Interleukin-1β (IL-1β) increases necrotic neuronal cell death in the CA1 area after induced status epilepticus (SE) in developing rats. However, it remains uncertain whether IL-1β has a similar effect on the hippocampal dentate gyrus (DG). In this study, we analysed the effects of IL-1β on 14-day-old Wistar rats experiencing DG neuronal death induced by SE.
MethodsSE was induced with lithium-pilocarpine. Six hours after SE onset, a group of pups was injected with IL-1β (at 0, 0.3, 3, 30, or 300ng/μL) in the right ventricle; another group was injected with IL-1β receptor (IL-1R1) antagonist (IL-1Ra, at 30ng/μL) of IL-1RI antagonist (IL-1Ra) alone, and an additional group with 30ng/μL of IL-1Ra plus 3ng/μL of IL-1β. Twenty-four hours after SE onset, neuronal cell death in the dentate gyrus of the dorsal hippocampus was assessed using haematoxylin–eosin staining. Dead cells showed eosinophilic cytoplasm and condensed and fragmented nuclei.
ResultsWe observed an increased number of eosinophilic cells in the hippocampal DG ipsilateral to the site of injection of 3ng/μL and 300ng/μL of IL-1β in comparison with the vehicle group. A similar effect was observed in the hippocampal DG contralateral to the site of injection of 3ng/μL of IL-1β. Administration of both of IL-1β and IL-1Ra failed to prevent an increase in the number of eosinophilic cells.
ConclusionOur data suggest that IL-1β increases apoptotic neuronal cell death caused by SE in the hippocampal GD, which is a mechanism independent of IL-1RI activation.
La interleucina 1β (IL-1β) aumenta la muerte neuronal necrótica debido al estado epiléptico (EE) en el área CA1 del hipocampo de ratas en desarrollo; sin embargo, se desconoce si ejerce un efecto similar en el giro dentado (GD) hipocampal. El objetivo de esta investigación fue analizar el efecto de IL-1β en la muerte neuronal inducida por el EE en el GD de ratas Wistar de 14 días de edad.
MétodosEl EE se indujo con el modelo de litio-pilocarpina. Seis horas después del inicio del EE, la IL-1β se inyectó intracerebroventricularmente (0, 0,3, 3, 30 o 300ng/μl); grupos adicionales se inyectaron con el antagonista natural del receptor tipo i (IL-1RI) de IL-1β (IL-1Ra, 30ng/μl) en ausencia o presencia de IL-1β (3ng/μl). La muerte neuronal se evaluó en la capa granular del GD 24h después del EE mediante la tinción de hematoxilina-eosina. Las células muertas se caracterizaron por presentar citosol eosinofílico y núcleos condensados y fragmentados.
ResultadosSe observó un incremento en el número de células eosinofílicas en el GD ipsilateral a la inyección de 3 y 300ng/μl de IL-1β en comparación con el grupo vehículo; en el GD contralateral se observó un efecto similar únicamente con 3ng/μl de IL-1β. La coadministración de IL-1β con el IL-1Ra no evitó el aumento en el número de células eosinofílicas.
ConclusiónLa IL-1β aumenta la muerte neuronal con morfología apoptótica provocada por el EE en el GD del hipocampo, mecanismo independiente de la activación del receptor IL-1RI.
According to the World Health Organization, approximately 50 million people around the world now have epilepsy, with a proportion estimated at 4 to 10 per 1000 people.1 The International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) define the condition as “a disorder of the brain characterised by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition”.2 Status epilepticus (SE) is a condition resulting either from the failure of the mechanisms responsible for terminating seizures or from the triggering of mechanisms that cause abnormally prolonged seizures. Depending on the type and duration of the seizure, the long-term consequences of SE include neuronal death, neuronal damage, and the alteration of neural networks.3 Epidemiological studies have revealed high incidence of SE in children,4–6 making this population particularly vulnerable to this type of epileptic activity and its consequences.
Status epilepticus can be studied experimentally through the use of animal models. The lithium-pilocarpine model is widely used in developing rats since it reproduces motor manifestations of SE and causes neuronal damage in several brain regions,7–10 with one of the most susceptible being the hippocampus (CA1 pyramidal layer and granular layer of the dentate gyrus).7,10,11 The hippocampus plays an important role in cognition and memory, and is characterised by a trisynaptic circuit which begins in the entorhinal cortex via the perforating pathway, with projections connecting to the granule cells of the dentate gyrus; these cells in turn project axons towards the CA3 pyramidal neurons, which in turn project to the CA1 area via the Schaffer collateral pathway.12
Current evidence suggests that neuroinflammation plays a major role in the pathophysiology of epilepsy and SE.13,14 People with epilepsy have been found to have high plasma concentrations of the proinflammatory cytokine interleukin 1β (IL-1β); analysis of surgically resected brain tissue has also identified cells displaying immunoreactivity to this cytokine.15–18 Higher IL-1β concentration and gene expression have also been found in developing rats shortly after SE induction.19–22 Experimental data have also shown IL-1β to have a pro-epileptic effect23–25; it has recently been demonstrated that this cytokine reduces the currents induced by γ-aminobutyric acid (GABA), the brain's main inhibitory neurotransmitter, in specimens from patients with temporal lobe epilepsy.26 Medel-Matus et al.27 observed that exogenous application of IL-1β increased SE-induced neuronal death in hippocampal area CA1; this effect was mediated by interleukin 1 receptor, type I (IL-1R1). However, it is unknown whether this cytokine has the same effect on other hippocampal areas, such as the dentate gyrus. The aim of our study was to evaluate the effect of intracerebral administration of different concentrations of IL-1β on the neuronal death observed in the dentate gyrus following SE, in addition to assessing the role of IL-1R1 in this effect through the administration of its natural antagonist (IL-1Ra).
Material and methodsExperimental subjectsThe study included male and female Wistar rats of postnatal age 14 days (P14) when seizures were induced (body weight: 25-30g), raised in the vivarium of the Brain Research Centre at Universidad Veracruzana. Both male and female rats were used, since no sexual dimorphisms have been detected in results for rats at that age and under the experimental conditions used. The parents of the rats used were procured from the company Rismart S.A. de C.V. (Mexico). During the mating period (5 days), the animals (one adult male and 2 adult females) were housed in transparent acrylic cages (20×30×50cm). The female adult rats were housed individually in transparent acrylic cages (15×24×37cm) during pregnancy and lactation. The day of birth was considered postnatal day zero (P0). Litter size was standardised to 8 pups per litter to prevent differences in body weight. The animals were kept with their mothers at ambient temperature and humidity levels, with 12:12 light–dark cycles starting at 8:00am and free access to food and water. All experiments observed domestic and international ethical standards, in accordance with the regulations in force (official Mexican guidelines NOM-062-ZOO-1999 on the use and care of experimental animals) and the National Research Council guide for the care and use of laboratory animals (2011 version).
Induction of status epilepticus with lithium-pilocarpineRats were injected with lithium chloride (3mEq/kg, i.p.) on day P13; 20 hours later, on day P14, SE was induced with pilocarpine chlorhydrate (100mg/kg, s.c.). Behavioural involvement of SE was monitored according to the scale proposed by Haas et al.28; only animals displaying this behaviour were included in the study.
Stereotactic surgerySix hours after pilocarpine clorhydrate administration, rats were anaesthetised with isoflurane (1.5%-2%), then placed in a sterotactic frame adapted for neonatal rats. The skull was exposed and a 2.4mm bit was used to drill a hole −4.0mm posterior to bregma and −1.6mm lateral to midline, targeting the right lateral ventricle. A 16mm, 25G (0.5mm) needle (Nipro) was inserted 3.5mm below the level of the skull to administer unilateral microinjections of the corresponding substances using a microinfusion pump (Pump 11 Elite, Harvard Apparatus, USA) with flow set at 0.2μL/min. After the injection was administered, the hole was covered with surgical bone wax (Ethicon Ltd.) and the skin was sutured. The rats were rehydrated with glucose solution (DX-5 solution, PiSA Agropecuaria; 5% glucose, 1mL, s.c.) and the wound was covered with a saturated picric acid solution to prevent maternal cannibalism.27 At all times, temperature was regulated using a temperature control system (model 41-90-8D, FHC); rat body temperature was monitored with a rectal thermometer, which was part of the same system. The entire procedure was performed in aseptic conditions; surgical equipment was treated with dry sterilisation (Germinator 500, Stoelting) prior to each procedure. When the rats had fully recovered, they were placed in the cages with their mothers. When the experiments were complete, histological study with haematoxylin and eosin staining was used to locate the injection site.
Administration of IL-1β and IL-1R1aWe produced a concentration-response curve for IL-1β to assess its effect on SE-induced neuronal death in developing rats. Six hours after the administration of pilocarpine clorhydrate, intracerebroventricular (i.c.v.) injections of IL-1β (recombinant rat, R&D Systems, #501-RL-010/CF) were administered at 4 different concentrations (0.3, 3, 30, and 300ng in 1μL pyrogen-free saline solution as a vehicle).27 Each animal received only one of these 4 concentrations. The control group was injected with the vehicle only. In order to evaluate the role of IL-1R1a in neuronal death, additional groups were created and administered 30ng of IL-1Ra (recombinant rat, R&D Systems, #1545-RA-025/CF) either alone or in combination with 3ng of IL-1β.
Preparation of tissues for histological studyTwenty-four hours after SE onset, rats were anaesthetised with an overdose of pentobarbital sodium (i.p.) and received transcardiac perfusions of saline solution (NaCl 0.9%) and 4% paraformaldehyde in a 0.1M phosphate buffer (pH 7.4). Brains were left in situ at 4°C overnight and subsequently extracted and post-fixed in the same solution for 2hours. The brains were dehydrated in a graded ethanol series (70%, 80%, 95%, and 100%) and xylene, and placed in paraffin blocks. Subsequently, 10μm coronal sections were obtained at the level of the dorsal hippocampus in order to carry out haematoxylin and eosin staining for the identification of dead cells in the dentate gyrus. Sections were also obtained at the level of the lateral ventricles in order to identify the injection site.
Haematoxylin and eosin stainingNeuronal damage in the dentate gyrus was evaluated by optical microscope using a modified version of haematoxylin and eosin staining.7 Dead cells were identified as those displaying shrinking or eosinophilia (pink-coloured cytoplasm), and whose nuclei displayed blue/purple colour (stained by haematoxylin), chromatin fragmentation (karyorrhexis, apoptotic morphology), or markedly pyknotic nuclei without fragmentation (necrotic morphology).10
The brain sections were deparaffinised using xylene and hydrated with a graded ethanol series (100%, 95%, and 70%). The sections were then placed for 10 minutes in Harris haematoxylin (Sigma–Aldrich Co.), washed in regular water, and placed in acid alcohol 70%. After several cycles of rinsing with distilled water followed by incubation in ammonium hydroxide 0.3%, the brain sections were counterstained in eosin in an acidified ethanol solution (eosin 0.01%; Sigma–Aldrich Co.) for one minute. The sections were finally dehydrated with ethanol and xylene and mounted in a non-aqueous medium (Permount, Fisher Scientific) for subsequent analysis with a Leica optical microscope. Dead cells were counted in the granular layer of the dentate gyrus of the dorsal hippocampus ipsilateral and contralateral to the injection site; counts were performed in 4 sections per individual to obtain a mean number of acidophilic cells. The same stain was used to verify the trajectory of the needle used in i.c.v. injections.
Statistical analysisThe results were analysed using one-way analysis of variance for independent groups with a post hoc Tukey test to identify differences between treatments. Statistical analysis was performed with the SigmaStat software, version 3.5. Results are expressed as the mean±standard error of the mean (SEM). Graphs were generated using GraphPad Prism version 5.0.
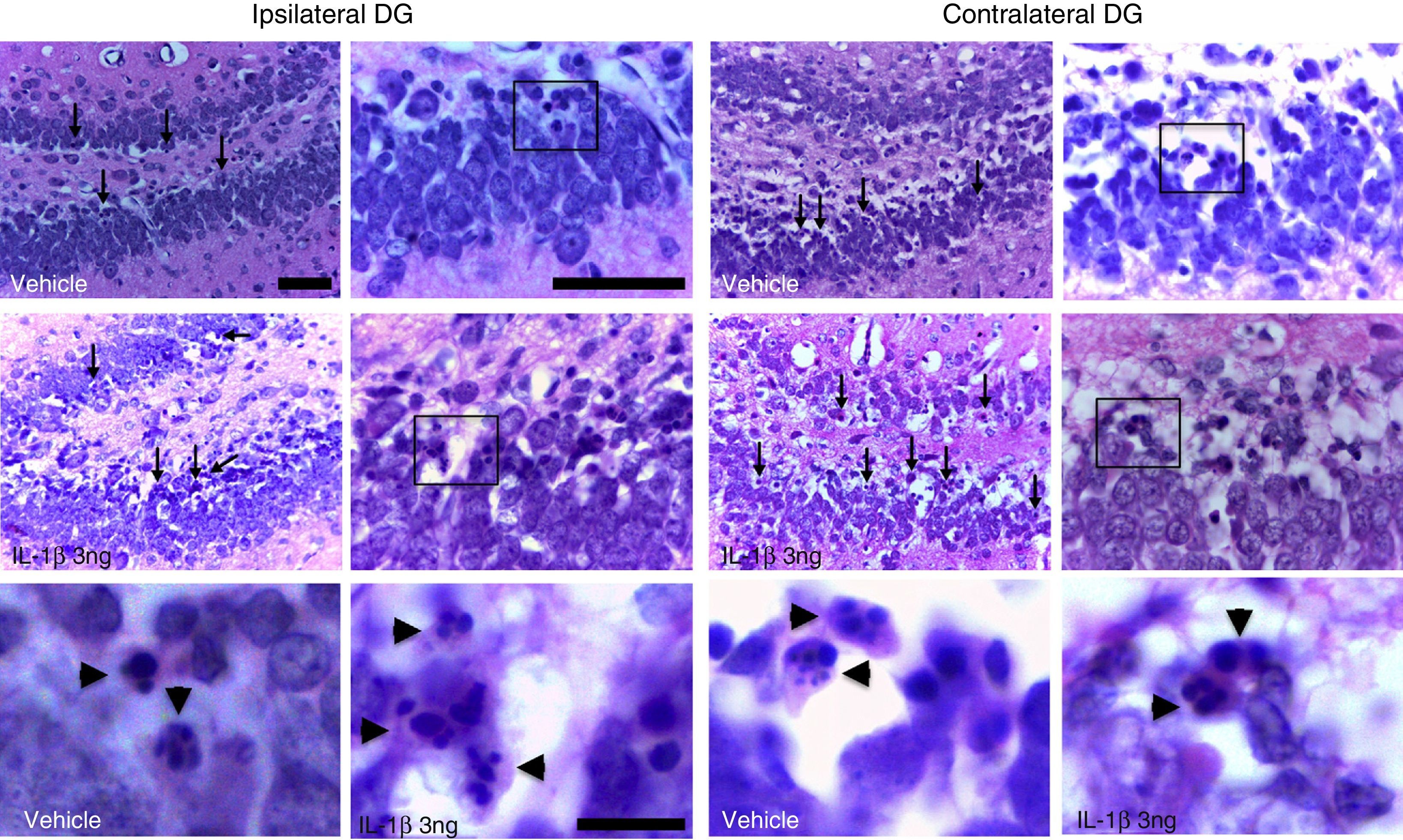
ResultsAll the rats in the study displayed generalised seizures and SE. Histological analysis revealed eosinophilic cells in the hippocampal dentate gyrus exhibiting highly condensed and fragmented nuclei, principally in the internal granular layer (Fig. 1).
Photomicrographs depicting the dentate gyrus regions ipsilateral (left panel) and contralateral (right panel) to the injection site in one animal treated with the vehicle and another treated with IL-1β at 3ng concentration. Arrows indicate some of the cells exhibiting eosinophilic cytosol and chromatin fragmentation. Boxes indicate the areas shown in high-magnification images. Note that eosinophilic cells display fragmentation of the nucleus, suggesting apoptotic cell death (arrowheads). Scale bars: 50μm (low and medium magnification) and 10μm (high magnification).
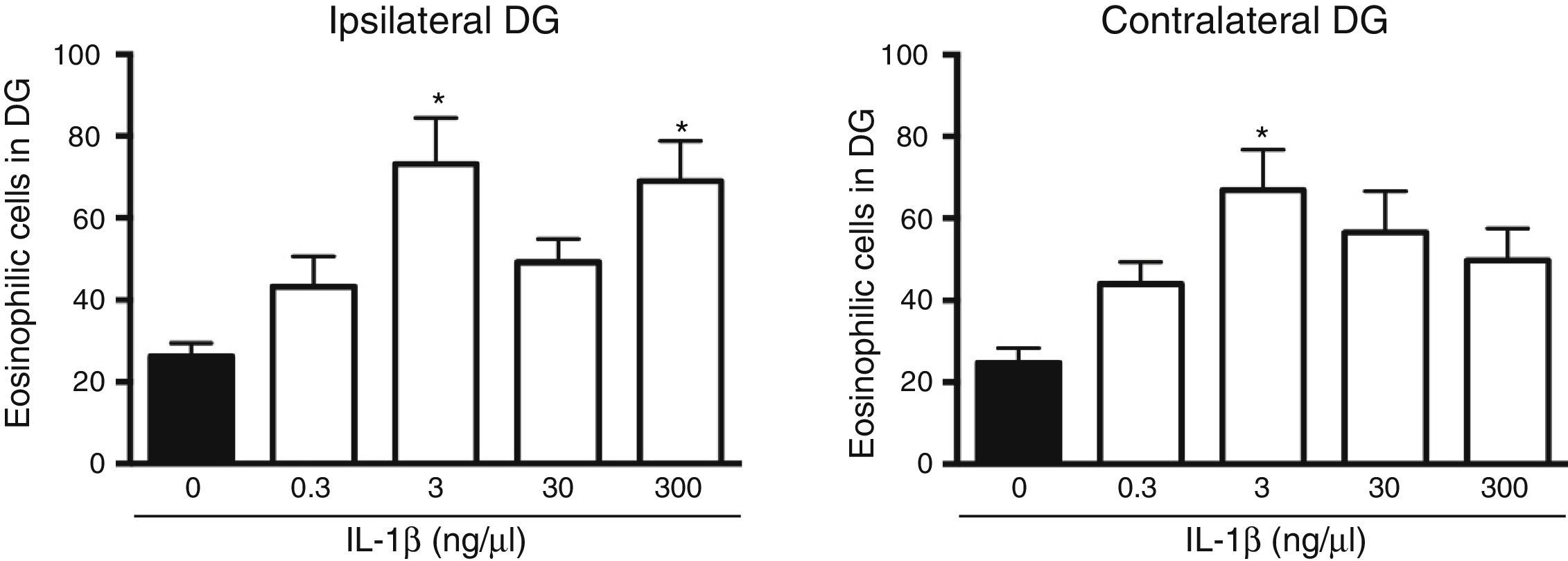
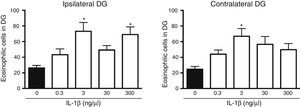
The analysis of variance showed significant differences between groups in the number of dead cells in the dentate gyrus ipsilateral to cytokine administration (F[4, 25]=5.8, P=.002). The results showed that the administration of IL-1β at 3 (73.2±11.3) and 300ng/μL (69±9.8) significantly increased (P=.003 and .007, respectively) the number of dead cells in the dentate gyrus ipsilateral to the injection site following SE, compared to the vehicle group (16.2±3.2). IL-1β at 0.3 and 30ng/μL did not affect neuronal death in the ipsilateral dentate gyrus following SE (Fig. 2). Statistical analysis also revealed significant differences between groups in the number of dead cells in the hippocampus contralateral to the administration site (F[4, 25]=4.078, P=.011); however, only the 3ng/μL preparation increased the number of dead cells observed after SE (66.9±9.91; P=.006), compared to the vehicle (24.7±3.6) (Fig. 2).
Concentration–response curve for the effect of IL-1β on status epilepticus-induced neuronal cell death in the hippocampal dentate gyrus (DG) ipsilateral (left panel) and contralateral (right panel) to the injection site. Bars represent mean±SEM number of eosinophilic cells in the vehicle group and for the different concentrations of IL-1β (n=6 per group). *P<.05 vs vehicle group.
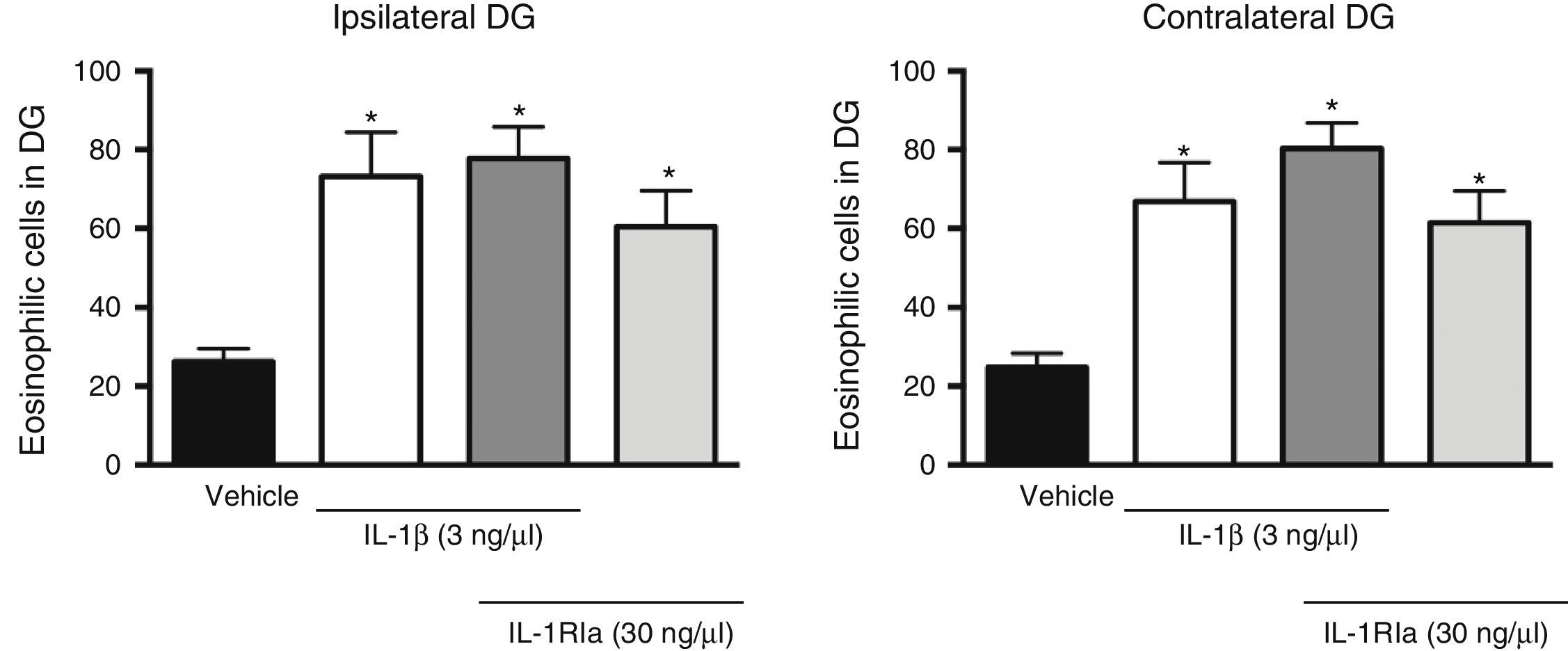
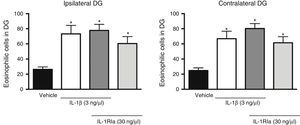
Analysis of variance found significant differences between groups in the effect of the IL-1β receptor antagonist on the increase caused by the cytokine in the number of eosinophilic cells in the dentate gyrus, both ipsilateral (F[3, 20]=7.62, P=.001) and contralateral (F[3, 20]=10.352, P=.001) to the injection site. However, the administration of IL-1Ra in combination with IL-1β did not prevent the increased neuronal death caused by the cytokine alone (P=.585). In fact, this combination was associated with a higher number of eosinophilic cells in the ipsilateral (77.8±7.9; P=.002) and contralateral dentate gyrus (80.3±6.5, P<.001), compared to the vehicle group. IL-R1a alone also promoted an increase in the number of eosinophilic cells in the ipsi- (60.5±9.2, P=.044) and contralateral hemispheres (61.5±8.1, P=.011), in comparison to the vehicle group (Fig. 3).
Effect of natural antagonist of IL-1β receptor (IL-1Ra) injected alone and in combination with IL-1β on the number of eosinophilic cells detected in the hippocampal dentate gyrus (DG) following SE. The left panel shows data for the hippocampus ipsilateral to the cytokine injection site; the right panel shows data for the contralateral hippocampus. Bars show mean±SEM number of eosinophilic cells in the vehicle group (veh), the 3ng IL-1β group, the IL-1β+IL-1Ra (3 and 30ng, respectively) group, and the IL-1Ra (30ng) group (n=6 per group). *P<.05 vs vehicle group.
Our results show that i.c.v. administration of IL-1β increases apoptotic neuronal death in the hippocampal dentate gyrus after SE, and that this is not mediated by IL-1R1, as its natural antagonist does not prevent this effect.
It has been widely reported that SE induced by the lithium-pilocarpine model causes neuronal death in the developing hippocampus.7–11 In 14-day-old rats, SE causes necrotic neuronal death in the CA1 area and apoptotic neuronal death in the internal granular layer of the dentate gyrus.10 Our findings are in line with previous studies, showing apoptotic cell death in the granular layer of the hippocampal dentate gyrus following SE, with or without the presence of IL-1β. In a study into the effect of i.c.v. administration of IL-1β on SE-induced neuronal death in the hippocampus of rats aged P14, Medel-Matus et al.27 found that administration of the cytokine at a concentration of 3ng/μL was associated to increased neuronal death in the CA1 area in both hippocampi following SE. Our results show that i.c.v. administration of IL-1β also increases cell death in another hippocampal region, the dentate gyrus. However, we observed a varying response in the hippocampi ipsi- and contralateral to the injection site: neuronal death increased in the dentate gyrus ipsilateral to the injection site with IL-1β at 3 and 300ng/μL, whereas this effect was only observed with a concentration of 3ng/μL in the contralateral hippocampus. The difference observed between hemispheres may be explained by a dilution effect, since the quantity of IL-1β ultimately reaching the contralateral hemisphere through the ventricular system following injection may have been insufficient to promote cell death.
Attempting to identify the mechanism by which IL-1β increases SE-induced neuronal death, we administered it in combination with the natural antagonist of its receptor; however, the antagonist did not prevent the effect, for which reason we consider that activation of the type 1 receptor can be ruled out. The previous report by Medel-Matus et al.27 found that IL-1β increased necrotic neuronal death in the CA1 area through the activation of the IL-1R1 receptor. The results of the present study show that while IL-1β does promote increased neuronal death in the hippocampus following SE, the mechanism involved may depend on the brain region affected. IL-1β may increase neuronal death in the dentate gyrus through an indirect mechanism, either by causing other cytokines to be synthesised and released29,30 or by promoting changes to cerebral blood flow31,32; these factors could lead to increased cell death. A recent report found that IL-1β concentrations ≥25ng/mL (pathophysiological concentrations) are associated to a decrease of up to 30% in GABAA-receptor-mediated neurotransmission in the tissues of patients with temporal lobe epilepsy, suggesting that the cytokine reduces inhibitory neurotransmission.26 IL-1β has also been observed to decrease the activity and to promote the endocytosis of glial glutamate transporters in the spinal cord.33 Both decreased GABAergic transmission and increased glutamatergic transmission could promote the SE-induced neuronal death observed in our study.
The dentate gyrus is the main pathway for information entering the hippocampus through the perforating pathway; cells in layers II and III of the entorhinal cortex project axons to the dentate gyrus, and granule neurons project axons towards the dendrites of the pyramidal neurons in the CA3 region.12 The dentate gyrus of the hippocampus is one of the best characterised neurogenic regions. The subgranular layer contains granule neuron precursor cells which are integrated into hippocampal circuits and show similar physiological properties to mature granule neurons,34 which migrate to the granular layer and project axons to the hilus and the CA3 area.35,36 Structural anomalies, including aberrant dendrite and mossy fibre development, have been observed in new cells during the development of epilepsy in animals.37 In granule cells, this leads to excitatory synapses with neighbouring cells, which can cause an eliptogenic focus.38 The development of abnormal circuits by granule cells is characteristic of temporal lobe epilepsy both in animals and in humans; the presence of these circuits contributes to dentate gyrus hyperexcitability.39–41 A previous study showed that dentate gyrus cells that died due to SE in developing rats expressed markers of immature neurons.10 The fact that IL-1β increases neuronal death in the granular layer suggests that a greater number of immature neurons may be dying due to SE. This would affect the functioning of the hippocampus, as there would be fewer viable cells for the generation of normal hippocampal circuits. However, it may also promote neurogenesis as a compensatory mechanism to recover the lost cells. Should this be the case, we cannot rule out the possibility of the new neurons establishing aberrant circuits, increasing hippocampal excitability and therefore promoting epileptic seizures or convulsions. Interestingly, selective inhibition of the interleukin converting enzyme (with selective inhibitor VX-765) delays kindling in rats; this effect is associated to the blockade of IL-1β production by astrocytes in the rat brain.25 These results suggest that IL-1β may promote ictogenesis or epileptogenesis, which in the case of SE may be associated to alterations to dentate gyrus function due to a process of neuronal damage or death.
Our study shows that i.c.v. administration of IL-1β increases SE-induced apoptotic neuronal death in the granular layer of the hippocampal dentate gyrus of 14-day-old rats. These results are evidence of the importance of identifying an anti-inflammatory treatment to reduce IL-1β levels in the central nervous system with a view to preventing SE-induced neuronal death in the hippocampus.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the Mexican National Council for Science and Technology for the basic research grant awarded to MLLM (CB-2008-106402) and for the research assistant grant awarded to ATP in partnership with Universidad Veracruzana (staff number 44250).
Please cite this article as: Rincón-López C, Tlapa-Pale A, Medel-Matus J-S, Martínez-Quiroz J, Rodríguez-Landa JF, López-Meraz M-L. La interleucina-1β aumenta la muerte neuronal en el giro dentado del hipocampo asociada al estado epiléptico en la rata en desarrollo. Neurología. 2017;32:587–594.