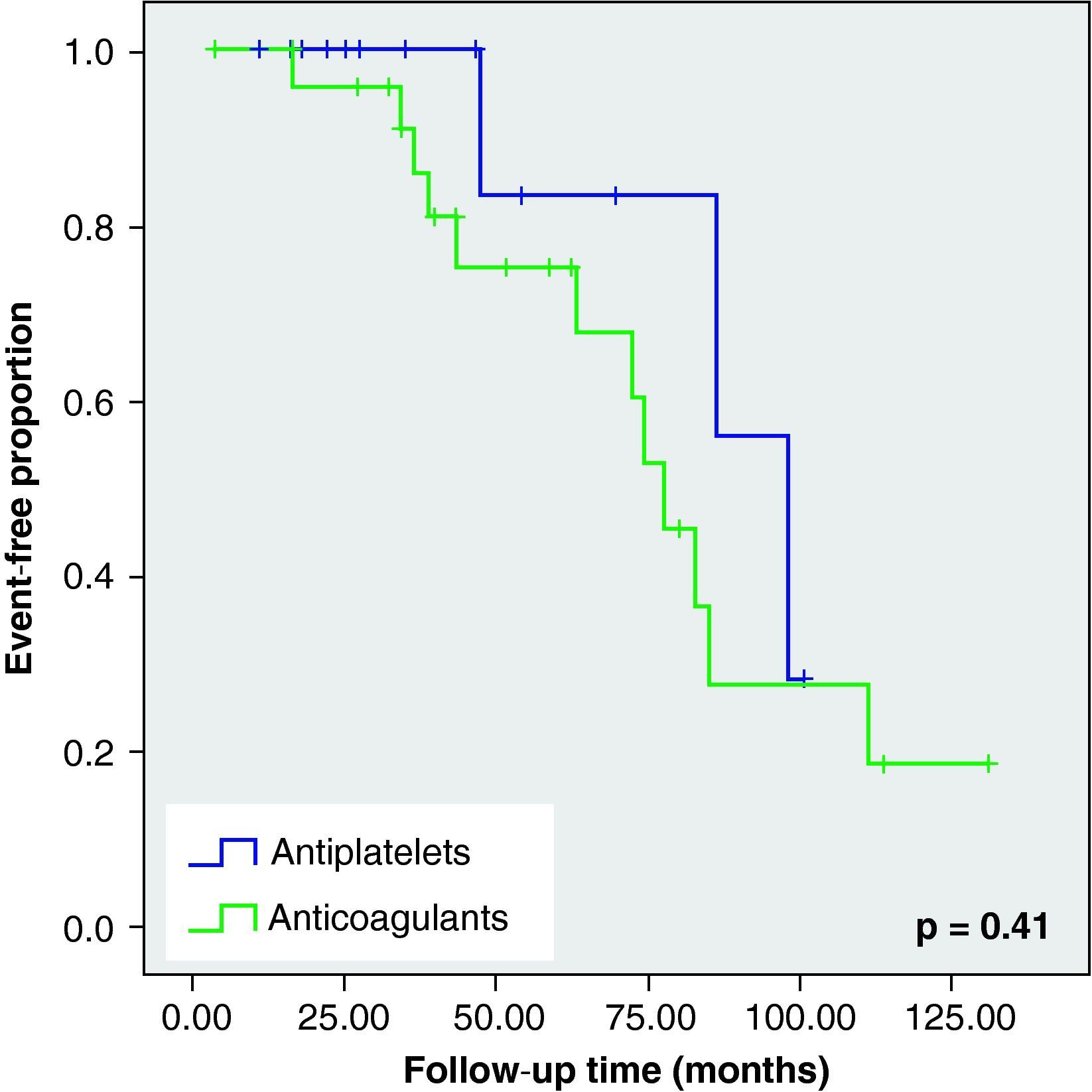
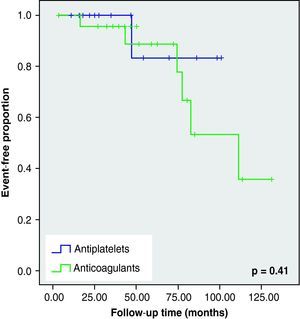
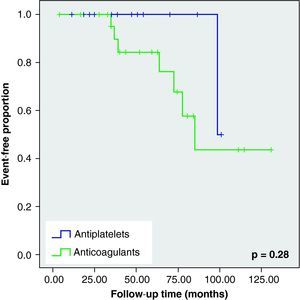
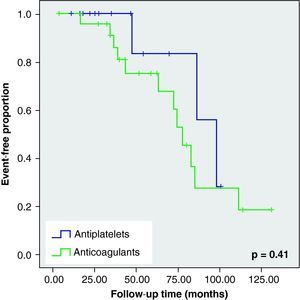
None of these differences in the new cerebrovascular events or deaths were statistically significant (Fig. 1).
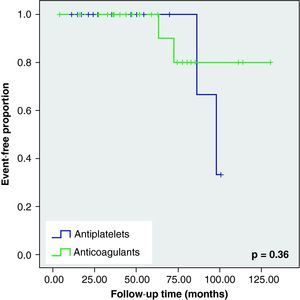
When we analysed the Kaplan–Meier survival curves, we observed the same trend towards a greater number of haemorrhagic events and death in the VKA group, although without reaching statistical significance (Figs. 2–5).
DiscussionMost studies on restart of VKA therapy focus on the acute phase or short-term safety of the resumption of treatment, but not on long-term prognosis.7–12
Although there have been some studies on the long-term safety of restarting antiplatelet therapy after intracerebral haemorrhage,13 this is the second literature review that compares the long-term prognosis of patients who restarted VKA treatment versus that of patients who changed to AP therapy after an ICH. Even though we found no statistically significant differences, our study suggests that the resumption of VKA treatment, despite being safe in most patients, carries an increased risk of spontaneous ICH recurrence. By contrast, the start of AP therapy is associated with an increased frequency of ischaemic events.
In our study, 24% of patients who resumed VKA treatment presented a new cerebral haemorrhagic event during a follow-up lasting over 4 years. This figure is higher than that described in the study by Claassen et al,14 who found that only 12.9% of patients who restarted treatment suffered a new ICH (taking into account both spontaneous and traumatic ICH). Follow-up duration was similar in both studies (about 50 months), as were the age distribution and percentage of patients with arterial hypertension and users of prosthetic heart valves. The difference in the number of recurrences could be explained by the different methodologies used to obtain clinical information. Thus, in our case, monitoring was conducted by reviewing clinical records and hospital follow-up reports, which could have led to a significant loss of asymptomatic patients who did not require emergency care or new specialised consultations. In contrast, in the study by Claassen et al,14 follow-up was conducted by a review of medical records and the sending of questionnaires which collected detailed information on current disability, use of VKA or AP and the recurrence of a cerebral event (ICH or CS) or other complications derived from the treatment; thus achieving less losses during follow-up.
Regarding the group who changed to AP, we observed a higher percentage of cerebral ischaemic events than in the VKA group (14.3% vs 8%, respectively). These figures were similar to those described in literature.14
In accordance with information published to date,14 when comparing both groups (anticoagulant and antiplatelet) we noted that those patients who resumed VKA treatment were younger, had a high risk of embolism (mechanical valves) or else had experienced a previous embolic event. These differences could be attributed to the fact that doctors who recommended which antithrombotic treatment to be followed opted for more effective options (albeit with greater risk) in younger patients, with a probable better functional recovery and longer life expectancy, whilst preferring to initiate antiplatelet therapy in patients with disabling sequelae. Moreover, the anticoagulation option seemed certain when the source of emboli was a high risk one, such as in the case of mechanical valve prostheses users. In addition, anticoagulation treatment was also likely to be restarted in patients who had suffered a previous thromboembolic event, since these patients were considered to have an increased risk of recurrences.15
In patients treated with VKA, the risk of ICH was related to the INR. However, in two-thirds of patients, ICH occurred within the therapeutic range.16 VKA treatment carried an annual ICH risk of 0.3% to 3.7% when the INR was between 2 and 4.5 units (considered the therapeutic range) versus 0.1% in placebo.17 By analysing the INR collected in the ICH index of our patients, we found that most patients were within the therapeutic range (mean 2.7±1.1).
In total, 73% of the patients in our study were hypertensive. This figure was expected, since hypertension is the most important risk factor for suffering ICH.17 The PROGRESS study18 showed that a strict control of blood pressure (BP) reduced the risk of suffering a first intracerebral haemorrhagic event by up to 50% and also seemed to decrease the risk of ICH recurrence. Therefore, although we were not able to assess this finding with the data obtained in our study, it would be logical to think that in those patients with deep ICH of hypertensive aetiology, the restart of anticoagulant treatment19 would be safer when accompanied by a good control of BP. Another risk factor strongly associated with the recurrence of ICH is a lobar location of the first haemorrhagic event,20 as it is related with the presence of cerebral amyloid angiopathy. Therefore, the resumption of VKA treatment in these patients should be carefully assessed.17 In our study, we found no differences between restarting treatment or changing it, when analysed according to ICH location.
In addition to arterial hypertension, ICH location and INR, other risk factors have been reported. These include age, presence of a microangiopathic burden – measured by the presence of leukoaraiosis or microbleeding in a cerebral MRI scan – or carrying the 2¿ or 4¿ alleles of apolipoprotein E.20 All these factors, many of them still being studied, will allow us to individualise future decisions on the start, reinstatement and withdrawal of anticoagulant therapy.
Our study has some important limitations. The first is the small number of patients evaluated, which made it more difficult to achieve statistical significance. This small sample number was due to the significant loss of patients during follow-up, both due to the considerable initial mortality of the disease and to the lack of subsequent hospital controls, since this is a retrospective study. Another important limitation was that the decision about antithrombotic treatment was not protocolised. Such a decision could have been influenced by the clinical status of each patient (in addition to the above mentioned factors). All these circumstances could lead to a selection bias which would be difficult to correct in a retrospective study, all the more so because the scores obtained in the Rankin scale and NIHSS scale were not recorded at discharge.
Despite this pathology being associated with significant morbidity and mortality and being the complication of anticoagulant therapy which generates most mortality and disability,21 studies conducted to date on the safety and benefit of restarting VKA treatment after an ICH are contradictory. These studies focus mainly on short-term safety or safety in the acute phase after an ICH.7–12 In order to resolve this dilemma, some authors have attempted to conduct a therapeutic approach using a decision-analysis model, indicating the restart of anticoagulation treatment or not depending on the location of the intracerebral haemorrhage, cardiovascular risk factors, and the indication of anticoagulant treatment.19 However, these recommendations should be taken with caution, as they are not based on clinical trials. In our study, we found a smaller number of cerebral embolic events, but also a trend towards increased risk of ICH recurrence and increased mortality, in those patients who restarted VKA treatment, contrary to what has been published in the literature to date.14 The data presented here are likely to be modified in the near future thanks to the advent of new oral thrombin inhibitors, which present a similar efficacy but a better safety profile than VKA treatment.22 For the time being, this therapeutic decision must be conducted in an individualised manner, taking into account the characteristics and clinical context of each patient and assessing the risk-benefit of administering or withdrawing treatment. Excluding users of prosthetic heart valves, patients with haemodynamically significant valvular diseases and those with severe renal disease, for whom there is probably no other treatment option, it is vitally important to conduct clinical trials that clarify the most appropriate therapeutic approach to be followed in patients with ICH associated with anticoagulant therapy, in order to base decisions on solid arguments.
Conflicts of interestsThe authors have no conflicts of interest to declare.
The authors wish to thank Ignasi Gich for his help with the statistical analysis.
Please cite this article as: Vidal-Jordana A, et al. Hemorragias intracerebrales en pacientes anticoagulados, ¿qué hacemos después? Neurología. 2012;27:136–42.
This work was presented as an oral communication at the 60th Annual Meeting of the Spanish Neurology Society.