To update the ad hoc Committee of the Cerebrovascular Diseases Study Group of The Spanish Neurological Society guidelines on prevention of ischemic stroke (IS) and Transient Ischaemic Attack (TIA).
MethodsWe reviewed the available evidence on ischaemic stroke and TIA prevention according to aetiological subtype. Levels of evidence and recommendation levels are based on the classification of the Centre for Evidence-Based Medicine.
ResultsIn atherothrombotic IS, antiplatelet therapy and revascularization procedures in selected cases of ipsilateral carotid stenosis (70–99%) reduce the risk of recurrences. In cardioembolic IS (atrial fibrillation, valvular diseases, prosthetic valves and myocardial infarction with mural thrombus) prevention is based on the use of oral anticoagulants. Preventive therapies for uncommon causes of IS will depend on the aetiology. In the case of cerebral venous thrombosis oral anticoagulation is effective.
ConclusionsWe conclude with recommendations for clinical practice in prevention of IS according to the aetiological subtype presented by the patient.
Actualizar las guías terapéuticas del Comité ad hoc del Grupo de Estudio de Enfermedades Cerebrovasculares de la SEN en el tratamiento preventivo de ictus isquémico (II) y ataque isquémico transitorio (AIT).
MétodosRevisión de evidencias disponibles sobre la prevención del ictus isquémico y AIT en función del subtipo etiológico. Los niveles de evidencia y grados de recomendación se han basado en la clasificación del Centro de Medicina Basada en la Evidencia.
ResultadosEn el II de origen aterotrombótico reducen el riesgo de recurrencias el tratamiento antiagregante y los procedimientos revascularizadores en casos seleccionados de estenosis carotidea ipsilateral (70-99%). La prevención de II de origen cardioembólico (fibrilación auricular, valulopatías, prótesis valvulares y en infarto de miocardio con trombo mural) se basa en el uso de anticoagulantes orales. En el II de origen inhabitual, las terapias preventivas dependerán dela etiología; en la trombosis venosa cerebral la anticoagulación oral es eficaz.
ConclusionesSe concluye con recomendaciones de práctica clínica en prevención de ictus isquémico y AIT adaptadas al subtipo etiológico de II que ha presentado el paciente.
In Spain, cerebrovascular diseases (CVD) are the leading cause of death in women, the third most frequent in men,1 and the most common cause of disability. The measures we describe here are designed to prevent both first-ever ischaemic strokes (IS) and future episodes in patients who have already suffered a stroke. We will also consider measures aimed at reducing overall vascular risk in these patients.2 Due to the length of this article, we decided to publish it in 2 parts. The first part presented a review of the risk factors for ischaemic stroke3 and any modifications that would need to be made to prevent IS. In this second part, we will specify different preventive treatments according to the stroke subtype. Recommendations for each section are shown in tables for better readability. Levels of evidence and recommendation grades are based on the classification proposed by the Centre for Evidence Based Medicine at the University of Oxford4 (Table 1). To facilitate reading, we have included transient ischaemic attack (TIA) in our definition of ischaemic stroke. This practice coincides with current criteria. Therefore, all recommendations included in these clinical practice guidelines are directed at preventing focal cerebral ischaemia in general, without distinguishing between cerebral infarct and TIA.
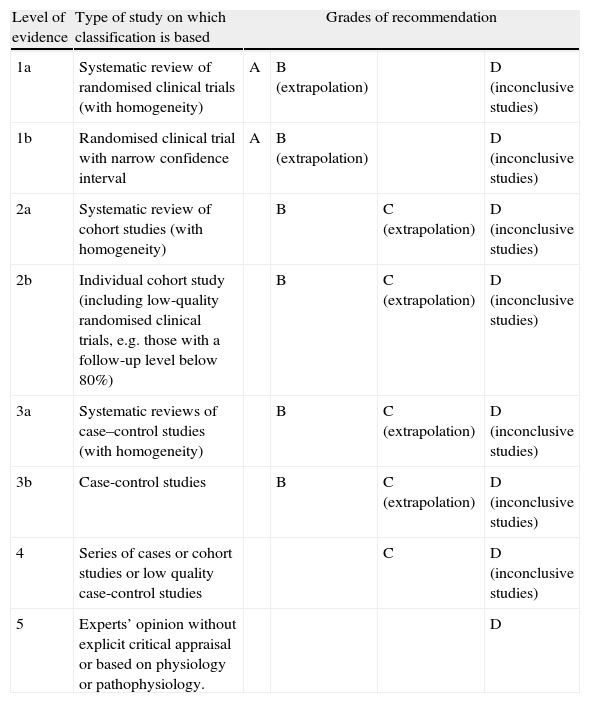
Levels of evidence and grades of recommendation.
| Level of evidence | Type of study on which classification is based | Grades of recommendation | |||
| 1a | Systematic review of randomised clinical trials (with homogeneity) | A | B (extrapolation) | D (inconclusive studies) | |
| 1b | Randomised clinical trial with narrow confidence interval | A | B (extrapolation) | D (inconclusive studies) | |
| 2a | Systematic review of cohort studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 2b | Individual cohort study (including low-quality randomised clinical trials, e.g. those with a follow-up level below 80%) | B | C (extrapolation) | D (inconclusive studies) | |
| 3a | Systematic reviews of case–control studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 3b | Case-control studies | B | C (extrapolation) | D (inconclusive studies) | |
| 4 | Series of cases or cohort studies or low quality case-control studies | C | D (inconclusive studies) | ||
| 5 | Experts’ opinion without explicit critical appraisal or based on physiology or pathophysiology. | D | |||
Source: Adapted from the Centre for Evidence Based Medicine (CEBM).1
Risk factors (RF) should be modified. In addition, antiplatelet drugs are recommended along with carotid endarterectomy or percutaneous transluminal angioplasty in selected patients.
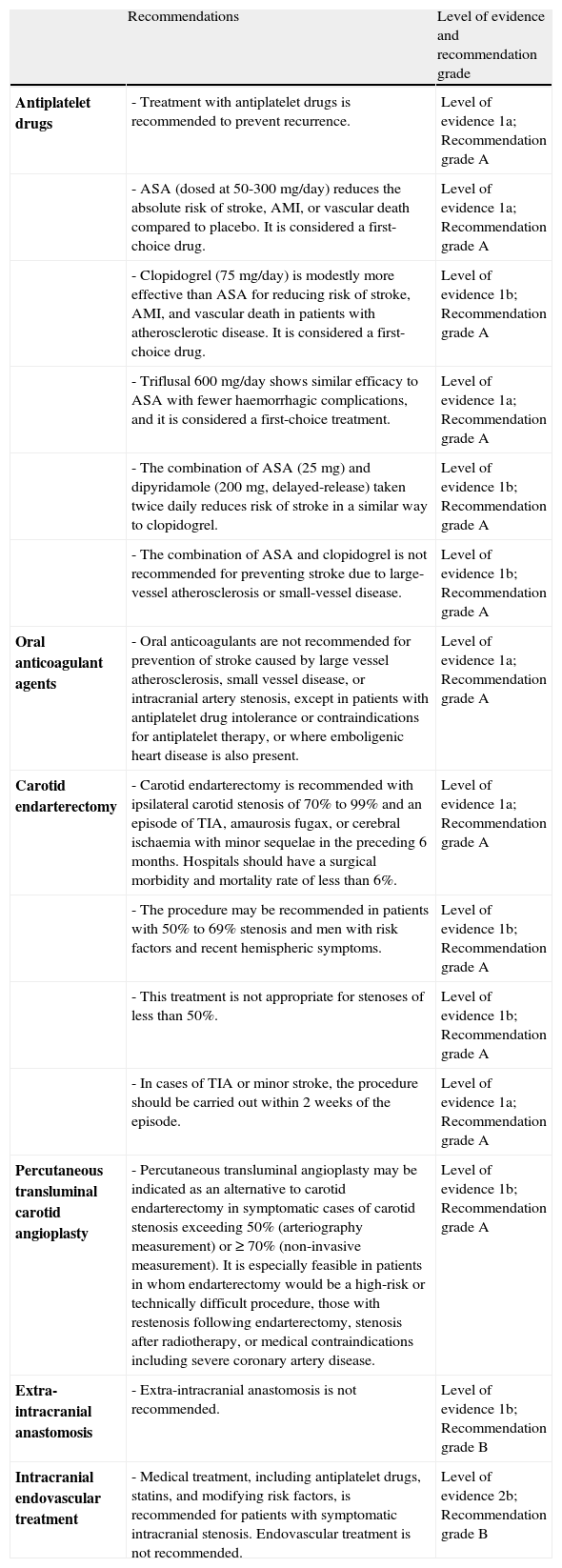
Recommendations. prevention of ischaemic stroke associated with large-vessel arteriosclerosis and small-vessel disease.
| Recommendations | Level of evidence and recommendation grade | |
| Antiplatelet drugs | - Treatment with antiplatelet drugs is recommended to prevent recurrence. | Level of evidence 1a; Recommendation grade A |
| - ASA (dosed at 50-300mg/day) reduces the absolute risk of stroke, AMI, or vascular death compared to placebo. It is considered a first-choice drug. | Level of evidence 1a; Recommendation grade A | |
| - Clopidogrel (75mg/day) is modestly more effective than ASA for reducing risk of stroke, AMI, and vascular death in patients with atherosclerotic disease. It is considered a first-choice drug. | Level of evidence 1b; Recommendation grade A | |
| - Triflusal 600mg/day shows similar efficacy to ASA with fewer haemorrhagic complications, and it is considered a first-choice treatment. | Level of evidence 1a; Recommendation grade A | |
| - The combination of ASA (25mg) and dipyridamole (200mg, delayed-release) taken twice daily reduces risk of stroke in a similar way to clopidogrel. | Level of evidence 1b; Recommendation grade A | |
| - The combination of ASA and clopidogrel is not recommended for preventing stroke due to large-vessel atherosclerosis or small-vessel disease. | Level of evidence 1b; Recommendation grade A | |
| Oral anticoagulant agents | - Oral anticoagulants are not recommended for prevention of stroke caused by large vessel atherosclerosis, small vessel disease, or intracranial artery stenosis, except in patients with antiplatelet drug intolerance or contraindications for antiplatelet therapy, or where emboligenic heart disease is also present. | Level of evidence 1a; Recommendation grade A |
| Carotid endarterectomy | - Carotid endarterectomy is recommended with ipsilateral carotid stenosis of 70% to 99% and an episode of TIA, amaurosis fugax, or cerebral ischaemia with minor sequelae in the preceding 6 months. Hospitals should have a surgical morbidity and mortality rate of less than 6%. | Level of evidence 1a; Recommendation grade A |
| - The procedure may be recommended in patients with 50% to 69% stenosis and men with risk factors and recent hemispheric symptoms. | Level of evidence 1b; Recommendation grade A | |
| - This treatment is not appropriate for stenoses of less than 50%. | Level of evidence 1b; Recommendation grade A | |
| - In cases of TIA or minor stroke, the procedure should be carried out within 2 weeks of the episode. | Level of evidence 1a; Recommendation grade A | |
| Percutaneous transluminal carotid angioplasty | - Percutaneous transluminal angioplasty may be indicated as an alternative to carotid endarterectomy in symptomatic cases of carotid stenosis exceeding 50% (arteriography measurement) or ≥ 70% (non-invasive measurement). It is especially feasible in patients in whom endarterectomy would be a high-risk or technically difficult procedure, those with restenosis following endarterectomy, stenosis after radiotherapy, or medical contraindications including severe coronary artery disease. | Level of evidence 1b; Recommendation grade A |
| Extra-intracranial anastomosis | - Extra-intracranial anastomosis is not recommended. | Level of evidence 1b; Recommendation grade B |
| Intracranial endovascular treatment | - Medical treatment, including antiplatelet drugs, statins, and modifying risk factors, is recommended for patients with symptomatic intracranial stenosis. Endovascular treatment is not recommended. | Level of evidence 2b; Recommendation grade B |
ASA reduces the absolute risk of stroke, acute myocardial infarction (AMI), or vascular death by 13% to 25% compared to placebo.5–7 This is a first-choice antiplatelet drug and the recommended dose is 100 to 300mg/day.8,9
ClopidogrelIn patients with atherosclerotic disease (AMI, stroke, or peripheral artery disease), ASA is slightly more effective for reducing the risk of stroke, AMI, and vascular death (decrease in relative risk of 8.7%) with fewer digestive tract haemorrhages and the same risk of neutropenia.10 A sub-analysis in the CAPRIE study found that clopidogrel was no more effective than ASA in patients with a prior stroke.10 However, clopidogrel was more effective in the subgroup of patients with a history of symptomatic atherosclerosis (prior IS or AMI).11 These observations should be considered with care, given that the CAPRIE study was not designed for subgroup analysis. Clopidogrel dosed at 75mg/day is considered a first-choice treatment, especially in patients with ASA intolerance.
TriflusalIn doses of 600mg/day, triflusal has a similar efficacy to that of ASA with fewer haemorrhagic complications.12–14 It is also considered a first-choice treatment.
DipyridamoleAlthough dipyridamole reduces stroke recurrence by 18% compared to placebo, it is not used in monotherapy because it has no effect on vascular death.15
CilostazolThe Second Cilostazol Stroke Prevention Study (CSPS2), conducted in Asian patients with IS, showed that cilostazol (100mg/12h) was noninferior and could even be superior to ASA (81mg/d) for preventing stroke recurrences due to being associated with a lower frequency of haemorrhagic complications.16 A recent Cochrane meta-analysis confirms that cilostazol was superior to ASA for preventing stroke recurrence in patients with IS.17
Other antiplatelet drugs under studySarpogrelateSarpogrelate's efficacy for secondary stroke prevention resembles that of ASA, and its haemorrhagic complication rate is lower.18
SCH 530348Follow-up on stroke patients was interrupted prematurely in the TRA-TIMI50 study as there was an increased risk of haemorrhage. The drug is still being investigated for patients with ischaemic heart disease or peripheral artery disease.
PrasugrelCompared to clopidogrel, prasugrel significantly reduced cardiovascular episodes in patients at moderate to high risk for acute coronary syndrome and treated with percutaneous coronary angioplasty. It is associated with a higher number of haemorrhagic complications; mortality was similar in both groups.19 This drug is not recommended in patients in early phases after stroke or TIA, and there have been no trials investigating its potential for secondary stroke prevention.
TicagrelorCompared to clopidogrel, ticagrelor reduced the number of AMI and vascular deaths or deaths due to other causes among patients with acute coronary syndrome. While there were no differences in the overall number of major haemorrhagic complications, the percentage of fatal cerebral haemorrhages was lower.20 No studies have been conducted to specifically examine secondary stroke prevention with ticagrelor.
Associations of antiplatelet drugsASA and dipyridamoleThe ESPS2 and ESPRIT studies were the first to identify the combination of ASA and dipyridamole (ASA/DPR) as being superior to ASA for preventing vascular episodes.21,22 However, the validity of these results has been called into question due to methodology problems. A systematic review showed that the ASA/DPR combination, compared to ASA alone, decreased the risk of a new, non-fatal stroke by 23%.23 The PRoFESS study compared clopidogrel 75mg/d to ASA 25mg/DPR 200mg (extended release, administered twice daily). That study found that ASA/DPR was not superior to clopidogrel in the areas of secondary stroke prevention,24 functional recovery,25 or the potential development of cognitive impairment.26
ASA and clopidogrelThe MATCH study examined the efficacy of the combination of ASA 75mg/d and clopidogrel 75mg/d (ASA/CLP), compared to clopidogrel alone, in patients with a history of stroke or TIA. The combination was not superior to clopidogrel and it was associated with a significantly higher risk of haemorrhage.27 Additionally, the CHARISMA28 study in patients with clinically evident vascular disease or multiple RFs did not show ASA/CLP to have greater efficacy for stroke recurrence reduction than ASA monotherapy. One of the objectives of the study Secondary Prevention of Small Subcortical Strokes (SPS3), currently underway, is to evaluate the efficacy of ASA/CLP in reducing stroke recurrence in patients with small vessel disease.
Other combinationsAdditional combinations of anti-platelet drugs have also been tested, including ASA/DPR/CLP and ASA/CLP with glycoprotein IIb/IIIa inhibitors. Data were insufficient in both cases to yield recommendations for stroke prevention.29
Oral anticoagulant agentsThe SPIRIT study30 compared high-intensity oral anticoagulants (3.0-4.5 INR) to ASA (30mg/d). It was terminated prematurely due to a high volume of haemorrhagic complications in the anticoagulant group. The WARSS31 study compared 325mg/d ASA to warfarin adjusted for an INR target of 1.4 to 2.8 and had identified no differences at the 2-year point. The ESPRIT study concluded that medium-intensity oral anticoagulants (2-3 INR) were no more effective than ASA for secondary prevention of IS, and that any possible protective effect was cancelled out by the number of haemorrhagic complications.32 One systematic review showed that anticoagulants (INR of up to 2.6) displayed no advantages compared to antiplatelet treatment with regard to either risk of death by vascular causes or overall mortality. Furthermore, intensive anticoagulant treatment (3-4.5 INR) is associated with a significant increase in total mortality and severe haemorrhagic episodes.33
Data from studies or specific cases of non-cardioembolic stroke appear below.
Stenosis of the intracranial arteriesThe WASID study for symptomatic intracranial stenosis was interrupted prematurely since warfarin showed higher rates of haemorrhagic complications and no benefits compared to ASA dosed at 1300mg/d.34
Basilar artery stenosisAn analysis of the WASID study suggested that these patients might benefit from anticoagulant treatment even though differences were not significant.35
Stroke recurrence despite antiplatelet treatmentOral anticoagulants have not shown any benefits in these patients.35
Vascular interventional proceduresExtracranial carotid artery stenosisCarotid endarterectomyEndarterectomy offers more advantages than medical treatment in cases of symptomatic carotid stenosis >70%. The NASCET study shows an improvement of 17% at the 2-year mark36 and ECST shows 14% improvement at the 3-year mark.37 For moderate cases of stenosis (50-69%), the benefit of carotid endarterectomy is less pronounced (4.5% reduction in absolute risk at 5 years)38; there is no significant benefit for stenosis <50%.38,39 In contrast, a meta-analysis that includes the ECST and NASCET studies points to higher benefits from endarterectomy in men, patients older than 75 years, and patients undergoing the procedure within 2 weeks of the stroke or TIA; it is less effective at later dates.40 It is currently indicated in patients with symptomatic carotid stenosis of 70% to 99% and an additional life expectancy of more than 5 years, provided that the hospital in question has a surgical morbidity and mortality rate of less than 6%.41–43 Endarterectomy may be considered in patients with symptomatic carotid stenosis of 50% to 69%, but certain variables may affect the risk-benefit balance: female sex (no clear benefit in clinical trials); initial manifestation as hemispheric IS (better results than in retinal ischaemia); or presence of a contralateral carotid occlusion, which is associated with a greater preoperative risk although benefits remain.42,43 Medical treatment without endarterectomy is indicted in patients with carotid stenosis <50%.41–43
Percutaneous transluminal angioplastyThis technique has shown good results in patients with fibromuscular dysplasia, lesions due to radiotherapy, or restenosis following endarterectomy. It has been suggested as a treatment alternative for stenosing atherosclerosis. The CAVATAS study showed similar efficacy and safety results for percutaneous transluminal angioplasty and endarterectomy.44 Technical advances include specific angioplasty balloons, stents, and most recently, distal protection devices. The SAPPHIRE study of carotid angioplasty and stenting showed that the technique was superior to endarterectomy in patients with a high level of surgical risk and symptomatic or asymptomatic carotid stenosis.45 However, early interruption of the EVA-3S study due to high rates of 30-day mortality or stroke in the carotid angioplasty group,46 and the SPACE study's failure to demonstrate that the technique was noninferior to carotid endarterectomy may constitute reasons to question carotid angioplasty as a safe alternative.47 However, long-term follow-up (2–4 years) has shown similar levels of reduction of ipsilateral IS for both techniques, although researchers stress the need to improve short-term safety results of carotid angioplasty and stenting.48,49 A meta-analysis indicates that endovascular treatment is associated with a modest increase in stroke and death rates 30 days after the procedure, with no significant intergroup differences in rates of incapacitating stroke or death at 30 days. On the other hand, surgical treatment is associated with a significant increase in cranial nerve palsy and AMI.50 The ICSS study51 found a 3.3% difference in absolute risk of stroke, death, or AMI at 120 days that pointed to endarterectomy as the superior technique. The CAVATAS study52 identified a higher incidence of non-perioperative stroke in patients with endovascular treatment (21.1%) than in those treated with endarterectomy (15.4%). This difference was observed even after the 2-year randomisation process, and it may be explained by the higher incidence of restenosis following angioplasty. Only a small group of patients in the CAVATAS study underwent stenting (22%). The true long-term incidence rate for post-stent restenosis is unknown. The CREST study, conducted in patients with high-grade stenosis, whether symptomatic (53%) or asymptomatic (47%), observed no significant differences regarding the study's main objective. During the periprocedural period, however, there was a greater risk of non-disabling stroke with stenting, and a greater risk of AMI with endarterectomy.53 One important consideration is that over time (4 years), the rates of stroke recurrence were very low with both treatment options. This may be partially explained by the high percentage of asymptomatic patients in this group. Factors associated with a higher risk of periprocedural stroke or death with carotid angioplasty include male sex, age >70 years, prior stroke, and having experienced a stroke rather than a TIA.48,54
In cases of symptomatic stenosis of the common carotid artery, the reasonable options are revascularisation by carotid angioplasty, direct arterial reconstruction, or extra-anatomic bypass grafting. However, available evidence is insufficient to make recommendations.55
Extracranial vertebral artery stenosis; subclavian artery stenosisIndications for revascularisation in cases of proximal vertebral artery stenosis are infrequent; generally speaking, the contralateral artery will supply the basilar artery sufficiently. In cases of stroke recurrence despite appropriate medical treatment, surgical or endovascular treatment may be considered. Evidence is insufficient to recommend one procedure over another.42,55 Surgical options include trans-subclavian vertebral endarterectomy, transposition of the vertebral artery to the ipsilateral common carotid artery, and reimplantation of the vertebral artery using a venous graft extension to the subclavian artery. The only randomised study on endovascular treatment in patients with vertebral artery stenosis was CAVATAS, which randomised 16 patients to receive either endovascular treatment and medical treatment, or medical treatment alone.56 In cases of cerebral ischaemia in the posterior cerebral territory due to subclavian artery stenosis, doctors may consider the possibility of a carotid-subclavian extra-anatomic bypass for cases with low surgical risk. Percutaneous angioplasty and stenting should be considered in cases with an elevated risk of surgical complications.55
Intracranial arterial stenosisThe WASID trial found a yearly risk of stroke of 11% despite the best medical treatment; this figure was 19% in the subgroup with stenosis ≥70%.34 These patients present a high risk of recurrent ischaemia in the territory of the stenosed artery. This is also seen in the vertebrobasilar territory (12% at 1 year and 15% at 2 years).57 In 2005, the FDA approved the use of the Wingspan stent system, which is effective in 96.7 to 98.8% of all cases and has a 30-day complication rate of 6.1 to 9.6%.58 In one European registry, 25 to 30% of patients experienced restenosis (mostly asymptomatic), while 4.8% experienced an incapacitating stroke. The mortality rate was 2.2%.59 Researchers recently halted the SAMMPRIS study (Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis). The study compared angioplasty and the Wingspan stent system associated with medical treatment (modification of risk factors and ASA/CLP combination treatment) to medical treatment alone in patients with symptomatic intracranial stenosis >70% and IS in the first 30 days. Recruitment was stopped after 451 patients, rather than the anticipated 764 since significant benefits were found for the medical treatment group, which yielded lower rates of stroke or death at 30 days (5.8% in the medical treatment group vs 14% in the Wingspan group).
Extra-intracranial arterial anastomosisApproximately 10% of patients with non-disabling stroke present occlusion or stenosis of the internal carotid artery or middle cerebral artery. Effects of this condition might be reduced by anastomosing the superficial temporal branch of the external carotid artery to an extra-intracranial bypass, although the only completed clinical trial yielded negative results.60 Regardless of the effectiveness this technique has displayed in selected patients with giant aneurysm of the internal carotid artery, or moyamoya disease in young patients, there are currently no indications for extra-intracranial anastomosis as a means of preventing stroke.61 Results from the clinical trial Carotid Occlusion Surgery Study, presented at ISC 2011, did not show surgery to be an effective means of preventing stroke recurrence at 2 years because of the low recurrence rate in the non-surgical group.
Ischaemic stroke of cardioembolic origin (Table 3)Nonvalvular atrial fibrillationCurrent treatment follows 2 strategies: prevention of thromboembolism using antithrombotic agents, and treatment of the FA itself, including stabilising both heart rate and rhythm. Furthermore, new non-pharmacological treatments are being developed to decrease risk of stroke in patients with non-valvular AF.62–64
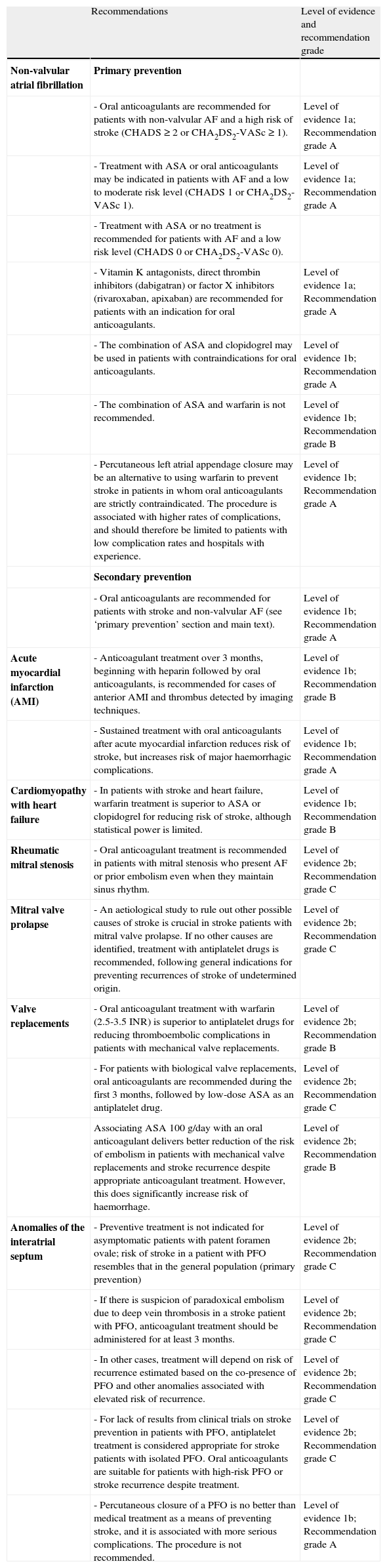
Recommendations for preventing ischaemic stroke of cardioembolic origin.
| Recommendations | Level of evidence and recommendation grade | |
| Non-valvular atrial fibrillation | Primary prevention | |
| - Oral anticoagulants are recommended for patients with non-valvular AF and a high risk of stroke (CHADS ≥ 2 or CHA2DS2-VASc ≥ 1). | Level of evidence 1a; Recommendation grade A | |
| - Treatment with ASA or oral anticoagulants may be indicated in patients with AF and a low to moderate risk level (CHADS 1 or CHA2DS2-VASc 1). | Level of evidence 1a; Recommendation grade A | |
| - Treatment with ASA or no treatment is recommended for patients with AF and a low risk level (CHADS 0 or CHA2DS2-VASc 0). | ||
| - Vitamin K antagonists, direct thrombin inhibitors (dabigatran) or factor X inhibitors (rivaroxaban, apixaban) are recommended for patients with an indication for oral anticoagulants. | Level of evidence 1a; Recommendation grade A | |
| - The combination of ASA and clopidogrel may be used in patients with contraindications for oral anticoagulants. | Level of evidence 1b; Recommendation grade A | |
| - The combination of ASA and warfarin is not recommended. | Level of evidence 1b; Recommendation grade B | |
| - Percutaneous left atrial appendage closure may be an alternative to using warfarin to prevent stroke in patients in whom oral anticoagulants are strictly contraindicated. The procedure is associated with higher rates of complications, and should therefore be limited to patients with low complication rates and hospitals with experience. | Level of evidence 1b; Recommendation grade A | |
| Secondary prevention | ||
| - Oral anticoagulants are recommended for patients with stroke and non-valvular AF (see ‘primary prevention’ section and main text). | Level of evidence 1b; Recommendation grade A | |
| Acute myocardial infarction (AMI) | - Anticoagulant treatment over 3 months, beginning with heparin followed by oral anticoagulants, is recommended for cases of anterior AMI and thrombus detected by imaging techniques. | Level of evidence 1b; Recommendation grade B |
| - Sustained treatment with oral anticoagulants after acute myocardial infarction reduces risk of stroke, but increases risk of major haemorrhagic complications. | Level of evidence 1b; Recommendation grade A | |
| Cardiomyopathy with heart failure | - In patients with stroke and heart failure, warfarin treatment is superior to ASA or clopidogrel for reducing risk of stroke, although statistical power is limited. | Level of evidence 1b; Recommendation grade B |
| Rheumatic mitral stenosis | - Oral anticoagulant treatment is recommended in patients with mitral stenosis who present AF or prior embolism even when they maintain sinus rhythm. | Level of evidence 2b; Recommendation grade C |
| Mitral valve prolapse | - An aetiological study to rule out other possible causes of stroke is crucial in stroke patients with mitral valve prolapse. If no other causes are identified, treatment with antiplatelet drugs is recommended, following general indications for preventing recurrences of stroke of undetermined origin. | Level of evidence 2b; Recommendation grade C |
| Valve replacements | - Oral anticoagulant treatment with warfarin (2.5-3.5 INR) is superior to antiplatelet drugs for reducing thromboembolic complications in patients with mechanical valve replacements. | Level of evidence 2b; Recommendation grade B |
| - For patients with biological valve replacements, oral anticoagulants are recommended during the first 3 months, followed by low-dose ASA as an antiplatelet drug. | Level of evidence 2b; Recommendation grade C | |
| Associating ASA 100g/day with an oral anticoagulant delivers better reduction of the risk of embolism in patients with mechanical valve replacements and stroke recurrence despite appropriate anticoagulant treatment. However, this does significantly increase risk of haemorrhage. | Level of evidence 2b; Recommendation grade B | |
| Anomalies of the interatrial septum | - Preventive treatment is not indicated for asymptomatic patients with patent foramen ovale; risk of stroke in a patient with PFO resembles that in the general population (primary prevention) | Level of evidence 2b; Recommendation grade C |
| - If there is suspicion of paradoxical embolism due to deep vein thrombosis in a stroke patient with PFO, anticoagulant treatment should be administered for at least 3 months. | Level of evidence 2b; Recommendation grade C | |
| - In other cases, treatment will depend on risk of recurrence estimated based on the co-presence of PFO and other anomalies associated with elevated risk of recurrence. | Level of evidence 2b; Recommendation grade C | |
| - For lack of results from clinical trials on stroke prevention in patients with PFO, antiplatelet treatment is considered appropriate for stroke patients with isolated PFO. Oral anticoagulants are suitable for patients with high-risk PFO or stroke recurrence despite treatment. | Level of evidence 2b; Recommendation grade C | |
| - Percutaneous closure of a PFO is no better than medical treatment as a means of preventing stroke, and it is associated with more serious complications. The procedure is not recommended. | Level of evidence 1b; Recommendation grade A |
The European Atrial Fibrillation Trial (EAFT) in stroke patients with non-valvular AF showed a 66% decrease in the IS recurrence rate in the warfarin group, vs a 15% decrease in the ASA group (300mg).65 The number needed to treat (NNT) to avoid one stroke per year is 14 for warfarin and 50 for ASA.66 While vitamin K antagonists (VKA) are effective, they pose significant problems in clinical practice and are consequentially often underused. Vitamin K antagonists may also be dosed incorrectly due to their delayed action and metabolism, narrow therapeutic range, the considerable variation in their metabolism process and numerous interactions with foods and other drugs, the presence of genetic polymorphisms that affect the dosing requirements, and the need for regular coagulation monitoring and frequent dose adjustments, which generates additional costs.62 As a result, only 10% to 18% of these patients have an appropriate INR.67,68 Risk of haemorrhage is another severe problem with VKA treatment. This risk can be reduced by treating AHT and monitoring INR.62 If the range is appropriate (2-3 INR), the risk of cerebral haemorrhage is 0.5% per year with a NNT of 200; this is acceptable when compared to the NNT of 14 for avoiding a stroke.66 We recommend using the HAS-BLED scale of risk of severe haemorrhage to assess the risk-benefit balance of oral anticoagulants. Patients with scores ≥3 are considered to be at high risk.69 On the other hand, despite risk of haemorrhage, oral anticoagulants show clear benefits in patients over 85 and with AF.70
New anticoagulants are currently available (thrombin and factor X inhibitors) that provide the added advantage of not requiring INR level monitoring. Dabigatran dosed at 100mg/12h has shown similar efficacy to warfarin treatment for stroke reduction. It is associated with a lower frequency of major haemorrhages; at doses of 150mg/12h, it is more effective than warfarin for stroke reduction with similar haemorrhage rates.71 Dabigatran is easy to administer, as it has set doses and does not require monitoring. Analysis of the subgroup with IS showed efficacy results for dabigatran that were similar to those for the total group, but tending towards superiority over warfarin. Both dabigatran dosages also showed a significantly lower risk of intracranial haemorrhage and haemorrhagic stroke.72 In a cost-effectiveness study in patients older than 65 years with a CHADS2 score ≥2, dabigatran proved itself to be an alternative to warfarin based on prices in the USA.73 Dabigatran has been approved by the FDA and by Health Canada, and it is pending approval in Europe. European and AHA guidelines63,74 place dabigatran on par with VKAs for stroke prevention in patients with AF. Doses of 110mg/12h are indicated in patients with a lower risk of cardioembolism or a higher risk of haemorrhage (very advanced age, poorly managed AHT, prior cerebral haemorrhage, neuroimaging evidence of leukoaraiosis or cerebral microhaemorrhage, or HAS-BLED score ≥3). Doses of 150mg/12h are indicated for patients with a higher risk of cardioembolism or INR below the therapeutic range despite VKA treatment, or where there are other VKA-related problems. For secondary prevention of cardioembolic stroke, 150mg/12h is an appropriate dose in patients with a HAS-BLED score < 3 or stroke recurrence despite correct treatment with VKAs. The dose of 110mg/12h is recommended for HAS-BLED scores ≥3. The AVERROES study75 in patients with AF and a VKA contraindication showed a significant impact by apixaban compared to ASA on the annual stroke or systemic embolism rate (1.6% vs 3.6%). Haemorrhage rates were similar for both treatments. The ROCKET AF study76,77 demonstrated that rivaroxaban dosed at 20mg every 24hours is noninferior to warfarin as a means of preventing stroke and peripheral embolism without increasing risk of haemorrhage. Analysis of the treated population found significant differences (1.71% vs 2.16%) with rivaroxaban appearing to be superior. Other anticoagulants currently being developed include edoxaban and betrixaban.
Antiplatelet drugsThe EAFT study65 showed that oral anticoagulants were clearly superior to ASA 300mg for secondary stroke prevention. The ACTIVE-A study showed that 2 antiplatelet drugs used in combination (ASA 75-100mg/CLP 75mg) in patients with a contraindication for VKAs achieved a 28% reduction in risk of stroke compared to ASA alone (75-100mg). However, there was a significant increase in risk of major haemorrhagic complications.78 In contrast, the ACTIVE W study found oral anticoagulants to be superior to the ASA 75-100mg/CLP 75mg combination for reducing stroke, systemic embolism, AMI, and vascular death.79
Combining oral anticoagulants and antiplatelet drugsIn cases of embolism recurrence despite appropriate anticoagulant treatment, empirical treatment calls for associating these drugs with ASA. Nevertheless, studies have found no differences in stroke or systemic embolism reduction through treatment with warfarin and ASA compared to warfarin alone, whereas the combination is associated with a significant increase in haemorrhagic complications.80 On the other hand, a significant reduction in the risk of death of vascular causes, TIA, non-fatal stroke, and systemic embolism has been observed with the combination of triflusal 600mg/day and moderate-intensity anticoagulants, without a significant increase in haemorrhagic complications.81 A long-term analysis of one patient subgroup found that the combination of triflusal 600mg/day and an oral anticoagulant was superior to anticoagulant only.82
Antiarrhythmic agentsA post hoc analysis of the ATHENA83 study in patients with persistent or paroxysmal AF found that dronedarone reduced risk of stroke by 36% regardless of presence or absence of antithrombotic treatment.84 Doctors should maintain anticoagulant treatment after stabilising cardiac rhythm in patients with an elevated risk of cardioembolism (CHADS2≥2).85 After cardiac ablation, the AF recurrence rate is 13% at 2 years, 21.8% at 3 years, 35% at 4 years, 46.8% at 5 years, and 54.6% at 6 years. Even patients who do not experience recurrence in the first year cannot be considered cured; follow-up studies show that 40% will experience recurrence.86
Other drug treatmentsUse of statins may prevent either appearance or recurrence of AF.87 Use of ACE inhibitors or ARBs to block the renin-angiotensin system has been shown to reduce the risk of de novo AF.88
Non-pharmacological treatmentsIn most patients with non-valvular AF, thrombi are located in the left atrial appendage. As a result, one possible approach would be excising that structure in selected patients with absolute contraindications to oral anticoagulants. In the PROTECT AF study, percutaneous closure of the atrial appendage was noninferior to warfarin for stroke prevention, although it yielded a higher rate of periprocedural complications.89
Other causes of cardioembolismEvidence regarding risk and recommendations for preventing stroke due to other cardioembolic causes are not as clear as in non-valvular AF or in other specific situations.
Acute myocardial infarction (AMI)Early onset of heparin treatment followed by oral anticoagulants decreased the risk of IS from 3% to 1% and yielded a >50% reduction in thrombus formation.42 A minimum of 3 months of oral anticoagulant treatment is recommended in anterior AMI with a thrombus detected by an imaging study.90 A meta-analysis comparing oral anticoagulants associated with ASA or used in monotherapy, ASA alone, or placebo found that while warfarin did not reduce mortality or re-infarction rates, it did decrease risk of stroke while also increasing risk of major haemorrhage.91
Cardiomyopathy with heart failureIn the Warfarin and Antiplatelet Therapy in Chronic Heart Failure trial (WATCH),92 warfarin was associated with a significantly lower stroke rate (0.6%) than ASA dosed at 162mg/day or clopidogrel at 75mg/day (2.3%). However, the study's statistical power is insufficient to establish which treatment is better. Another study, Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF), is currently underway in patients with LVEF ≤ 35%.93
Rheumatic mitral stenosisOral anticoagulant treatment is recommended in patients with mitral stenosis and AF or prior embolism, even when they maintain sinus rhythm.94
Mitral valve prolapseThere are no available clinical trials in patients with stroke and mitral valve prolapse. Stroke patients with mitral valve prolapse will require an aetiological study to rule out other possible causes of stroke. If no other causes are identified, antiplatelet treatment is recommended according to general indications for preventing recurrences of stroke of undetermined origin. Oral anticoagulants should be prescribed when patients experience recurrence despite ASA treatment, evidence of prior systemic embolism, or associated AF.
Valve replacementsThe indication for antithrombotic treatment depends on the type of replacement and associated RFs. As a means of reducing thromboembolic complications, warfarin has been shown to be superior to 2 different antiplatelet regimens (ASA dosed at 650 or 990mg/day + dipyridamole 150-225mg/day or ASA 650mg/day + pentoxifylline 800mg/day).95 Oral anticoagulants are indicated for mechanical valves; however, even when INR levels are appropriate (2.5–3.5), there is still a yearly risk of thromboembolism of 1-2%. Valves in the mitral position are at more risk than those in the aortic position. INR should therefore remain between 2.5 and 3.5 for mechanical valves in the mitral position, but lower, between 2.0 and 3.0, for bivalvular or Medtronic Hall valves in the aortic position, provided that the factors mentioned above are not present. For biological valves, oral anticoagulants are recommended during the first 3 months after surgery, which is when emboli are most likely to appear (before the valve is completely endothelialised). Subsequently, doctors may consider long-term antiplatelet therapy with low-dose ASA (75-100mg), provided that the patient does not have associated RFs such as AF, history of thromboembolism, left ventricular dysfunction, or a hypercoagulable state. Oral anticoagulants are recommended in these cases.94 Associating low doses of ASA (100mg/day) or dipyridamole with an oral anticoagulant is more effective for reducing risk of embolism in patients with mechanical valve replacements and stroke recurrence despite appropriate anticoagulant treatment. However, this does significantly increase risk of haemorrhage.96
Patent foramen ovale (PFO) and atrial septal aneurysm (ASA)Preventive treatment is not indicated for asymptomatic patients with patent foramen ovale; risk of stroke in a patient with PFO resembles that in the general population.90,97 If doctors suspect paradoxical embolism due to deep vein thrombosis in a stroke patient with PFO, anticoagulant treatment should be administered for at least 3 months. In other cases, treatment will depend on the risk of recurrence, which is estimated based on co-presence of PFO and other anomalies that are associated with an increased yearly risk of recurrence. Such anomalies include hypercoagulable state or co-presence of an ASA; the significance of the latter is more debatable.98–100 PICSS, a sub-study of the WARSS clinical trial, observed no differences in recurrence rates among patients with cryptogenic stroke and PFO treated with either aspirin or oral anticoagulants.101 For lack of results from clinical trials on stroke prevention in patients with PFO, antiplatelet treatment is considered appropriate for stroke patients with isolated PFO. Oral anticoagulants are suitable for patients with high-risk PFO or stroke recurrence despite treatment. Results from the CLOSURE-I study102 show that percutaneous closure of PFO is nonsuperior to medical treatment for stroke prevention, and that its complication rate is higher. The RESPECT and REDUCE trials are still underway, and at present, percutaneous closure of PFO is not recommended except in clinical trials.103
Infarcts of rare causes (Table 4)In this section, we consider only the main aetiologies implicated in IS of rare causes in our setting. These include artery dissection, prothrombotic states, atheromatous plaque in the aortic arch, and lastly, thrombosis of dural sinuses.
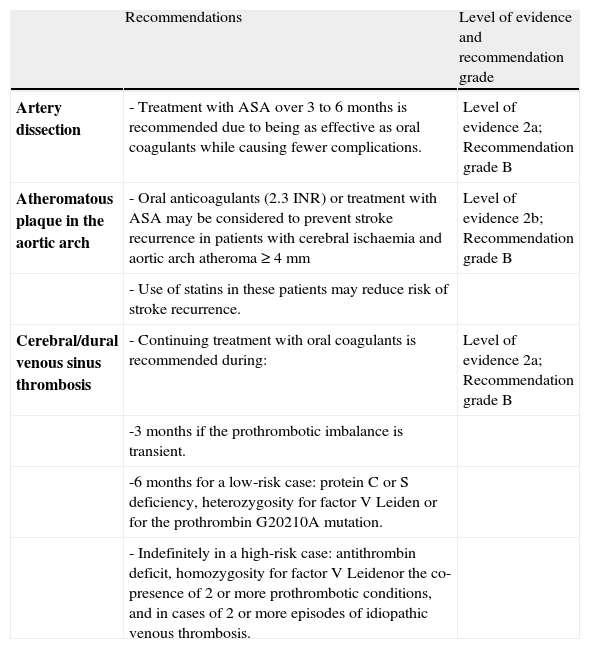
Recommendations for preventing ischaemic stroke of rare origin.
| Recommendations | Level of evidence and recommendation grade | |
| Artery dissection | - Treatment with ASA over 3 to 6 months is recommended due to being as effective as oral coagulants while causing fewer complications. | Level of evidence 2a; Recommendation grade B |
| Atheromatous plaque in the aortic arch | - Oral anticoagulants (2.3 INR) or treatment with ASA may be considered to prevent stroke recurrence in patients with cerebral ischaemia and aortic arch atheroma ≥4mm | Level of evidence 2b; Recommendation grade B |
| - Use of statins in these patients may reduce risk of stroke recurrence. | ||
| Cerebral/dural venous sinus thrombosis | - Continuing treatment with oral coagulants is recommended during: | Level of evidence 2a; Recommendation grade B |
| -3 months if the prothrombotic imbalance is transient. | ||
| -6 months for a low-risk case: protein C or S deficiency, heterozygosity for factor V Leiden or for the prothrombin G20210A mutation. | ||
| - Indefinitely in a high-risk case: antithrombin deficit, homozygosity for factor V Leidenor the co-presence of 2 or more prothrombotic conditions, and in cases of 2 or more episodes of idiopathic venous thrombosis. |
There is no evidence regarding treatment of carotid or extracranial vertebral dissection; analysis of studies performed with either ASA or oral anticoagulants does not reveal significant differences between these regimens.104–106 A prospective study showed a higher frequency of major haemorrhages with antithrombotic treatment (antiplatelet, 1%; anticoagulant, 2%) than of IS (0.3%).104 As efficacy is similar, it would be reasonable to use the drug with the lowest haemorrhagic risk, that is, the antiplatelet drug. Doctors believe that maintaining treatment during 3 to 6 months is appropriate, although no studies support this.42 A systematic review of cases of carotid or vertebral artery dissection treated with angioplasty and stenting concluded that this technique is safe.107 However, no conclusions can be drawn regarding its efficacy, since there are no studies comparing this option to medical treatment.
Prothrombotic statesDeep vein thrombosis is an indication for short- or long-term anticoagulation therapy, depending on the clinical and haematological circumstances.108 Although there are guidelines establishing general recommendations for acquired hypercoagulable states, there is no specific evidence regarding secondary stroke prevention.42 Oral anticoagulants are recommended in cases of protein C, S, and antithrombin III deficiency, especially in high-risk situations and for secondary prevention. For secondary prevention in patients with lupus anticoagulant, anticoagulant treatment is indicated, although there are no appropriately designed studies that assess the efficacy of that treatment compared to antiplatelet treatment. The APASS study revealed that the presence of antiphospholipid antibodies was not associated with an increased risk of stroke recurrence or with a different response to treatment with ASA 325mg/day or warfarin with an INR of 1.4-2.8.109 Based on these data, aspirin and low-intensity anticoagulation may be considered preventive treatments in patients with antiphospholipid antibody positivity after a first ischaemic stroke. Oral anticoagulants for an INR of 2-3 are recommended for cases of antiphospholipid syndrome.42
Atheromatous plaque in the aortic archAtheromatous plaque in the aortic arch is associated with an elevated risk of stroke recurrence and death, especially if plaques measure ≥4mm or they are complex (presence of ulceration or mobile components) despite treatment with ASA or oral anticoagulants.110,111 In a retrospective study, use of statins was independently associated with less stroke recurrence.112 The Aortic Arch Related Cerebral Hazard Trial (ARCH), currently underway, compares warfarin to ASA/CLP combination therapy.113 Oral anticoagulants or ASA treatment may be considered to prevent recurrent stroke in patients with ASA and atheromatous plaque in the aortic arch measuring 4mm or larger.114
Cerebral/dural venous sinus thrombosisTreatment with oral anticoagulants should be maintained for at least 3 months to avoid rethrombosis following the early-phase anticoagulant treatment.115,116 The decision to extend the treatment over the long term will depend on whether or not a prothrombotic risk factor is detected. If this risk factor is transient, anticoagulants may be discontinued after 3 months. For lower-risk situations, such as protein C or S deficiency, or heterozygosity for factor V Leiden or for the prothrombin G20210A mutation, anticoagulants may be maintained during 6 months. In contrast, for high-risk situations such as antithrombin deficiency, homozygosity for factor V Leiden, or co-presence of 2 or more prothrombotic conditions, long-term anticoagulation is recommended. This is also true in cases of multiple episodes of idiopathic venous thrombosis.117,118
Ischaemic stroke of undetermined originIn cryptogenic IS, antiplatelet drugs are recommended as the primary treatment method. Some authors recommend anticoagulants when strokes recur despite treatment with antiplatelet drugs. In cases of IS of undetermined aetiology due to an incomplete work-up, missing data must be obtained so as to start the most suitable treatment. For IS of several possible aetiologies, treatment should be tailored to the cause with the highest risk of recurrence and act on all causes where possible.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ad hoc committee of the SEN Study Group for Cerebrovascular Diseases formed to draw up clinical practice guidelines for stroke.
Coordinator: Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid.
Exuperio Díez-Tejedor (Coord), Hospital Universitario La Paz, Madrid; Blanca Fuentes (Secretary), Hospital Universitario La Paz, Madrid; María Alonso de Leciñana, Hospital Universitario Ramón y Cajal, Madrid; José Álvarez-Sabin, Hospital Universitari Vall d’Hebron, Barcelona; Juan Arenillas, Hospital Universitario Clínico de Valladolid; Sergio Calleja, Hospital Universitario Central de Asturias, Oviedo; Ignacio Casado, Hospital San Pedro, Cáceres; Mar Castellanos, Hospital Josep Trueta, Girona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Antonio Dávalos, Hospital Universitari Germans Trias i Pujol, Badalona; Fernando Díaz-Otero, Hospital Universitario Gregorio Marañón, Madrid; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; José Antonio Egido, Hospital Clínico Universitario San Carlos, Madrid; Juan Carlos Fernández, Hospital Universitario Dr. Negrín, Las Palmas; Mar Freijo, Hospital Universitario de Basurto, Bilbao; Blanca Fuentes, Hospital Universitario La Paz, Madrid; Jaime Gállego, Hospital General de Navarra, Pamplona; Andrés García Pastor, Hospital Universitario Gregorio Marañón, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; Francisco Gilo, Hospital Universitario La Princesa, Madrid; Pablo Irimia, Clínica Universitaria de Navarra, Pamplona; Aida Lago, Hospital Universitario La Fe, Valencia; José Maestre, Hospital Universitario Virgen de las Nieves, Granada; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Joan Martí-Fábregas, Hospital de la Santa Creu i Sant Pau, Barcelona; Patricia Martínez-Sánchez, Hospital Universitario La Paz, Madrid; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Carlos Molina, Hospital Universitari Vall d’Hebron, Barcelona; Ana Morales, Hospital Universitario Virgen de la Arrixaca, Murcia; Florentino Nombela, Hospital Universitario La Princesa, Madrid; Francisco Purroy, Hospital Universitario Arnau de Vilanova, Lérida; Marc Ribó, Hospital Universitari Vall d’Hebron, Barcelona; Manuel Rodríguez-Yáñez, Hospital Clínico Universitario, Santiago de Compostela; Jaime Roquer, Hospital del Mar, Barcelona; Francisco Rubio, Hospital Universitario de Bellvitge, Barcelona; Tomás Segura, Hospital Universitario de Albacete, Albacete; Joaquín Serena, Hospital Josep Trueta, Gerona; Patricia Simal, Hospital Clínico Universitario San Carlos, Madrid; Javier Tejada, Hospital Universitario de León, León; José Vivancos, Hospital Universitario La Princesa, Madrid.
José Álvarez-Sabín, Hospital Universitari Vall d’Hebron, Barcelona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; José Larracoechea, Hospital de Cruces, Bilbao; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Jorge Matías-Guiu, Hospital Clínico Universitario San Carlos, Madrid; Francisco Rubio, Hospital de Bellvitge, Barcelona.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, ParkhomenkoA, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators.Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011 Sep 15;365(11):981-92.
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GLJr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ; SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011 Sep 15;365(11):993-1003.
Author affiliations and members of the committee are listed in the Addendum.
Please cite this article as: Fuentes B, Gállego J, Gil-Nuñez A, Morales A, Purroy F, Roquer J, et al. Guía para el tratamiento preventivo del ictus isquémico y AIT (II). Recomendaciones según subtipo etiológico. Neurología. 2014;29:168–183.