Cortical motor areas are influenced not only by peripheral sensory afferents and prefrontal association areas, but also by the basal ganglia, specifically the striatum. The dorsomedial striatum (DMS) and dorsolateral striatum are involved in both spatial and stimulus-response learning; however, each of these areas may mediate different components of learning. The aim of the study is to determine the effect of electrolytic lesion to the DMS on the learning and performance of sexual behaviour and locomotor activity in male rats.
MethodOnce the subjects had learned to perform motor tests of balance, maze navigation, ramp ascent, and sexual behaviour, they underwent electrolytic lesion to the DMS. Five days later, the tests were repeated on 2 occasions and researchers compared performance latencies for each test.
ResultsAverage latency values for performance on the maze and balance tests were higher after the lesion. However, the average values for the ramp test and for sexual behaviour did not differ between groups.
ConclusionsElectrolytic lesion of the DMS modifies the performance of locomotor activity (maze test and balance), but not of sexual behaviour.
Las áreas motoras corticales no solo son influenciadas por aferencias sensitivas periféricas y áreas de asociación prefrontales, sino también por los ganglios basales, específicamente el estriado. El estriado dorsomedial (EDM) y el estriado dorsolateral están involucrados en el aprendizaje espacial y el aprendizaje estímulo-respuesta; sin embargo, cada una de estas zonas pudiera mediar distintos componentes del aprendizaje. El propósito del estudio es determinar el efecto de la lesión electrolítica del EDM sobre el aprendizaje y ejecución de la conducta locomotora y sexual en ratas macho.
MétodoUna vez que los sujetos aprendieron a ejecutar las pruebas motoras de equilibrio, laberinto, rampa de ascenso y la conducta sexual, se realizó la lesión electrolítica del EDM. Cinco días después se realizaron las pruebas en 2 ocasiones más y se compararon las latencias de ejecución de cada prueba.
ResultadosDespués de la lesión, los valores promedio de latencia, incrementaron durante la ejecución de las pruebas de laberinto y equilibrio. Sin embargo, los valores promedio en la prueba rampa y conducta sexual, no aportaron efectos contrastantes entre los grupos.
ConclusionesLa lesión electrolítica del EDM modifica la ejecución de la actividad locomotora (prueba de laberinto y equilibrio), pero no la ejecución de la conducta sexual.
Evidence suggests that the basal ganglia are involved in such non-motor functions as learning and memory.1,2 Findings from studies of animal models with electrolytic and pharmacological lesions support this hypothesis.3–9 In some mammals, the dorsal striatum, including the caudate nucleus and putamen, has traditionally been considered to be involved in motor control.10 However, it has also been associated with sensorimotor integration,11 cognitive function,12 learning,2,13 and some aspects of attentional performance.14,15
According to several studies, the dorsal striatum may play a major role in associative learning (classical conditioning, that is, associating an unconditioned stimulus with a response).1,16,17 More specifically, manipulation of the dorsal striatum has been found to alter a wide range of learning tasks requiring spatial information processing in the Morris water maze18 and the four-arm cross-maze.19
However, the striatum is not a homogeneous structure: several areas may be distinguished by their biochemistry20,21 and connectivity (afferent and efferent projections).16,22
Rats with dorsolateral striatum lesions have displayed impaired discriminant ability on conditional discrimination tasks and poor performance on simple discrimination tasks. These findings are consistent with the idea that the dorsolateral striatum is involved in stimulus-response (S-R) learning and conditioned place preference learning.23,24 Lesions to the dorsomedial striatum (DMS) have no effect on conditioned place preference learning.23 However, evidence suggests that the poorer performance on discrimination tasks in the post-lesion period (after having acquired the task) may affect S-R learning.16,23
The striatum is a key structure in motor function; it has recently been linked to such functions as S-R learning, place preference learning, and spatial information processing, all of which are involved in sexual behaviour. Questions remain as to whether the DMS is responsible for the learning of locomotor patterns involved in sexual behaviour. The purpose of our study was to evaluate the effect of electrolytic lesions to the DMS on previously acquired sexual behaviour and locomotor activity.
Material and methodsSubjects and housingOur study included sexually experienced male Wistar rats (250-350g) and ovariectomised female Wistar rats (200-250g). The male rats selected for these experiments had ejaculated in at least 2 of 4 tests with ejaculation latencies of less than 15minutes. Female receptivity was induced with exogenous steroids dissolved in canola oil: estradiol benzoate (10μg) and progesterone (500μg) injected subcutaneously 48 and 4hours before each test, respectively. Animals were housed in transparent acrylic boxes (50cm×30cm×20cm) containing sawdust bedding. They were kept with a 12×12hour inverted light-dark cycle (lights were turned on at 20.00); food (LabDiet, Prolab RMH 2500) and water (Xallapan) were provided ad libitum. All experiments followed the official Mexican guidelines (NOM-062-ZOO-1999) and the ethical standards published by the Society for Neuroscience.
Experimental groupsA total of 21 rats were randomly allocated to 3 groups: 7 rats served as controls (control group), 7 received bilateral electrolytic anodal lesions in the DMS (lesion group), and 7 underwent the same surgical procedure without the electrolytic lesions (sham-operated group). All male rats were sexually experienced.
Maze testWe constructed an eight-arm radial maze out of transparent plexiglass. All arms (35cm×10cm×20cm) provided access to a central octagonal area made of plexiglass (each side measured 10cm). The receptive female rat was always placed in the same arm; plastic mesh served to prevent contact between the male rat and the receptive female. Male rats were tested every 3 days during one week to allow them to learn the skills necessary to reach the female. After this training period, male rats were tested twice a week for 2 weeks: one week before surgery and in the week following the procedure. Females were always placed in the arm of the maze before the test began and the male rat was allowed into the maze 5minutes later. Movements of the male rats were recorded throughout the test with a videocamera (Sony DCR-SR20) and analysed with The Observer XT software (Noldus Information Technology, USA). This test analysed latency to reach the female.
Horizontal bar test (balance)The horizontal bar test was conducted as described in the study by Ortiz-Pulido et al.25 We used 2 transparent acrylic boxes measuring 40cm×30cm×15cm and elevated 20cm off the floor. Boxes were set 45cm apart and joined by a horizontal wooden bar measuring 2.5cm wide. A receptive female was placed in one of the boxes with plastic mesh to prevent her from crossing the bar; the male rat was placed in the other box. From that distance, male rats were able to detect sexual cues from the female, exit the box, cross the bar, and reach the box where the female was located. We analysed time to leave the box and time to cross the bar and reach the box where the female rat was kept. The same procedure was repeated in the ramp test (ascending bar).
Ramp testWe used 2 acrylic boxes measuring 40cm×30cm×15cm; one of them was placed on the floor and the other 30cm above floor level. This test was also conducted as described in the study by Ortiz-Pulido et al.25
Sexual behaviour testAfter completing each of the tests (maze test, horizontal bar test, and ramp test) rats engaged in copulation during the third dark period. Males were placed in the testing arena, a plexiglass cylinder measuring 50cm in diameter and 50cm high. After an adaptation period of 5minutes, a receptive female was introduced in the cylinder. We analysed the following parameters: number of mountings (nM), number of intromissions (nI), number of ejaculations, and ejaculation latency; we also calculated the intromission index (nI/[nM+nI]). Once the ejaculation pattern had been analysed, the test was concluded and rats were returned to their boxes.
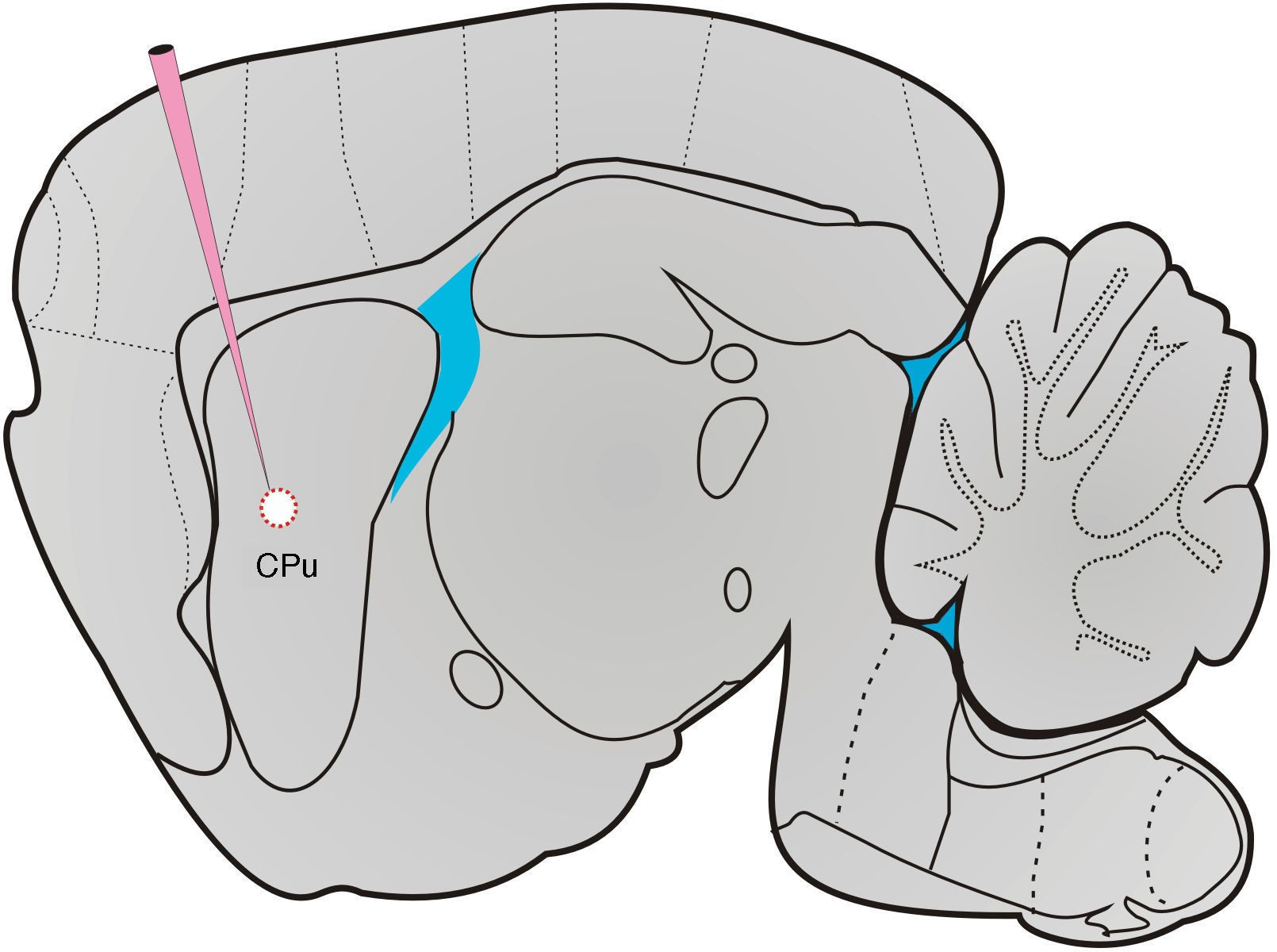
Surgery and electrolytic lesionMale rats allocated to the lesion and sham-operated groups were anaesthetised with a mixture of ketamine (50mg/kg) and xylazine (8mg/kg). They were hydrated with subcutaneous injections of physiological saline (0.9%) every 30minutes. During deep anaesthesia, animals were placed in a stereotaxic instrument (Stoelting Co., USA) to make a midline incision exposing the skull from lambda to the posterior edge. After drilling 2 holes in pertinent locations in the skull, researchers introduced 2 single-shaft stainless steel microelectrodes measuring 250μm in diameter (FHC) to cause anodal electrolytic lesions to the dorsomedial area of the striatum according to the stereotactic coordinates of the rat brain atlas created by Paxinos and Watson,26 AP=0.0mm, L=+3.0/−3.0mm, V=−5.5mm from bregma. Sham-operated rats were treated identically; electrodes were also introduced bilaterally but not used to provoke electrolytic lesions. In the lesion group, electrical pulses (0.5mA/20s) were delivered using a stimulator (Grass S40, Astro-Med Inc., USA) connected to a constant current unit (Grass CCU1, Astro-Med Inc., USA). Microelectrodes were withdrawn and the incision sutured 5 to 10minutes after microelectrode placement in sham-operated rats and electric current delivery in lesioned rats. Animals recovered from anaesthesia in an incubator, after which they were housed in individual boxes in the vivarium.
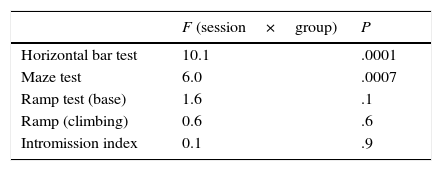
HistologyAfter all tests had been conducted, sham-operated and lesioned rats were deeply anaesthetised with pentobarbital sodium (60mg/kg) and killed by transcardial perfusion. Brains were removed and placed in a fixation solution for 24hours. Researchers prepared 40μm coronal and sagittal sections at −24°C. Slices were stained with cresyl violet, mounted on gelatin-coated slides, and covered with glass coverslips using Permount. We took microphotographs of the specimens using a microscope (Olympus PROVIS AX70, Olympus Co., Japan) to analyse lesion sites and dimensions (Fig. 1).
Statistical analysisThe response variable (latency) was processed using repeated measures ANOVA to analyse the first 4 sessions of locomotor training for each of the tests (maze, horizontal bar, and ramp tests). We subsequently analysed the last pre-intervention session and the 2 post-intervention sessions. The statistical model included a fixed factor (latency) with 3 levels (electrolytic lesions to the DMS, sham operation, and no intervention); data on latency times were gathered from the maze, horizontal bar, and ramp tests. We tested for normal distribution and homogeneity of variance.
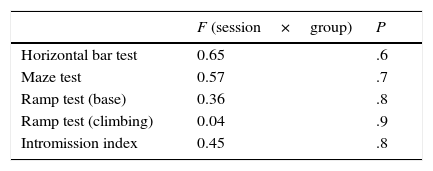
ResultsIn this study, rats were assigned to 3 different groups before completing 4 training sessions for different locomotor tests and for sexual behaviour. Contrary to our expectations, performance on the horizontal bar test was similar for all groups and sessions; there was no interaction effect between session and group. Latency times in the maze test were similar with respect to fixed factors, again with no interaction effect between session and group. The ramp test, which assessed balance, revealed a similar pattern for latency times; there were no factor or interaction effects. Session-group interaction did not significantly affect the intromission index (Table 1).
Response latency time and intromission index in locomotor and sexual behaviour tests, respectively, during 4 sessions in the lesioned, sham-operated, and control groups.
| F (session×group) | P | |
|---|---|---|
| Horizontal bar test | 0.65 | .6 |
| Maze test | 0.57 | .7 |
| Ramp test (base) | 0.36 | .8 |
| Ramp test (climbing) | 0.04 | .9 |
| Intromission index | 0.45 | .8 |
Response variables were analysed after 4 sessions of locomotor training; we compared results from the last training session to those of 2 additional sessions conducted after the intervention.
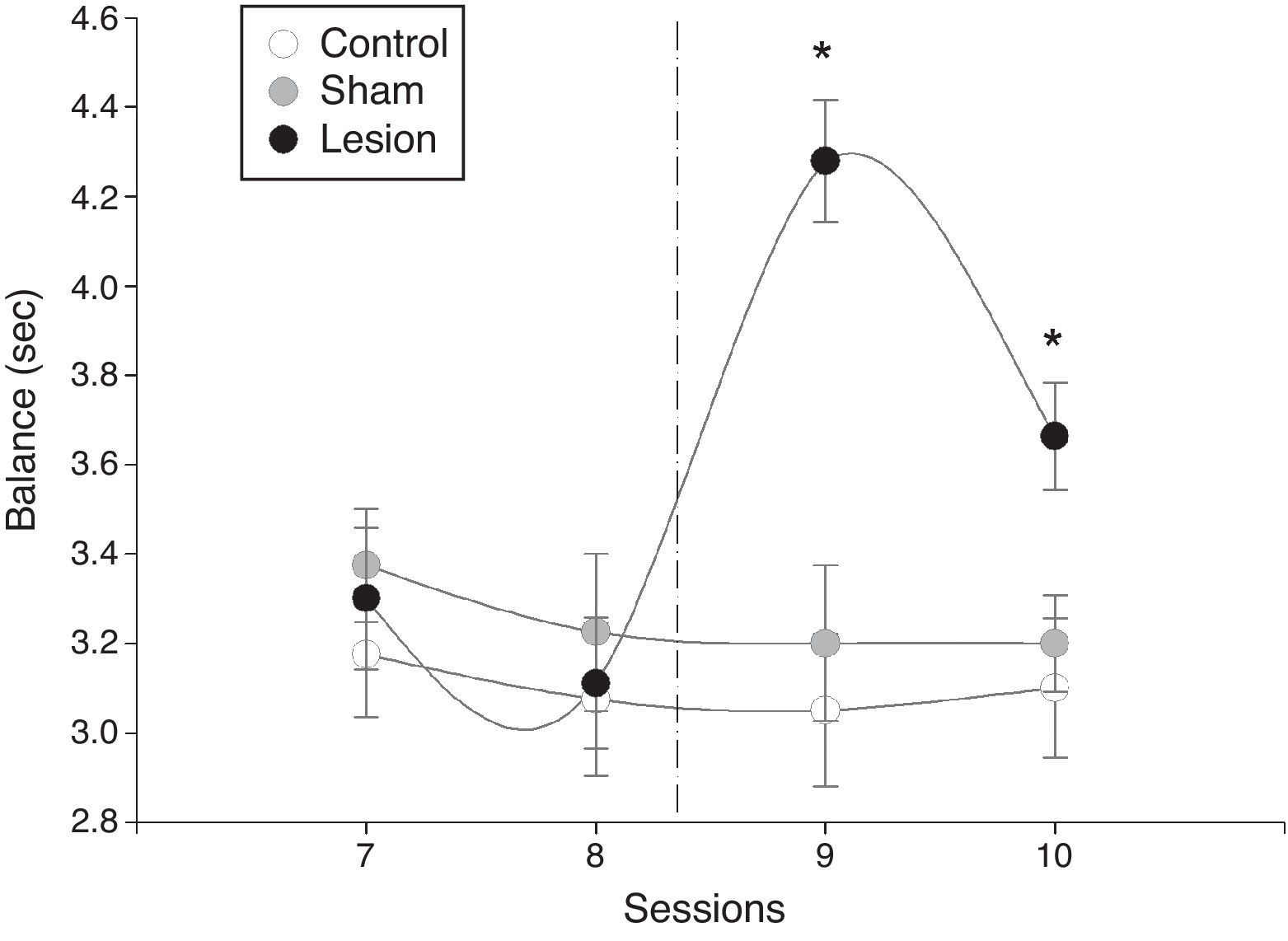
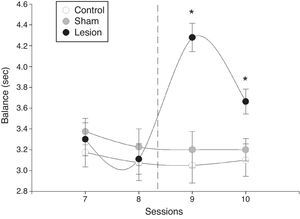
There were significant intergroup differences in mean latency time during the balance test (F=16, P=.0001). Furthermore, significant differences were also apparent between sessions in the lesion group (F=10, P=.0003), whereas the sham-operated and control groups displayed no differences (Fig. 2). In the lesion group, however, differences in mean latency time before and after the intervention were significant: mean latency time increased dramatically in the first session after the intervention and subsequently decreased (Fig. 2).
Mean latency time±SD to complete the horizontal bar test (balance). The time it took male rats to cross the bar and reach the receptive female decreased progressively in the sessions preceding electrolytic damage and increased significantly after the intervention compared to times by sham-operated and control rats.
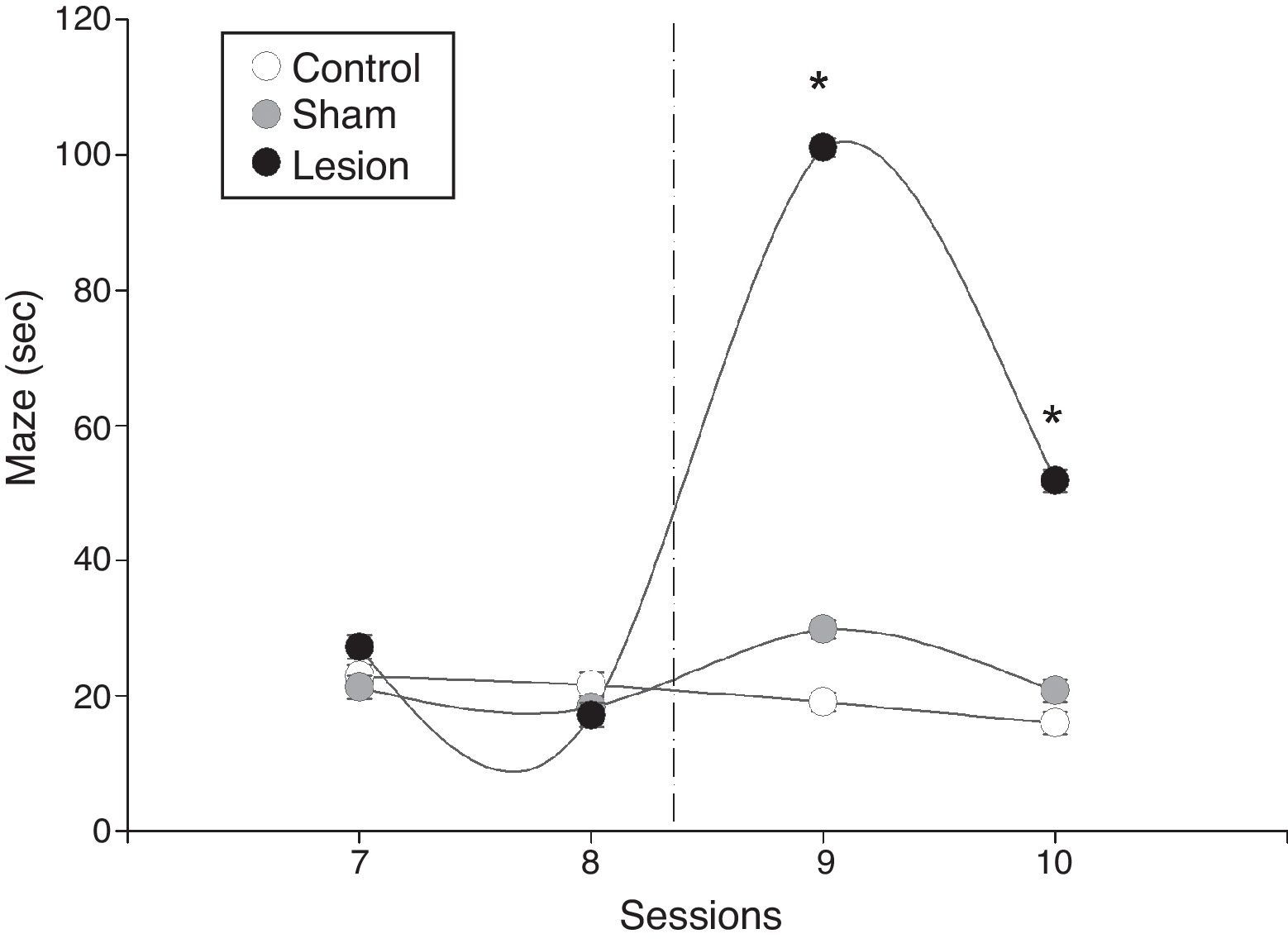
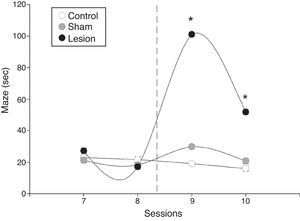
Significant inter-group differences in mean latency times were also found in the maze test (F=9.2, P=.001). We found significant differences between sessions in the lesion group (F=9.1, P=.0006); the session-group interaction analysis also detected differences between pre-lesion and post-lesion values, but not in the sham-operated and control groups (Fig. 3). Mean latency time after electrolytic lesions was significantly higher in the lesion group than in the sham-operated and control groups, and remained higher in the last session after the intervention (Fig. 3).
However, mean latency times on the ramp test (time to start climb) were not indicative of intergroup differences (F=3.4, P=.06). We did find significant differences between sessions (F=9.7, P=.0005) due to the time effect regardless of the group: session-group interaction was homogeneous and had no effect (Table 2). The same was true for the ramp test (time to climb), with no significant differences between groups (F=1.8, P=.19). However, differences between sessions were significant, which points once more to a time effect rather than to an effect of treatment (F=3.5, P=.04), as shown by the lack of session-group interaction. After electrolytic lesions, session-group interaction had no effect on the intromission index (Table 2).
Response latency time and intromission index in locomotor and sexual behaviour tests, respectively, during 3 sessions in the lesioned, sham-operated, and control groups (before and after electrolytic damage to the DMS).
| F (session×group) | P | |
|---|---|---|
| Horizontal bar test | 10.1 | .0001 |
| Maze test | 6.0 | .0007 |
| Ramp test (base) | 1.6 | .1 |
| Ramp (climbing) | 0.6 | .6 |
| Intromission index | 0.1 | .9 |
Our results suggest that while the DMS is involved in previously acquired locomotor activity, lesions to this structure do not affect locomotor performance. The role of the DMS is unclear. Although it has fundamentally been associated with motor activity,10 several studies suggest that it also contributes to S-R learning.1,2,16 Earlier studies have shown that animals with lesions to the dorsolateral striatum,27,28 and no lesions to the DMS,23 display impaired acquisition of tasks involving S-R learning.
The main finding in our study is that bilateral lesions to the DMS in rats resulted in poor performance of 2 locomotor tasks, leading to increased latency to initiate behaviour. All animals in our study were sexually experienced and trained for the locomotor tests (maze, horizontal bar, and ramp tests). During training sessions, latency times decreased progressively in all groups as animals developed the skills to complete the tasks (Table 1). After the intervention, however, latency times for the maze and horizontal bar tests increased considerably in the lesion group compared to the control and sham-operated groups (Figs. 2 and 3, Table 2).
Our findings are in line with those reported by Rogers et al.,15 who described increased latency times in Pavlovian appetitive conditioning after inducing lesions to the DMS. Deficits resulted not only from altered attentional performance but also from impaired attentional control and faulty response (for example, inhibitory response), both of which are necessary for task completion.15
Increased latency times after damage to the DMS in animals that had previously acquired a behaviour are attributed to motor rather than motivational problems. The lack of an effect on sexual behaviour patterns after lesion induction suggests that motivation is not affected in these animals. Baunez and Robbins29 found that striatal dopamine loss in rats significantly increased response latency times, as well as omissions and perseverative behaviour. Therefore, successful completion of a task (maze or horizontal bar tests) requires organising multiple cognitive components and motor processes, including visual attention, and modulating responses, while locating the visual, auditory, or olfactory targets that facilitate the appropriate behaviour.15 Our study of sexual behaviour in animals with DMS damage displayed no alterations in the intromission index. Our results are supported by those from similar studies reporting no changes in those behaviours which had been learnt before lesion induction.27,28
Our findings agree with the hypothesis that different areas of the striatum are involved in the execution of specific visuospatial tasks.
Striatal damage prior to the acquisition of sexual behaviour may result in impaired learning or performance. Evidence suggests that DMS damage before a task has been assimilated results in learning delays.23,24
In our study, only 2 sessions were conducted after inducing DMS lesions. Latency times in the maze and horizontal bar tests decreased in the last session, indicating recovery, although they remained higher in the lesion group than in the control and sham-operated groups. This decrease may be due to plasticity; further sessions should be conducted after induction of electrolytic lesion to confirm this hypothesis.
FundingThis study was completed as a part of ROP's doctoral thesis under CONACyT grant ID 207997. It received funding from PROMEP-México PTC-195.
Conflicts of interestThe authors have no conflict of interest to declare.
We would like to thank Drs Grecia Herrera Meza and Armando J. Martínez-Chacón for their comments on our drafts of this manuscript and their help with statistical analysis.
Please cite this article as: Ortiz-Pulido R, Hernández-Briones ZS, Tamariz-Rodríguez A, Hernández ME, Aranda-Abreu GE, Coria-Avila GA, et al. Efecto de la lesión electrolítica del estriado dorsomedial sobre la conducta sexual y actividad locomotora de la rata. Neurología. 2017;32:278–283.