The diagnosis of vascular parkinsonism (VP) is based on a series of clinical criteria and neuroimaging findings. An increase in transcranial Doppler ultrasonography pulsatility index (PI) has been described as a frequent finding in patients with VP. We aimed to confirm this association and to determine the PI value with the highest sensitivity and specificity for diagnosis of VP.
MethodPI was determined in all patients admitted to Hospital Universitari i Politècnic La Fe due to parkinsonism between January 2012 and June 2016. We assessed the probability of having VP based on PI values in patients with a definite diagnosis of either VP or idiopathic Parkinson's disease (IPD). A ROC curve was created to determine the PI value with the highest sensitivity and specificity.
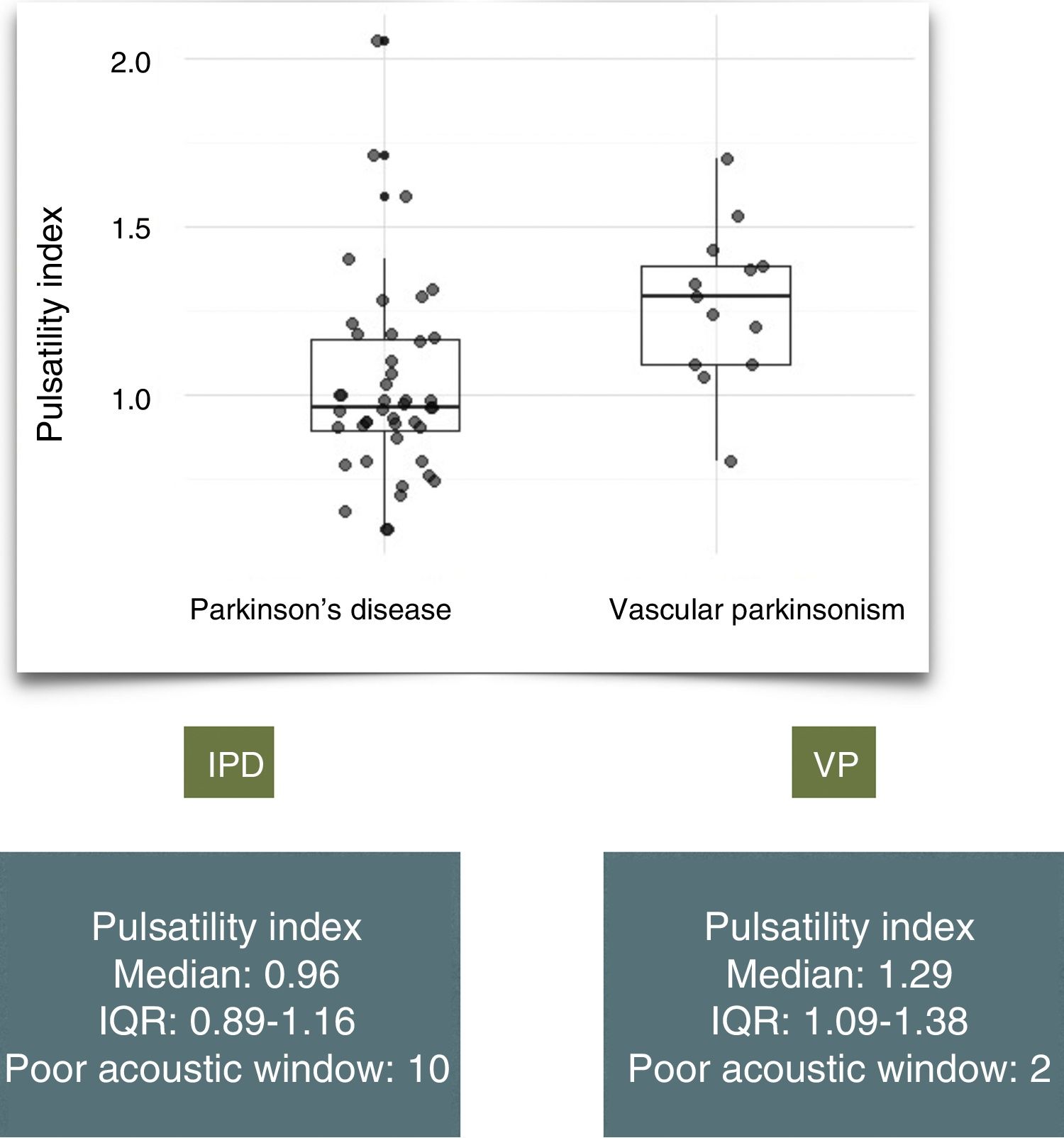
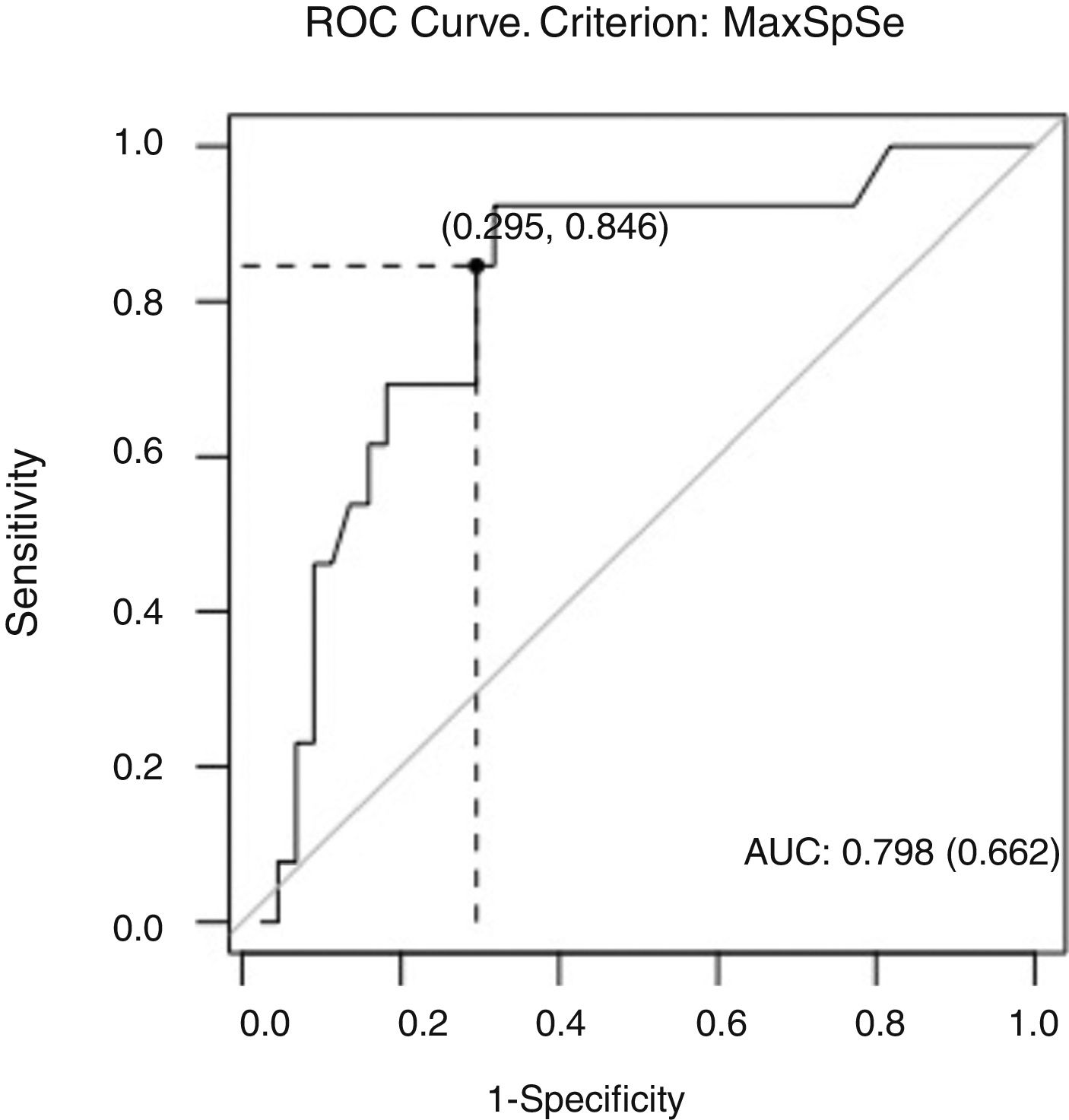
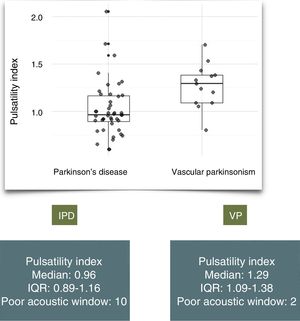
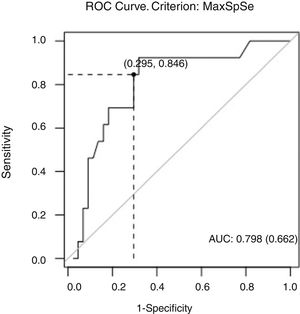
ResultsWe assessed a total of 146 patients with suspected parkinsonism; 54 (37%) were diagnosed with IPD and 15 (10%) with VP. Patients with VP were significantly older than those with IPD (mean age of 79 vs 68.5, P=.00144) and had a higher PI (median of 1.29 [IQR: 1.09–1.38] vs 0.96 [IQR: 0.89–1.16], P=.01328). In our sample, a PI of 1.09 conferred 84% sensitivity and 70% specificity.
ConclusionsIn our series, the PI was significantly higher in patients with VP than in those with IPD. We therefore support the use of transcranial Doppler ultrasonography for the initial assessment of elderly patients with akinetic-rigid syndrome.
El diagnóstico de parkinsonismo vascular (PV) se realiza en función de una serie de criterios clínicos y hallazgos de neuroimagen compatibles. El aumento en el índice de pulsatilidad (IP) determinado por doppler transcraneal se ha descrito como hallazgo en los pacientes con PV. El objetivo de este trabajo ha sido comprobar en nuestro laboratorio de neurosonología dicha relación y determinar el valour de IP con mayor sensibilidad y especificidad para el diagnóstico de PV.
MétodoSe realizó una medición del IP en todos los pacientes remitidos por parkinsonismo al Hospital Universitari i Politècnic La Fe entre enero de 2012 y junio de 2016. En los pacientes finalmente diagnosticados de PV y enfermedad de Parkinson idiopática (EPI) estudiamos la probabilidad de padecer PV en función del valour IP, y elaboramos una curva ROC para determinar el valour de IP con mayor sensibilidad y especificidad.
ResultadosSe evaluaron 146 pacientes remitidos por parkinsonismo; 54 (37%) fueron diagnosticados de EPI y 15 (10%) de PV. La media de edad fue mayor en el grupo de PV (79 vs 68,5; p=0,00144). Encontramos un IP mayor en los pacientes con PV: mediana 1,29 (Q1-Q3: 1,09–1,38) vs 0,96 (Q1-Q3 0,89–1,16), p=0,01328. En nuestra muestra un valor de IP de 1,09 presenta una sensibilidad y especificidad del 84% y del 70% respectivamente.
ConclusionesEn nuestra serie el IP es significativamente mayor en los pacientes con PV que con EPI. Apoyamos la inclusión sistemática del doppler transcraneal en la valoración inicial de pacientes con síndrome rígido-acinético.
Diagnosis of vascular parkinsonism (VP) is complex, relying on clinical and radiological variables that enable its differentiation from idiopathic Parkinson's disease (IPD).
The main review studies1,2 on the subject describe the condition as an extrapyramidal syndrome in a patient with several cardiovascular risk factors, older than the typical age of onset of IPD, who presents ischaemic lesions in neuroimaging studies.
The clinical characteristics of the VP phenotype include3: rigidity, predominantly affecting the lower limbs, with symmetrical distribution and incomplete response to levodopa; lower incidence of resting tremor and presence of such symptoms of pyramidal tract involvement as hemiparesis and hyperreflexia; and frequent association with pseudobulbar syndrome or dementia from symptom onset.4,5
The use of neurosonology6 has become increasingly important in the diagnosis of parkinsonism since increased hyperechogenicity in the mesencephalic substantia nigra was associated with IPD. This sign is widely validated as a marker of neurodegeneration, helping in the differentiation of IPD from atypical parkinsonism.7
It has been proposed that the pulsatility index (PI) in the arteries of the circle of Willis, determined by transcranial Doppler ultrasonography, is elevated in patients with VP, and that this helps to differentiate VP from IPD.
The aim of this study is to analyse the association between PI and likelihood of presenting VP in our population, and to determine the convergent validity (sensitivity, specificity, and predictive values) of this variable using a ROC curve.
As a secondary aim, we studied the difference in substantia nigra hyperechogenicity (SNH) between patients with VP and IPD.
MethodsWe included all patients referred to the Hospital Universitari i Politècnic La Fe movement disorder unit due to parkinsonism between January 2012 and June 2016.
The criterion for suspicion of parkinsonism was the presence of at least one of the clinical signs established by the UK Parkinson's Disease Society Brain Bank criteria: bradykinesia, asymmetric resting tremor, axial rigidity or stiffness in the limbs, or postural instability.
A diagnosis of VP was established when the following criteria were met: (1) presence of 2 or more cardiovascular risk factors (arterial hypertension, diabetes mellitus, dyslipidaemia, history of smoking); (2) ischaemic lesions in neuroimaging (lacunar infarcts, territorial infarcts, extensive leukoaraiosis); (3) minimal or non-sustained levodopa response; and (4) absence of clinical signs leading to suspicion of atypical parkinsonism.
Considering the lack of universal criteria, we adapted our definition of VP from the studies by Winikates and Jankovic,8 and Ziljmans.9
Diagnosis of probable or possible IPD was established according to the criteria by Hughes and colleagues.
Patients with a history of treatment with neuroleptics or history of severe head trauma in the year prior to diagnosis were excluded from our analysis.
At the beginning of each patient's follow-up, we performed biochemical and neuroimaging studies and a transcranial duplex ultrasound with determination of the PI in both middle cerebral arteries, and measured the hyperechogenic area (mm2) of the substantia nigra through both transtemporal windows.
The highest value found for each variable was used to compare diagnoses.
We analysed the demographic characteristics and the PI and SNH ultrasound values for patients finally diagnosed with IPD or VP (Table 1).
We used the “pROC”10 statistical package in the RStudio 1.0.143 programming environment to analyse data (“R: a language and environment for statistical computing.” R Foundation for Statistical Computing, Vienna, Austria; available from: https://www.R-project.org).
Using a univariate logistic regression model, we determined patients’ likelihood of being diagnosed with VP (variable response vs IPD) as a function of the PI value obtained.
We plotted a ROC curve to determine the PI value with the highest sensitivity and specificity for diagnosing VP.
Using a multivariate mixed linear regression model, we analysed the difference in the SNH area as a function of the covariables diagnosis, age, and sex.
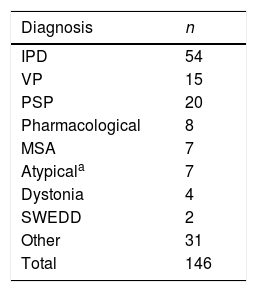
ResultsWe assessed a total of 146 patients referred due to parkinsonism; 54 were diagnosed with IPD (37%) and 15 with VP (10%). The main definitive diagnoses in the remaining 77 patients were atypical parkinsonism (20 with progressive supranuclear palsy, 7 with multiple system atrophy, and 7 with unspecified Parkinson plus syndrome) and tremor or extrapyramidal symptoms of pharmacological origin (8 patients) (Table 2).
Diagnoses in the cohort of patients assessed due to parkinsonism.
| Diagnosis | n |
|---|---|
| IPD | 54 |
| VP | 15 |
| PSP | 20 |
| Pharmacological | 8 |
| MSA | 7 |
| Atypicala | 7 |
| Dystonia | 4 |
| SWEDD | 2 |
| Other | 31 |
| Total | 146 |
IPD: idiopathic Parkinson's disease; MSA: multiple system atrophy; PSP: progressive supranuclear palsy; SWEDD: scans without evidence of dopaminergic deficit; VP: vascular parkinsonism.
Sex distribution between patients with IPD and those with VP was similar (women: 48% vs 53%, P=.9494), although we observed a significant difference in age (P=.00144), with a median age of 79 in the VP group (IQR: 74.4-81.5) and 68.5 in patients with IPD (IQR: 58.2-74).
We did not compare the number of basal ganglia lesions in each diagnostic group as these were not systematically quantified.
The median PI value was 1.29 in patients with VP (IQR: 1.09-1.38) and 0.96 in patients with IPD (IQR: 0.89-1.16) (Fig. 1). A similar proportion of patients in each group presented poor acoustic window (13% vs 18%, P=.67). We identified a significant difference in PI value between groups (P=.01328).
The cut-off PI value on the ROC curve was 1.09, giving 84% sensitivity and 70% specificity. The area under the curve was 0.798, suggesting good overall accuracy of the test (Fig. 2). We found no differences in SNH values between diagnostic groups (P=.14) or according to age (P=.91).
DiscussionDifferential diagnosis between IPD and VP continues to represent a challenge. In addition to the clinical features mentioned in the introduction, differentiation largely relies on the presence of MRI findings,11,12 especially periventricular lesions and abnormalities in the subcortical white matter.
In our cohort of patients with parkinsonism, we found a significant proportional relationship between PI in proximal segments of the middle cerebellar artery and the probability of diagnosis of VP. Several authors have proposed evaluating PI as a measure to support the vascular aetiology of parkinsonism.5 Studies reporting transcranial Doppler ultrasound results in patients with VP (compared to IPD) show higher PI values, as well as a higher frequency of intracranial stenosis; the latter is not addressed in our study. These findings were also correlated with increased presence of leukoaraiosis and subcortical lacunar infarcts on MRI.
The main hypothesis explaining increased PI in these patients is the increased resistance in the distal (arteriolar) vascular bed, causing small vessel disease in patients with VP. The increase in overall resistance would cause a relative increase in the peak systolic velocity compared to the mean velocity in the branches of the circle of Willis. This finding is consistent with the concept of VP as a small vessel disease. The anatomical pathology study of patients with VP, compared to that of patients with IPD, shows more extensive atrophy of the cortical gyri, dilated ventricles,13 atherosclerosis in large intracranial vessels, and higher prevalence of findings compatible with small vessel disease (hyalinosis in the tunica media, enlargement and gliosis of the perivascular spaces), regardless of sex or age at death.
On the other hand, it has been suggested that the incidence of cerebrovascular events is lower in patients with IPD than in the general population.14,15 This theory is still controversial.16
Although the increased PI may be explained, at least partially, by the presence of ischaemic lesions in the parenchyma, the pathophysiology of the akinetic-rigid syndrome has not yet been associated with any specific locus of lacunar lesions. Yamanouchi and Nagura4 report greater pallor and decreased oligodendrocyte density in the frontal white matter of patients with VP when compared with age- and sex-matched controls without parkinsonism. However, no differences were found in the quantity or distribution of vascular lesions in the basal ganglia. Furthermore, several case reports describe the acute onset of parkinsonism in the context of a focal lesion,17,18 each at a different location (territorial, thalamus, substantia nigra).
In our neurosonology laboratory, the PI value 1.09 was established as a cut-off point with good sensitivity (84%) and specificity (70%) for the diagnosis of VP. This sensitivity value is comparable to that of SNH for diagnosing IPD, as reported by Tsai et al.5 (83%), although PI is less specific, probably due to the prevalence of increased values in elderly patients.
We observed an abnormally high number of patients with atypical parkinsonism. This overrepresentation is explained by the fact that the sample was gathered from a movement disorder specialist unit at a tertiary hospital. The percentages of patients with VP and IPD, however, are similar to those usually reported in the literature.
Contrary to expectations, the multivariate analysis revealed no significant differences in SNH as a function of diagnosis or age.
In conclusion, there is a continuing need to establish more reliable diagnostic criteria, given the implications for response to dopaminergic treatment and progression. The inclusion of PI as one of these criteria would require studies to be performed, systemically including transcranial Doppler ultrasonography in the initial assessment of patients with akinetic-rigid syndrome.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caba LM, Ferrairó JIT, Torres IM, Costa JFV, Muñoz RB, Martin AL. El índice de pulsatilidad intracraneal elevado apoya el diagnóstico de parkinsonismo vascular frente a enfermedad de Parkinson idiopática. Neurología. 2020;35:563–567.
Part of this study was presented orally at the 68th Annual Meeting of the Spanish Society of Neurology.