Although citrulline is produced by nitric oxide (NO) synthase upon activation of the NMDA glutamate receptor, nitrite and nitrate (NOx) concentration is considered the best marker of NO synthesis, as citrulline is also metabolised by other enzymes. This study analyses the correlation between human cerebrospinal fluid NOx and citrulline concentrations in order to determine the extent to which citrulline reflects NO synthesis and glutamatergic neurotransmission.
MethodsParticipants were patients with acute neurological diseases undergoing lumbar puncture (n=240). NOx and amino acid concentrations were determined by HPLC.
ResultsNOx concentrations did not vary significantly where infection (P=.110) or inflammation (P=.349) were present. Multiple regression analysis showed that NOx concentration was correlated with glutamine (r=−0.319, P<.001) and citrulline concentrations (r=0.293, P=.005) but not with the citrulline/arginine ratio (r=−0.160, P=.173). ANCOVA confirmed that NOx concentration was correlated with citrulline concentration (F=7.6, P=.007) but not with the citrulline/arginine ratio (F=2.2, P=.136), or presence of infection (F=1.8, P=.173) or inflammation (F=1.4, P=.227). No association was found between NOx and arginine or glutamate concentrations.
ConclusionThe results suggest that CSF citrulline concentration reflects NOx synthesis to some extent, despite the contribution of other metabolic pathways. In addition, this study shows that glutamine is an important modulator of NO synthase activity, and that arginine and glutamate are not correlated with NOx.
Aunque la citrulina es producida por la sintasa del óxido nítrico (NO) al activarse el receptor glutamatérgico NMDA, nitritos y nitratos (NOx) son considerados los mejores marcadores de síntesis del NO pues citrulina es también metabolizada por otras enzimas. En este estudio se realizó un análisis de correlaciones de concentraciones de citrulina y NOx en líquido cefalorraquídeo humano para evaluar la proporción en que la citrulina refleja la síntesis del NO y la neurotransmisión glutamatérgica.
MétodosSe incluyeron pacientes con trastornos neurológicos agudos a los que se realizó punción lumbar (n=240). Se determinó la concentración de NOx y aminoácidos por HPLC.
ResultadosNOx no fue diferente por la presencia de infección (p=0,110) o inflamación (p=0,349). La regresión múltiple mostró que NOx correlacionó con glutamina (r=−0,319, p<0,001) y citrulina (r=0,293, p=0,005) pero no con el cociente citrulina/arginina (r=−0,160, p=0,173). El ANCOVA confirmó que NOx está asociado con citrulina (F=7,6; p=0,007) pero no con el cociente citrulina/arginina (F=2,2; p=0,136), ni con la infección (F=1,8; p=0,173) o la inflamación (F=1,4; p=0,227). NOx no correlacionó con arginina ni con glutamato.
ConclusionesEsto sugiere que citrulina refleja parte de la síntesis del NO a pesar de la contribución de otras vías metabólicas. Además, este estudio muestra que la glutamina es un modulador importante de la actividad de la NOS, y que arginina y glutamato no correlacionan con la concentración de NOx.
Nitric oxide synthase (NOS; EC 1.14.13.39) simultaneously synthesises citrulline and nitric oxide (NO), in equimolar amounts.1,2 This explains why citrulline and the citrulline-to-arginine (Cit/Arg) ratio have been used in different studies as markers of NO synthesis.3,4 However, since other metabolic pathways also alter citrulline concentration, its reliability as a marker of NO synthesis has been questioned.5 Other studies suggest that citrulline may be even more sensitive to NOS activity in the human central nervous system (CNS).4,6
Nitrites and nitrates (NOx) are the most widely used markers of NOS activity, particularly in in vivo studies. NOx are regarded as the most reliable markers of NO synthesis; NOx concentrations in the brain are reported to be correlated with NOS activity.7,8 However, the correlation with citrulline concentration has not been addressed.
The usefulness of citrulline concentrations for estimating NO synthesis is much debated, given that the amino acid is metabolised by various enzymes.5 For example, dimethylarginine dimethylaminohydrolase (DDAH; EC 3.5.3.18) produces citrulline by hydrolysis of methylarginines; these are competitive NOS inhibitors.9,10 NOS production is induced by cytokines10,11 and inhibited by arginine, citrulline, and NO produced by inducible NOS (iNOS).12,13
Aside from citrulline synthesis, the only known reaction in which the amino acid serves as a substrate is arginine synthesis via argininosuccinate synthetase (ASS; EC 6.3.4.5), occurring in the CNS (and other systems). ASS is stimulated by citrulline and proinflammatory stimuli, increasing NO synthesis.15–17 This enzyme catalyses aspartate and citrulline condensation in the rate-limiting step of arginine biosynthesis15,18; however, ASS activity is also regulated by NO since the enzyme is inhibited by nitrosylation.19
Further research should aim to determine the extent to which citrulline concentration reflects NO synthesis in the human CNS. This study analyses how different markers of the synthesis of NO and related amino acids are correlated with human CSF NO concentration and citrulline metabolism, to evaluate the extent to which different metabolic pathways alter NOx and citrulline concentrations, with a view to determining whether these may be used as markers of NO synthesis.
MethodsOur cross-sectional study was approved by the research ethics committee at the Mexican National Institute of Neurology and Neurosurgery. All patients and their families received written information on the purpose of the study, and participants signed informed consent forms. Our study complies with the ethical standards of the Declaration of Helsinki; all tests performed were strictly necessary for diagnosis and standard clinical management.
The sample comprised consecutive patients attending the emergency department. Patients with acute neurological diseases compatible with CNS infection were evaluated with standard techniques including EEG, CT, MRI, lumbar puncture, and general laboratory analyses.
All patients underwent ELISA testing for HIV; those testing positive underwent a complementary analysis to confirm the diagnosis. Diagnosis of viral encephalitis in HIV-negative patients was based on the following criteria: (1) healthy individuals with clinical signs of acute brain disorder (fever, seizures, altered level of consciousness, psychomotor agitation, or lethargy); (2) abnormal EEG results, with generalised slowing of background activity, or abnormal MRI findings (cerebral oedema, hyperintensities on T2-weighted sequences, increased contrast uptake in cortical gyri); (3) CSF analysis results suggestive of inflammation, with increased protein levels and cell counts; and (4) negative bacterial and fungal CSF cultures, and negative results from tests for anti-cysticercus antibodies. A diagnosis of acute primary HIV encephalitis was made when other pathogens were ruled out and patients showed positive serological results for HIV.
Tuberculous meningitis was diagnosed in patients with a compatible CSF profile and a positive culture for Mycobacterium tuberculosis, or positive PCR results for M. tuberculosis together with CSF adenosine deaminase levels above 7IU/L. A diagnosis of cryptococcal meningitis was established in patients with a positive culture for Cryptococcus neoformans. Diagnosis of bacterial meningitis was based on a positive CSF culture for pathogenic bacteria and compatible symptoms. Neurocysticercosis was identified in patients with typical lesions in imaging studies and positive CSF test results for anti-cysticercus antibodies. CNS inflammation was defined as a cell count≥8cells/mm3; this criterion is commonly used in our centre.
The professionals performing the clinical and biochemical tests were blind to one other's assessment. CSF samples were drawn by lumbar puncture after performing a CT scan; none of the patients presented complications secondary to the procedure. NOx and amino acid concentrations were determined by high-performance liquid chromatography (HPLC), as described in the literature.4,20
For amino acids, samples were collected in glass tubes, which were stored at −80°C. CSF was subsequently thawed and filtered with a 0.45-μm-pore size nitrocellulose membrane. Samples were derivatised with o-phthalaldehyde/mercaptoethanol and injected into an Adsorbosphere OPA-HS column (100mm×4.6mm, 5μm particle size; Alltech Associates) connected to a quaternary pump (1100 series; Agilent Technologies). The mobile phase consisted of a buffer solution of sodium acetate (50mmol/L, pH 5.9) and HPLC grade absolute methanol (MetOH), at a flow rate of 1.5mL/min. We used a linear gradient of 12%-22% MetOH for 27minutes. Signals were recorded at wavelengths of 232nm for excitation and 455nm for emission with Chemstation A.10.02 (Agilent Technologies).
For NOx analysis, samples were collected in glass tubes and stored at −80°C. They were subsequently thawed and filtered with a 0.45-μm-pore size nylon membrane and injected into a LiChrosorb C18 column (250mm×4.6mm, 5μm particle size; Alltech Associates), connected to an isocratic pump (1200 series, Agilent Technologies). The mobile phase consisted of an octylamine solution (pH 6.4) at a flow rate of 1.2mL/min. Signals were recorded at wavelengths of 228nm with Chemstation A.10.02 (Agilent Technologies).
Statistical analysisWe performed the Kolmogorov-Smirnov test for normality and the Levene test for homogeneity of variance. CSF variables were compared with the Mann-Whitney U test. Sex-related differences were evaluated with the Fisher exact test. Bivariate correlations were analysed using the Pearson correlation coefficient. Predictors of NOx concentration were identified by multiple linear regression and ANCOVA using log-transformed values for NOx and Cit. Results are expressed as medians (interquartile range); statistical significance was set at a bilateral p-value <.05.
ResultsA total of 240 individuals were initially selected. To reduce the heterogeneity of the sample, we excluded 12 patients who did not have a definitive diagnosis by the time of data analysis, and an additional 51 patients with primary psychiatric disorders. We only analysed neurological diseases, as these were the most frequent diagnoses in our population.
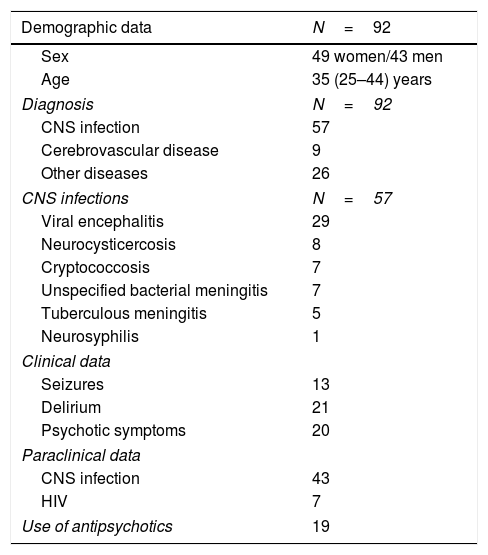
A total of 177 patients were diagnosed with neurological diseases, of whom 92 underwent biochemical analysis. The most frequent diagnosis was neuroinfection, with viral encephalitis being the most prevalent. The final sample included 92 patients, who were divided into 4 groups according to presence of CNS infection or inflammation (Table 1): 18 patients had infection and no signs of inflammation, 4 patients showed CNS inflammation (mainly demyelinating) without infection, 39 patients had infection and inflammation, and 31 patients displayed neither infection nor inflammation.
Demographic and clinical characteristics of the study population.
| Demographic data | N=92 |
|---|---|
| Sex | 49 women/43 men |
| Age | 35 (25–44) years |
| Diagnosis | N=92 |
| CNS infection | 57 |
| Cerebrovascular disease | 9 |
| Other diseases | 26 |
| CNS infections | N=57 |
| Viral encephalitis | 29 |
| Neurocysticercosis | 8 |
| Cryptococcosis | 7 |
| Unspecified bacterial meningitis | 7 |
| Tuberculous meningitis | 5 |
| Neurosyphilis | 1 |
| Clinical data | |
| Seizures | 13 |
| Delirium | 21 |
| Psychotic symptoms | 20 |
| Paraclinical data | |
| CNS infection | 43 |
| HIV | 7 |
| Use of antipsychotics | 19 |
This classification was used because inflammation can present in the absence of infection (as in cerebrovascular diseases), whereas some infections (such as those associated with viral encephalitis) may present without signs of inflammation in CSF cytochemical analyses.
Biochemical analysisNo differences in NOx concentrations were observed with regard to sex (women: 104μM [52-190] vs men 71μM [56-126]; P=.150) or presence of delirium (present: 76 [58-122] vs absent: 89 [51-188]; P=.469), CNS infection (present: 66 [49-150] vs absent: 106 [59-204]; P=.110), CNS inflammation (present: 66 [53-192] vs absent: 96 [55-172]; P=.349), symptoms of psychosis (present: 73 [55-122] vs absent: 88 [54-205]; P=.337), HIV infection (present: 60 [30-364] vs absent: 86 [55-174]; P=.639), cerebrovascular disease (present: 82 [50-319] vs absent: 84 [54-174]; P=.789), seizures (present: 89 [43-155] vs absent: 84 [56-181]; P=.583), or treatment with antipsychotics (present: 88 [51-141] vs absent: 82 [55-181]; P=.529).
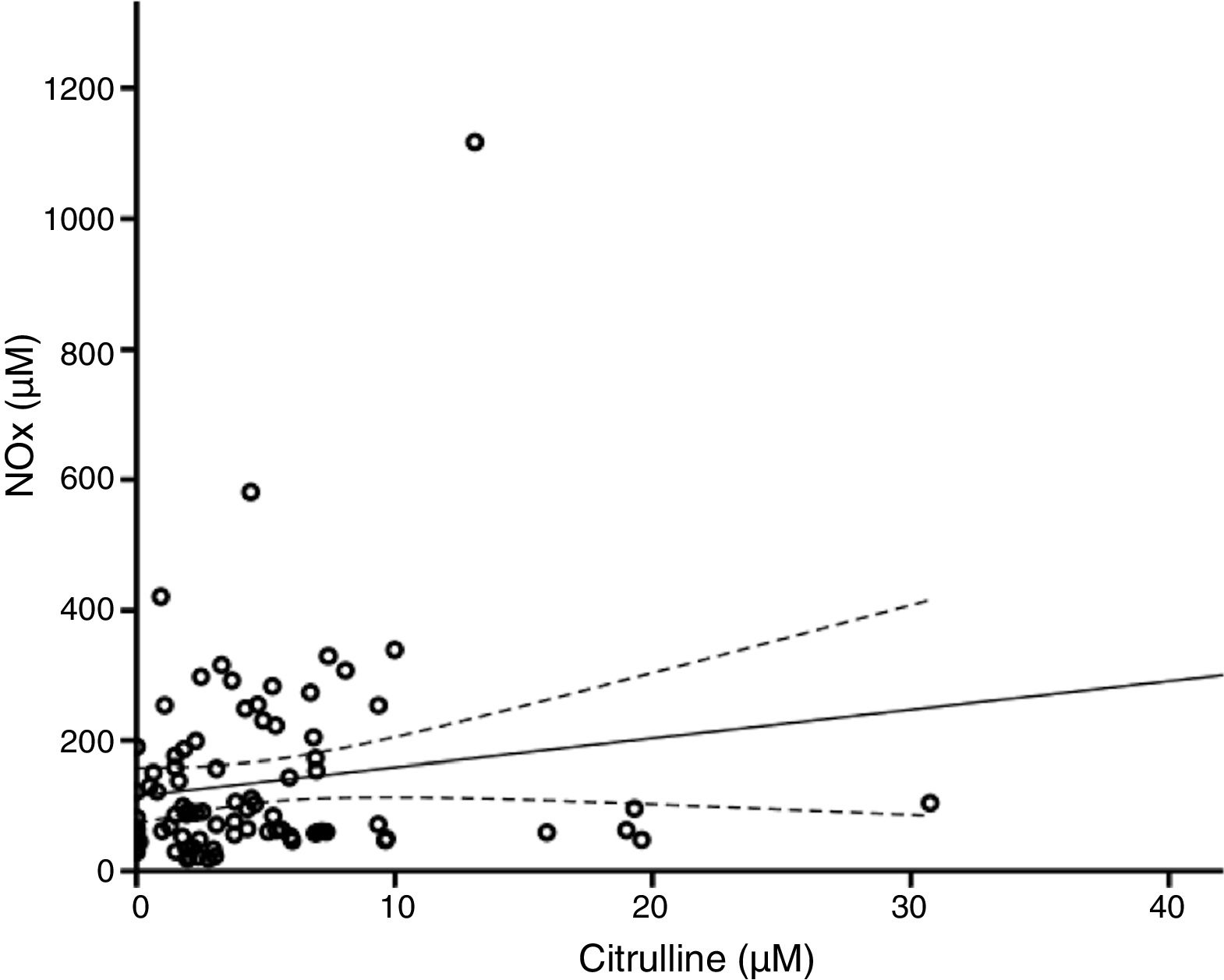
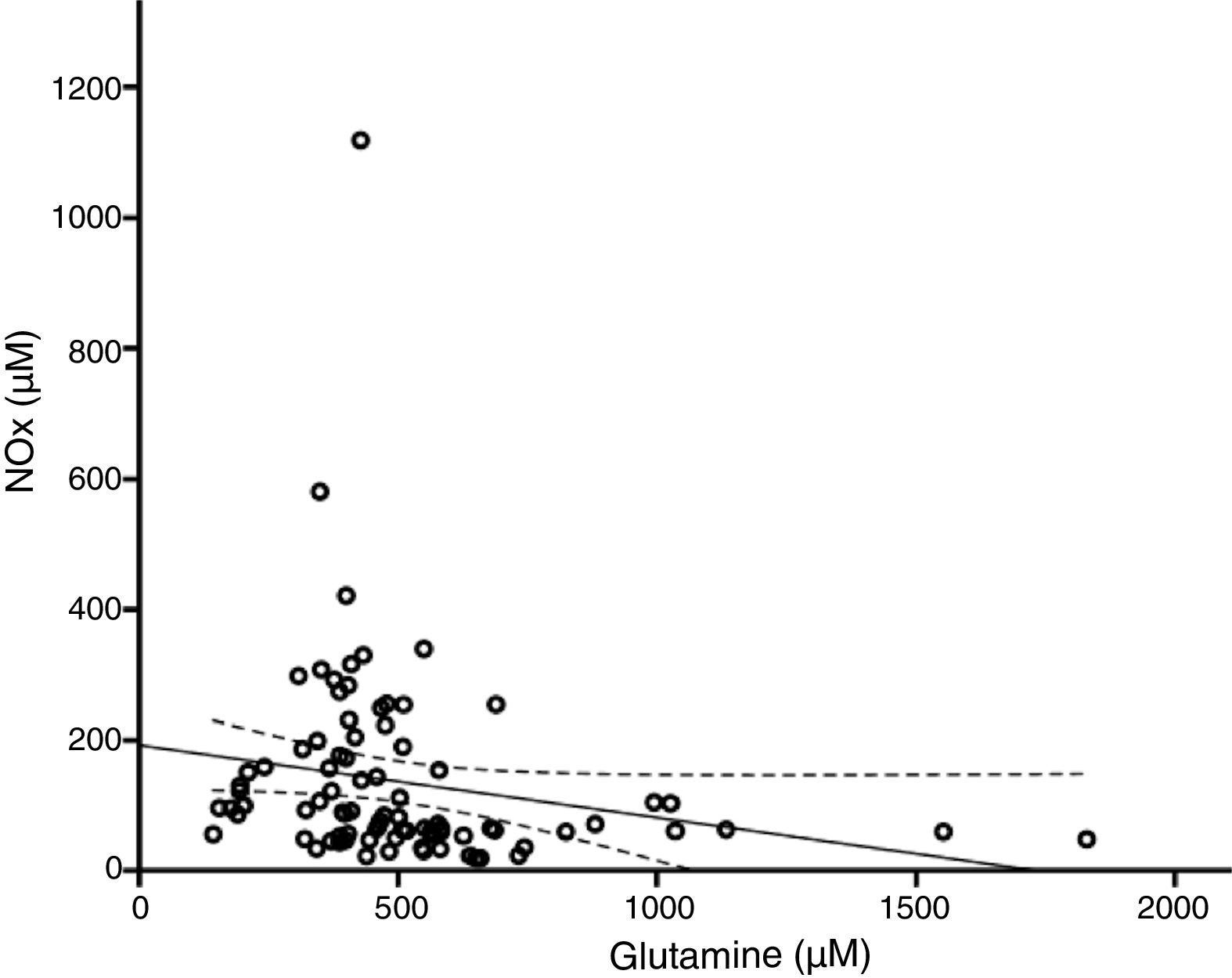
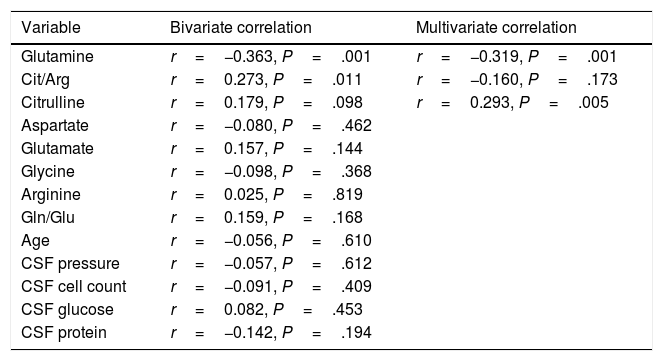
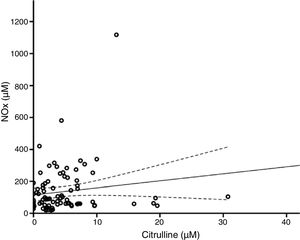
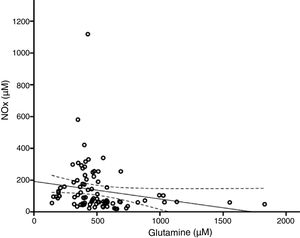
According to the bivariate analysis, NOx concentration was correlated with glutamine concentration and the Cit/Arg ratio; the correlation with citrulline concentration bordered on significance. The correlation between NOx and citrulline or glutamine is expressed by the equations NOx=4.3*Cit+115.5 and NOx=−111.0*Gln+192.3; the y-intercept of the Cit-NOx linear function was 115.5μM (SD=21.4; 95% CI, 72.8-158.2). No correlation was observed with age; concentrations of aspartate, glutamate, glycine, or arginine; glutamine-to-glutamate (Gln/Glu) ratio; CSF pressure; CSF cell count; or CSF protein level (Table 2).
Bivariate and multivariate correlations between CSF NOx concentration and several potential predictors.
| Variable | Bivariate correlation | Multivariate correlation |
|---|---|---|
| Glutamine | r=−0.363, P=.001 | r=−0.319, P=.001 |
| Cit/Arg | r=0.273, P=.011 | r=−0.160, P=.173 |
| Citrulline | r=0.179, P=.098 | r=0.293, P=.005 |
| Aspartate | r=−0.080, P=.462 | |
| Glutamate | r=0.157, P=.144 | |
| Glycine | r=−0.098, P=.368 | |
| Arginine | r=0.025, P=.819 | |
| Gln/Glu | r=0.159, P=.168 | |
| Age | r=−0.056, P=.610 | |
| CSF pressure | r=−0.057, P=.612 | |
| CSF cell count | r=−0.091, P=.409 | |
| CSF glucose | r=0.082, P=.453 | |
| CSF protein | r=−0.142, P=.194 |
Only variables with a P-value <.100 in the bivariate analysis were included in the multivariate analysis.
Cit/Arg: citrulline-to-arginine ratio; Gln/Glu: glutamine-to-glutamate ratio.
The multiple linear regression model (r=0.440, F=5.76; P=.001) showed that NOx concentration was significantly correlated with citrulline (Fig. 1) and glutamine concentrations (Fig. 2); the correlation with the Cit/Arg ratio was not significant, however. According to the multiple linear regression analysis, citrulline and glutamine concentrations independently explain 8% (r2=0.085) and 10% (r2=0.101) of the variance in NOx concentrations, respectively. The complete model explains 19% (r2=0.193) of the variance in NOx concentrations (Table 2).
ANCOVA confirmed that NOx concentration was associated with glutamine (F=17.1; P<.001) and citrulline concentrations (F=7.6; P=.007), but not with the Cit/Arg ratio (F=2.2; P=0.136) or presence of CNS infection (F=1.8; P=.173) or inflammation (F=1.4; P=.227).
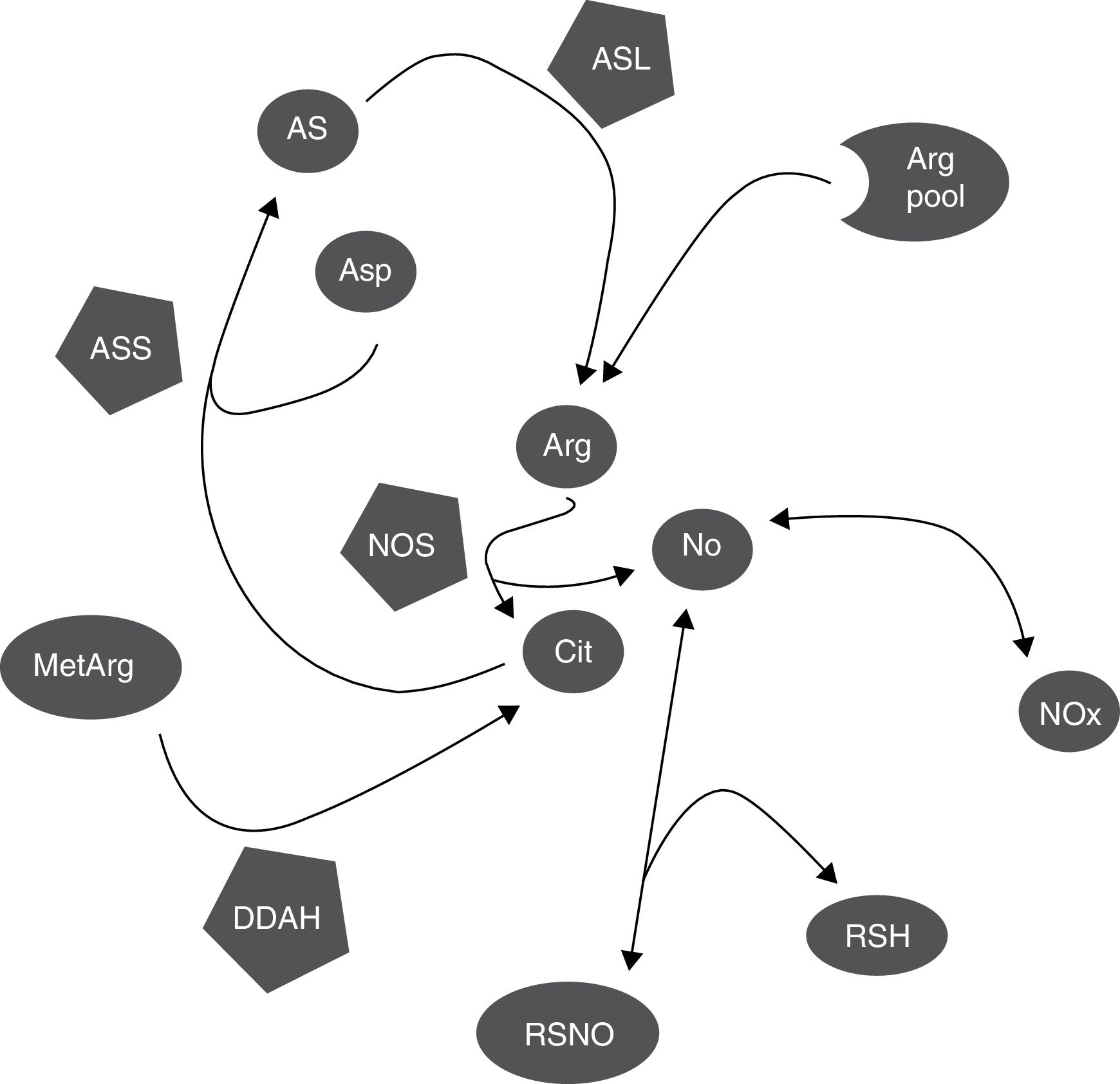
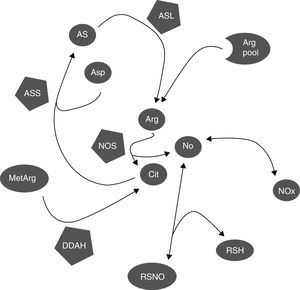
DiscussionCitrulline and nitrites/nitrates are markers of nitric oxide synthesisNOS simultaneously synthesises citrulline and NO, in equimolar amounts (Fig. 3).1,2 This explains why citrulline and the Cit/Arg ratio have so frequently been used as markers of NO synthesis.3,4 However, other metabolic pathways may also alter citrulline concentrations (Fig. 3); further research is necessary to determine the true extent to which citrulline concentration reflects NO synthesis.
Biochemical mechanisms that determine the correlation between markers of NO synthesis. NOS uses a small proportion of the pool of arginine for NO synthesis; citrulline and NO are generated in equimolar amounts and NO is oxidised into NOx in the same proportion. NOx can be reduced to NO by the action of several haemoproteins. NO can be trapped by thiols and released by nitrosothiols, regulating NOS activity through negative feedback inhibition. Citrulline can be synthesised by DDAH during hydrolysis of methylarginines, which inhibit NOS, and used by ASS for arginine synthesis. NO also regulates these processes, since both DDAH and ASS are inhibited by nitrosylation.
Arg: arginine; Arg pool: pool of arginine; AS: argininosuccinate; ASL: argininosuccinate lyase; asp: aspartate; ASS: argininosuccinate synthetase; cit: citrulline; DDAH: dimethylarginine dimethylaminohydrolase; MetArg: methylarginines; NO: nitric oxide; NOx: nitrites and nitrates; RSH: thiol; RSNO: nitrosothiol.
The fact that citrulline, or any other metabolite, is a marker of NOS activity implies not only that it is produced by the enzyme, but also that alterations in citrulline concentration accurately reflect changes in NOS activity. Thus, citrulline may act as a marker of NOS activity if concentrations change in parallel with NOS activity, irrespective of whether the amino acid is produced by the enzyme.
According to our regression analysis, when citrulline concentration equals 0μM, NOx concentration is 115.5μM; this suggests that NOx concentration is independent of citrulline metabolism. In fact, the gradient of the regression curve is 4.3μM NOx (indicating that 1μM of citrulline is equivalent to 4.3μM of NOx in our population), which supports the hypothesis that the concentration of these markers depends on multiple mechanisms of synthesis and degradation.
If citrulline is produced by NOS, its concentration should be similar to that of NO; however, the latter has a very short half-life.21,22 This explains why NOx content constitutes a better correlate of NOS activity.8,23 The interaction between NO and such haemoproteins (Fig. 3) as haemoglobin leads to NO oxidation, resulting in the production of NOx; these are used as markers of NO synthesis.4,24
In theory, citrulline concentration changes in parallel to NOx concentration. In fact, our results show that citrulline concentration is positively correlated with NOx concentration (Fig. 1). This points to CSF citrulline concentration as a good marker of NO synthesis in the human CNS, as previously suggested.3,4,25 The limitations of this hypothesis are discussed later in the article.
Other metabolic pathways also alter citrulline concentration; these include ASS activity (which recycles citrulline concentration; Fig. 3).18,26 ASS activity is stimulated by citrulline (as well as proinflammatory stimuli), increasing NO synthesis.15–17 NO inhibits ASS activity by nitrosylation19; therefore, increased NOS activity probably inhibits ASS activity and increases (or at least, does not decrease) citrulline concentration (Fig. 3). However, even in this case, the increase in citrulline concentration would be caused (indirectly) by NOS activity.
This mechanism enables citrulline concentration to increase to an even greater extent than NOx during NO production. The amino acid may therefore be a more sensitive marker of changes in NO concentration than NOx.4 However, ASS malfunction increases CSF citrulline concentrations, as occurs with citrullinaemia type II27; therefore, we cannot rule out the influence of the enzyme on our results.
Furthermore, ASS is not the only enzyme able to alter citrulline concentration. DDAH and NOS are the main enzymes synthesising citrulline in the CNS (Fig. 3).10,14 DDAH generates citrulline by methylarginine hydrolysis.9,10 NOS is induced by cytokines10,11 and inhibited by arginine, citrulline, and NO produced by iNOS.12,13 The enzyme is also inhibited by nitrosylation during iNOS activity, resulting in decreased citrulline synthesis (Fig. 3). This mechanism therefore cannot explain the positive correlation between citrulline and NOx concentrations (Fig. 1); this stands in contrast with the results of other studies.5 These mechanisms may have occurred in our patients: increased iNOS activity has been reported in the CSF of patients with CNS inflammatory processes (multiple sclerosis),28 as is the case with our patients.
Furthermore, citrulline concentration is better correlated with NOx levels than with the Cit/Arg ratio. This may be due to the fact that arginine concentrations can remain unchanged in patients with such metabolic disorders as renal insufficiency, given that arginine is more strongly correlated with protein degradation than with amino acid metabolism.29,30 Arginine levels may remain stable even during NO synthesis.31 Only 1% of the total available arginine is used for NO synthesis (Fig. 3)5,32; therefore, we may hypothesise that neither the Cit/Arg ratio nor arginine concentration accurately reflect NOS activity.
Not only citrulline is altered by NOS-independent mechanisms. Nitrites can be reduced to NO by the interaction of different metalloproteinases, such as NOS itself (Fig. 3), causing vasodilation and reducing arterial blood pressure.33,34 Furthermore, NO can interact with glutathione and other thiols, forming different molecules, including nitrosothiols and N2O (Fig. 3),35,36 limiting NO formation. In fact, the resulting nitrosothiols can release NO, increasing NOx concentration (Fig. 3). Citrulline and NOx concentrations can therefore be altered by mechanisms independent of NOS activity.
The correlation between citrulline and NOx concentrations did not depend on the presence of CNS infection or inflammation; this may be because all NOS isoforms synthesise both metabolites in equimolar amounts. According to the literature, cytokines induce iNOS expression in the context of infection or inflammation.10,37 Neuronal NOS may also be induced by cytokines21; NO can therefore be produced by both enzymes during infection or inflammation.
Our results support a direct correlation between citrulline and NO synthesis, independent of the presence of infection or inflammation. CSF NOx concentration is reported to remain unchanged in patients with CNS infection38,39; however, this is not the case with patients with CNS inflammation (multiple sclerosis).28 Infectious and inflammatory processes may have different biochemical profiles.
Glutamine and nitric oxide are reciprocally regulatedThere is growing evidence of the inverse relationship between NO and glutamine; our study also supports this observation (Fig. 2). Glutamine (200μM) inhibits ASS activity40,41 and NO synthesis.42 By this mechanism, glutamine reduces the intracellular arginine concentration, NO release, and vasorelaxation.24,43,44 This may be linked to the fact that diets rich in glutamine reduce plasma NOx concentration.45,46
The studies mentioned previously address endothelial NOS activity; however, glutamine can modulate other isoforms. For example, glutamine reduces iNOS expression,47 NO synthesis, and protein nitration.48,49 Other researchers have suggested that glutamine increases NO synthesis50,51; however, our results with neurological patients do not support this hypothesis.
NO regulates glutamine synthesis. Glutamate receptor activation and NO synthesis reduce glutamine synthetase (EC 6.3.1.2) activity through tyrosine nitration, whereas NOS inhibition has the opposite effect,52,53 increasing the concentrations of this amino acid.54
This inverse correlation may be related mainly to iNOS activity: increased glutamine concentrations have been observed in some brain regions of iNOS knockout mice.55 iNOS is very likely to be present in the samples analysed in our study, due to the inflammatory component of neurological diseases.28
Furthermore, NO causes non-enzymatic deamidation of glutamine peptide residues in the presence of O2,56 decreasing glutamine concentration.
The literature reports an inverse correlation between these biochemical markers and cognitive assessment scale scores in neurological patients; reciprocal modulation may therefore play a major role in brain physiology.57
ConclusionsOur results do not allow us to completely rule out the involvement of ASS and DDAH in the regulation of citrulline concentration, but do show that CSF citrulline concentration constitutes a reliable correlate of NO synthesis in the human CNS. The mechanisms underlying the association between the 2 markers may change depending on the brain region or stage of the disease. Furthermore, our results show that glutamine modulates NO synthesis in the CNS and may explain the “arginine paradox.” The importance of citrulline and glutamine concentrations for NO synthesis must be interpreted with caution since both metabolites may be modulated by NOS-independent mechanisms.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez-Neri I, Ramírez-Bermúdez J, Ojeda-López C, Montes S, Soto-Hernández JL, Ríos C. Las concentraciones de glutamina y citrulina reflejan la síntesis del óxido nítrico en el sistema nervioso humano. Neurología. 2020;35:96–104.