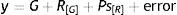Tremulous jaw movement (TJMs) in rats can be induced pharmacologically by striatal dopaminergic manipulation or electrolytic lesion of ventrolateral striatum (VLS). This tremor has neurochemical, anatomical and electromyographic (EMG) characteristics similar to those of tremor in Parkinson patients. However, the EMG characteristics of tremors generated by electrolytic lesion to the VLS have not yet been studied.
MethodThis study used electromyography to describe tremulous jaw movement generated by bilateral electrolytic lesion in the VLS and compared it to tremors induced using subchronic IP treatment with haloperidol, a dopaminergic D2 receptor antagonist. The experimental groups contained rats with a lesion in the ventrolateral striatum and rats on subchronic haloperidol treatment; the control group received only the vehicle. The EMG signal from the temporal muscle was recorded at baseline and during TJMs in all groups.
ResultsTMJ frequencies were heterogeneous among the groups. Rats with VLS lesion showed higher amplitude and frequency values than the haloperidol-treated rats. Amplitudes at baseline also differed among the groups.
ConclusionsWe conclude that TMJs associated with electrolytic lesion to the VLS show a higher frequency and amplitude than tremors induced by haloperidol. This may be related to the way striatum neurons are affected.
El temblor mandibular (TM) en la rata es inducido farmacológicamente por la manipulación dopaminérgica estriatal y por lesión del estriado ventrolateral (EVL). Este temblor tiene características neuroquímicas, anatómicas y electromiográficas similares al temblor que presentan los pacientes con parkinsonismo. Pero se desconocen las características electromiográficas de los temblores generados por la lesión electrolítica del EVL.
MétodoEn ese estudio, se describió electromiográficamente el temblor mandibular generado por la lesión electrolítica bilateral del EVL y se comparó con el inducido por el tratamiento subcrónico (i.p.) con haloperidol, neuroléptico de selectividad alta como antagonista dopaminérgico del receptor D2. A ratas con lesión en la región ventrolateral del estriado, con un tratamiento subcrónico de haloperidol, y a un grupo control que solo recibió el vehículo, se les registró la actividad electromiografía del músculo temporal en condiciones basales y durante los TM.
ResultadosLa distribución de frecuencias del TM entre los grupos varió, puesto que las ratas con la lesión en el EVL mostraron TM de mayor amplitud y frecuencia EMG que las ratas tratadas con el haloperidol. La amplitud en condiciones basales difirió en los distintos grupos de ratas.
ConclusionesSe concluye que los TM asociados a la lesión electrolítica del EVL son de mayor amplitud y frecuencia que los generados por haloperidol, esto puede estar relacionado con el tipo de afectación estriatal.
Onset of mandibular tremor or tremulous jaw movement (TJM) in rats has been linked to changes in striatal mechanisms.1,2 Most TJM cases are induced by changes in striatal dopaminergic neurotransmission, which in turn are caused by dopaminergic antagonism3–5 or by the effect of dopamine depletion in the ventrolateral striatum elicited by 6-hydroxydopamine (6-OHDA). Both conditions lead to TJMs.
The lateral part of the striatum is associated with motor and sensorimotor function; the ventrolateral area in particular is involved in motor control of the mouth area and limbs.8,9 As a result, the ventrolateral part of the striatum is specifically implicated in TJM.7 That area receives dopaminergic pathways from the compact substantia nigra,10,11 which contains both neurotransmitters and neuromodulators such as ACh and DA.12
TJM characteristically occurs in phasic bursts of repetitive jaw movements that are not triggered by any particular stimulus13 and have a frequency distribution of 3 to 7Hz.14 As a result, the neurochemical, anatomical, and EMG findings in rodent TJM are comparable with tremor recorded in parkinsonian patients,13–15 and TJM associated with the manipulation of dopamine in striatal mechanisms therefore provides a functional rat model for analysing certain changes in basal ganglia.
In this context, and given evidence showing that the ventrolateral striatum is the area directly related to the origin of TJM, we hypothesise that electrolytic lesion to the VLS will cause jaw movements that differ electromyographically from those derived from dopaminergic antagonism by means of subchronic (14-day) treatment with IP haloperidol. The purpose of this study was to describe electromyographic readings from TJMs generated by bilateral electrolytic lesion to the VLS and to compare them to TJMs induced by subchronic treatment with IP haloperidol in Wistar rats.
Materials and methodsExperimental subjectsTwenty-two male rats ranging in weight from 250 to 350g were housed individually in transparent acrylic cages measuring 44cm×34cm×20cm, each of which contained 5cm sterile sawdust bedding (Harlan, Mexico). Rats were kept with ad libitum feeding and a 12×12hour inverted light–dark cycle (lights were turned on at 20.00). All experiments were carried out in strict accordance with official Mexican guidelines for the care and use of laboratory animals (NOM-062-ZOO-1999) and Guide for the Care and Use of Laboratory Animals (NIH, Washington D.C., USA).
Study groupsRats were assigned at random to 3 different groups. The first group (n=8) received IP haloperidol during 14 days (1.5mg/kg) and the second group (n=8) received IP saline vehicle (1.5ml/kg) during the same period. The haloperidol dose and treatment regimen were calculated based on the descriptions by Salamone et al.16 and Trevitt et al.17 The third group (n=6) consisted of animals subjected to a bilateral electrolytic lesion in the VLS.
After 14 days, all rats treated with haloperidol (RBI Research Biochemicals International, Natick, MA, 01760, USA) exhibited TJM. The lesion group presented the same response at 24hours; a 10-minute video recording of the response was made for each study group prior to EMG testing. Subsequently, only 2 rats from each group were selected at random to undergo electromyographic measurement of TJM and contrast responses to pharmacological and electrolytic changes. Rats with VLS lesion were treated with flunixin meglumine as an analgesic (2.5mg/kg) and enrofloxacin as an antibiotic (5mg/kg) in addition to subcutaneous fluid therapy with an electrolyte solution (Hartmann's solution 0.9%).
Electrolytic lesion to the ventrolateral striatumRats were anaesthetised with mixture of ketamine (60mg/kg, Bayer) and xylazine (8mg/kg, Pisa). While animals were under deep anaesthesia, researchers drilled burr holes using the following stereotaxic coordinates18: anterior–posterior (AP)=8.74mm anterior to lambda, lateral (L)=±4.4mm, and ventral (V)=3.40mm from the interaural line. Using a stereotaxic instrument (Stoelting Co, USA), researchers then placed a single shaft stainless steel microelectrode 250μm in diameter (Stoelting Co, USA). The electrode delivered 2.5mA direct anodal current during 30seconds using a Grass model S48 stimulator (Astro-Med Inc., USA) connected in series to a Grass model CCU1 constant current unit (Astro-Med Inc., USA).
Implanting electrodes for electromyography recordingsRats in all 3 groups were fitted with bipolar silver wire electrodes (Grass EW10AG) in the right temporal muscle 24hours before electromyography. These electrodes measured 254μm in diameter and were approximately 3cm long with a 3mm exposed segment at the end. Electrode leads were routed subcutaneously towards the rostral part of the skull and cemented with dental acrylic (MDC Dental).
Recording tremulous jaw movementResearchers recorded waking EMG activity. Animals with VLS lesion were assessed 48hours after lesion induction. Assessment of animals treated with haloperidol or saline vehicle took place on day 14 of treatment.
Implanted electrodes were connected to a Grass 15A54, 15LT amplifier (Astro-Med Inc., USA) and simultaneously to a Grass AM9 audio monitor (Astro-Med Inc., USA). The amplified analogue signal was relayed through an interface to a PVA-16 PolyVIEW Adaptor Unit to be digitised and then to a PC to calculate and analyse each animal's responses using the Grass PolyVIEW Data Acquisition and Analysis System 16 v 1.0.
PerfusionAfter the EMG recording, all animals were injected with high-dose sodium pentobarbital anaesthesia (0.6ml/kg; Sedalpharma, Mexico). Transcardial perfusion was then performed to extract the brain, which was then cryoprotected in graded sucrose concentrations (10%, 20%, and 30%) diluted in a 0.1M phosphate buffer. Researchers used a cryostat at −24°C (LEICA CM1850) to make coronal slices 60μm thick and verify lesion location in those animals with lesions.
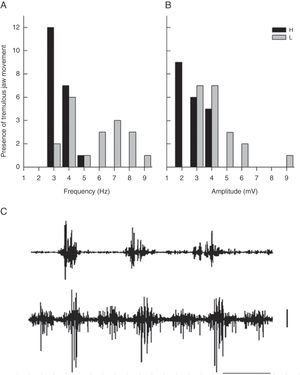
Statistical analysisResearchers prepared graphs of the distribution of amplitude frequencies and EMG activity frequencies in the temporal muscle at baseline and during TJM in rats and recorded modal values. We also created general linear models with a design of main and nested (hierarchical) factors as indicated for analyses with pseudoreplications19:
Here, y is the response variable (frequency and amplitude), G is the group, R is the rat, and Ps is pseudoreplication of EMG trace. In this analysis, the same model was adjusted by the amplitude of baseline activity in all groups. Response variables were fitted to Poisson-distributed data with a log-link function. All analyses were performed using JMP 6 statistical software (SAS Inc, Cary NC, USA, 2005).
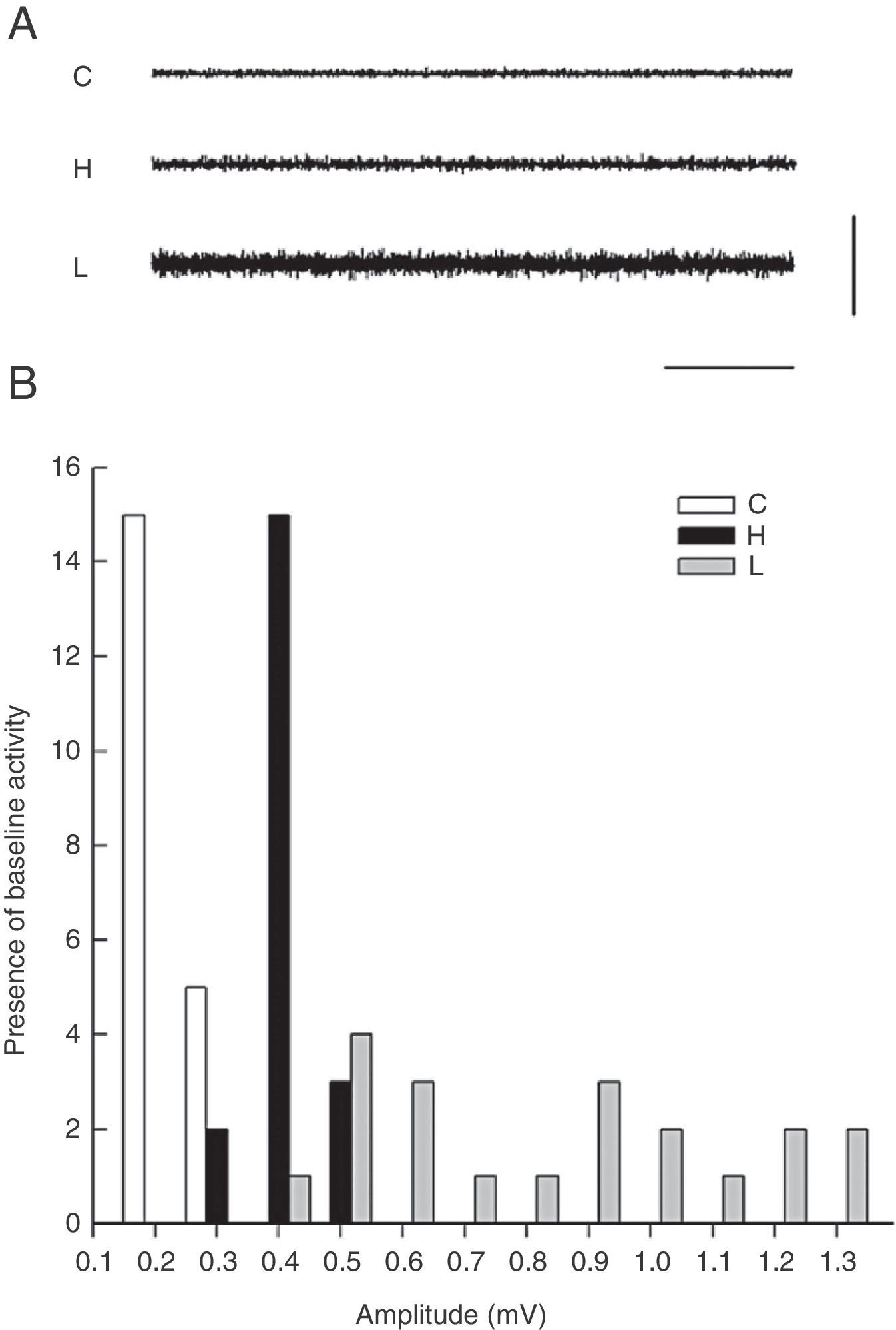
ResultsElectromyography recordings of baseline activityBaseline temporal muscle activity in the control, haloperidol, and electrolytic VLS groups showed different response patterns as evidenced in the 1-second EMG trace (Fig. 1A). Amplitude in control group animals ranged from 0.2 to 0.3mV, while amplitude in the haloperidol group ranged from 0.3 to 0.5mV. Rats with VLS lesions demonstrated the greatest amplitude in baseline activity, from 0.4 to 1.3mV (Fig. 1B).
(A) One-second EMG trace showing baseline activity in the temporal muscle for control group (C), haloperidol group (H), and electrolytic VLS lesion (L). Horizontal calibration: 200ms. Vertical calibration: 1mV. (B) Amplitude of baseline activity (mV) during the EMG recording in the temporal muscle in the control group (C), the haloperidol group (H), and the electrolytic VLS lesion group (L).
The modal value of the amplitude in temporal muscle baseline activity differed for each of the 3 groups. The mode was 0.2mV for control group rats, 0.4mV for haloperidol group rats, and 0.5mV for rats in the lesion group (Fig. 1).
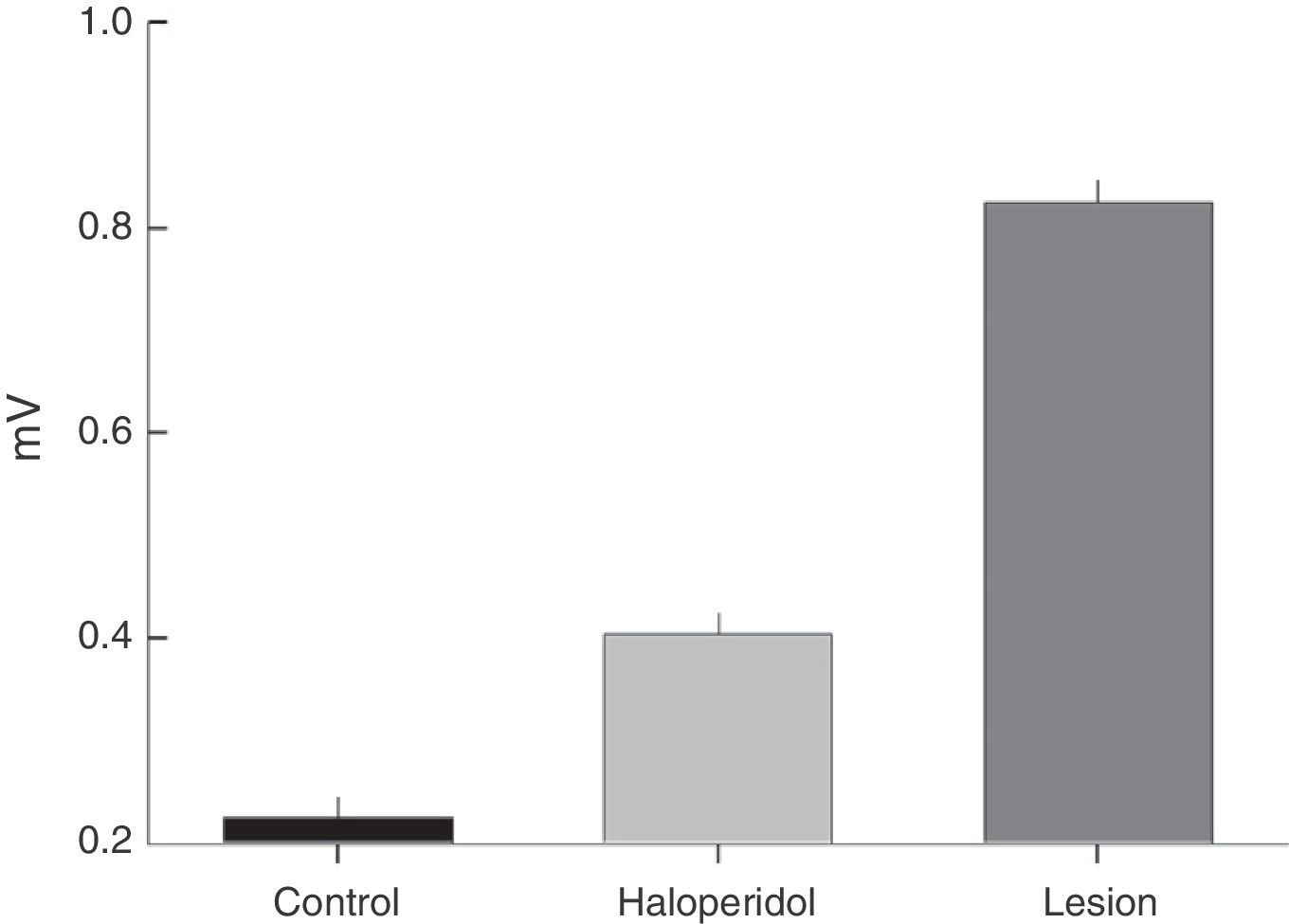
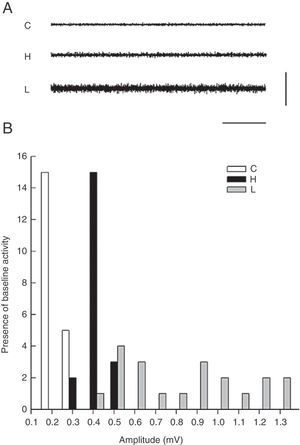
Electromyographic comparison of baseline activityWe found differences in mean amplitude of baseline EMG activity upon comparing the 3 groups (χ2=6.6, P<.03). Nested factors in the model did not exert an effect (P>.05) given that the greatest amplitude was observed in the group with VLS lesion and not among animals treated with haloperidol. Both of these study groups contrasted with the control group, which showed less activity at 0.3mV (Fig. 2).
Electromyographic recordings during tremulous jaw movementsThe VLS lesion induced TJMs in all animals as of 24hours after the intervention; systemic exposure to 1.0mg/kg haloperidol also elicited TJMs in all animals by day 14 of treatment.
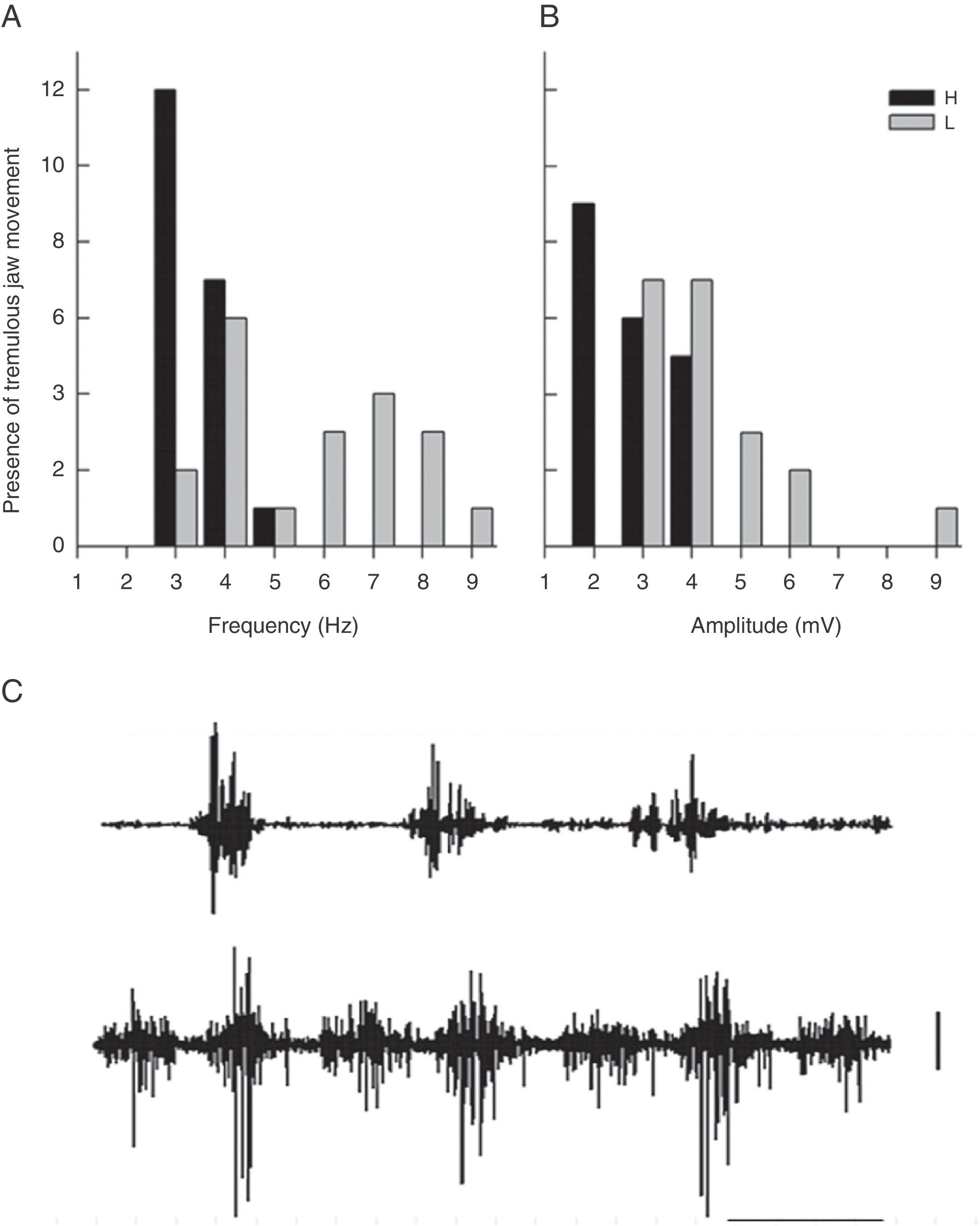
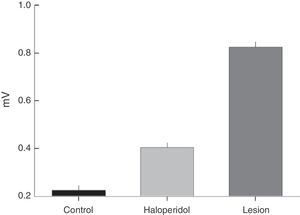
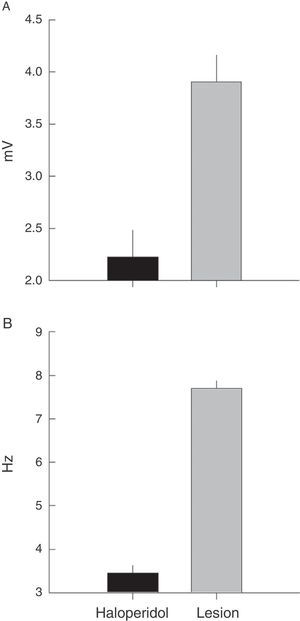
The frequency distribution for TJM varied between the groups; rats with a ventrolateral striatum lesion displayed higher EMG amplitude and frequency than rats treated with haloperidol (Fig. 3). The modal value of TJM in animals exposed to haloperidol (H) fell in the frequency category of 3Hz and tremor was only recorded within the interval of 3 to 5Hz (Fig. 3A and C). In contrast, rats subjected to VLS lesion displayed 7 categories of TJM frequency between 3 and 9Hz; the mode corresponded to the 4Hz category (Fig. 3A).
(A) Frequency (Hz) of EMG activity in the temporal muscle during tremulous jaw movement in the haloperidol group (H) and the electrolytic VLS lesion group (L). (B) Amplitude (mV) of EMG activity in the temporal muscle during tremulous jaw movement in the haloperidol group (H) and the electrolytic VLS lesion group (L). (C) One-second sample of EMG trace showing tremulous jaw movements in the haloperidol group (H), and electrolytic VLS lesion group (L). Horizontal calibration: 200ms. Vertical calibration: 1mV.
EMG activity recordings taken during TJM in rats treated with haloperidol showed amplitudes between 2 and 4mV, while rats with VLS lesion displayed amplitudes between 3 and 9mV (Fig. 3B and C). Furthermore, modal values for TJM occurrence also differed; among animals treated with haloperidol, amplitude was 2mV, while amplitude was recorded in the categories of 3 and 4mV for rats with VLS lesion (Fig. 3B).
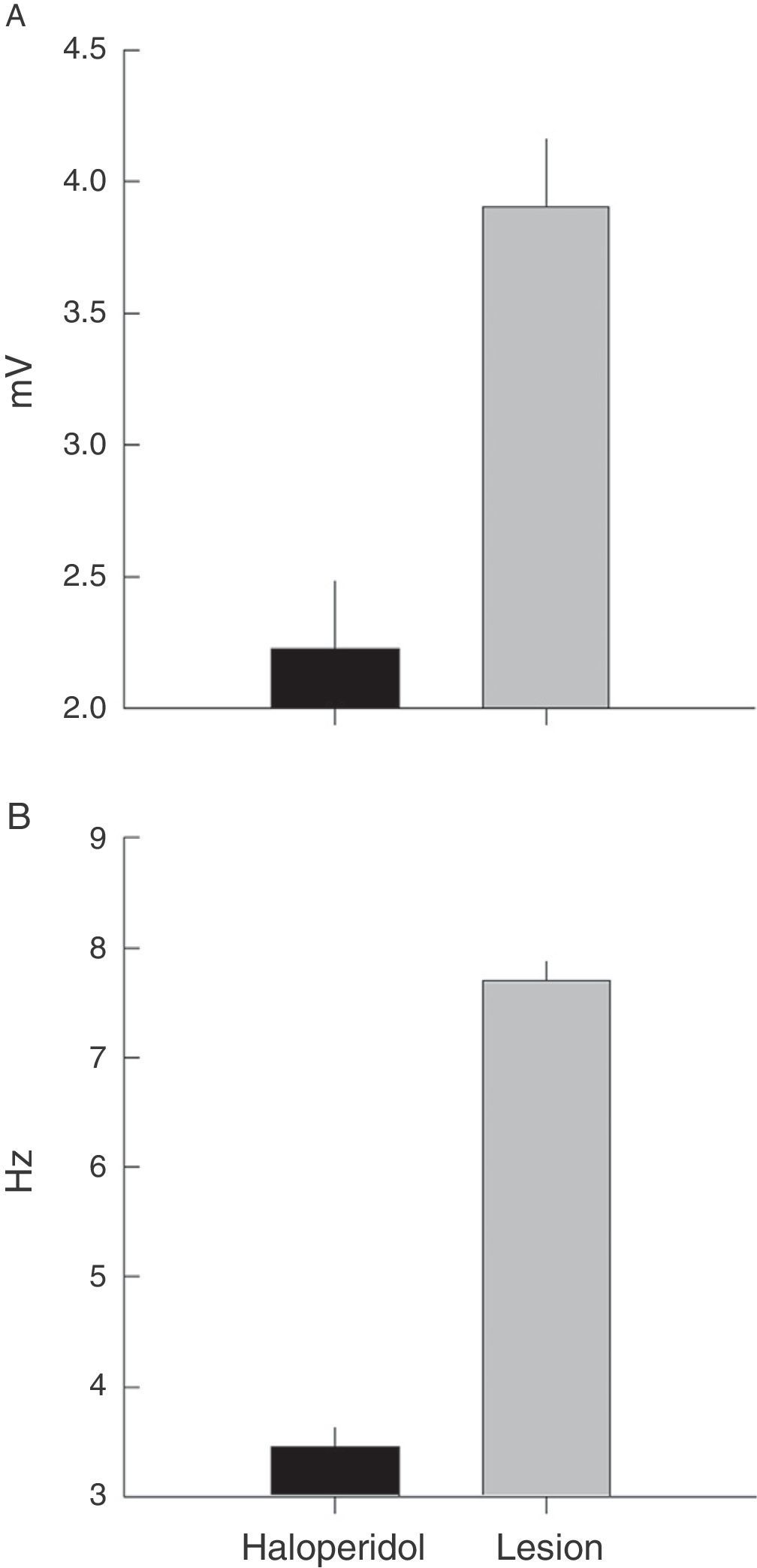
Electromyographic comparison of tremulous jaw movementsMean values for tremor amplitude (mV) varied between the haloperidol group and the VLS lesion group (χ2=10.3, P<.001). Nested factors in the model did not contribute to the contrast effect (P>.05), which shows that the highest mean amplitude was in subjects with VLS lesion and not in animals treated with haloperidol (Fig. 4A).
Frequency recordings (Hz) also showed the same type of response when the two rat groups were contrasted (χ2=31, P<.001). In this model, the effect of individuals nested within the group provided contrast (χ2=7, P<.03) and pseudoreplications did not vary when nested by rat (χ2=1.7, P<.9). A higher mean frequency value was therefore also recorded in rats with VLS lesion than in rats treated with haloperidol (Fig. 4B).
DiscussionThe EMG description of tremulous jaw movements caused by bilateral electrolytic lesion to the VLS or by subchronic treatment with IP haloperidol shows clear differences between the frequency and amplitude patterns.
TJMs are generated when the ventrolateral region of the striatum suffers an electrolytic lesion. This result is similar to that recorded in a study of dopamine depletion with 6-hydroxydopamine that induced TJM6,7 and attributed motor control over the mouth and limbs to the ventrolateral striatum.8,9
The VLS is the critical striatal subregion in which the mechanisms of DA and ACh receptors and adenosine interact to regulate TJMs.5,12 It is within this context that TJMs may be induced by manipulating a number of different pharmacological and neurochemical conditions, and also by means of electrolytic lesion in this region, as shown by this study.
TJMs caused by lesion to the VLS behave similarly to those described by Salamone et al.13 in animals that have undergone dopaminergic manipulation. Both conditions generate periodic bursts of vertical oscillations of the lower jaw without there being any particular stimulus. These oscillations are observed during the jaw-closing phase and the transition between jaw closing and opening, as described by Cousins et al.14 in rats with TJM induced with IP tacrine (acetylcholinesterase inhibitor). Furthermore, EMG activation is coherent with that reported in the temporal muscle under different functional conditions, such as chewing food14 or when teeth are chattering (unpublished data, Herrera-Meza).
TJM induced by electrolytic lesion to the VLS shows specific frequencies and amplitudes in the EMG study. According to EMG findings from each group of rats, tremor presents within a frequency range of 3 to 9Hz and with an amplitude of 3 to 9mV. This jaw activity shows frequency and amplitude values that are greater than those observed in tremor caused by dopaminergic antagonism elicited by prolonged administration of IP haloperidol (3–5Hz and 2–4mV). The frequency pattern for jaw activity during lesion-induced TJM is consistent with EMG findings from TJM described by Salamone et al.13 in the rat parkinsonian model, and with the resting tremor (3 to 7Hz) observed in human parkinsonism.20,21 It does not resemble the frequency characteristic of tardive dyskinesia (1–2Hz).22
The EMG description showed that baseline activity of motor neurons that innervate the temporal muscle intensifies in the presence of a VLS lesion or in response to subchronic treatment with haloperidol. The amplitude of baseline activity is greater in animals with a bilateral electrolytic lesion in the VLS than in rats subjected to subchronic treatment with IP haloperidol. As a result, we understand that EMG characteristics of tremors and the increase in amplitude of baseline muscle activity may be related to the type of changes occurring in the striatum. Haloperidol is a neuroleptic drug that is relatively highly selective for the D2 receptor and causes extrapyramidal effects. Dopaminergic antagonism with that drug acts on the basal ganglia, especially the striate and related nuclei, and it also probably affects striatal neurons expressing D2 receptors in the indirect pathway. The electrolytic lesion destroys striatal neurons in the ventrolateral area of the striatum. This is the main point of entry for the nigrostriatal dopaminergic pathway in which the mechanisms of DA and ACh receptors interact with adenosine,5,12,13 which may cause generalised striatal damage with an effect on related nuclei.
The EMG description of tremulous jaw movements due to electrolytic VLS lesion shows that this activity displays specific frequency and amplitude traits that can be distinguished from those observed in rats on subchronic treatment with haloperidol. However, lesion-induced tremors correspond to the tremulous jaw movements that result from a number of neurochemical and pharmacological conditions, as Salamone et al. have described.13
In summary, both the variation in baseline activity observed in these groups and the rhythmic activation pattern of the temporal muscle during TJM, induced either by VLS lesion or haloperidol, provide evidence that striatal mechanisms are related to motor neurons that innervate the trigeminal nerve. Although few test subjects were used to provide this description, we were able to determine the response patterns for TJMs induced by VLS lesion. EMG findings for frequency and amplitude in tremulous jaw movements caused by VLS lesion differ from the frequency and amplitude measured in animals treated with haloperidol. This variability may be attributed to the type of striatal change elicited.
FundingsThis study was completed as a part of GHM's doctoral thesis under CONACyT grant ID 377111. It received funding from PROMEP-México PTC-195 and the Mexican Ministry of Science and Innovation [PSI2011-29181].
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Dr J. F. Rodríguez Landa and Dr A. J. Martínez-Chacón for their comments on our drafts of this manuscript.
Please cite this article as: Herrera-Meza G, Manzo J, Hernández ME, Miquel M, García LI. Inducción del temblor mandibular por lesión electrolítica del estriado ventrolateral y por el tratamiento subcrónico con haloperidol en rata macho: un contraste electromiográfico. Neurología. 2014;29:416–422.