Drug-resistant epilepsy (DRE) is a top-priority social health problem which requires early individual treatment due to its dramatic repercussions for the patient and society.
DevelopmentThe International League Against Epilepsy (ILAE) has recently defined DRE as that in which the seizures are not controlled after having correctly taken two appropriate and well tolerated anti-epileptic drugs, with lack of control being understood as the appearance of seizures within one year or in a period less than three times the inter-seizure interval before starting treatment. This International Society recommends a rapid and detailed assessment of all patients in an Epilepsy Unit. A Clinical Epilepsy Unit (CEU) is understood as a group of professionals who, acting in collaboration, have the diagnosis and treatment of the patient with epilepsy as their primary objective. CEUs in Spain may be stratified into different levels depending on the activity carried out in each of them. The specific epilepsy clinic is considered the fundamental type of CEU and includes the necessary figure of an expert in epilepsy. Prolonged video-monitoring is performed in medical CEUs. In medical-surgical CEUs epilepsy surgery with varying degrees of difficulty is also performed.
ConclusionsAll CEUs must cooperate with consensus protocols, and there must be a two-way flow between them. Stratification of CEUs increases efficacy and efficiency, due to there being a sufficient number of them to ensure easy access by all patients with epilepsy.
La epilepsia resistente a fármacos antiepilépticos (ERF) constituye un problema socio-sanitario de primer nivel, que debe ser individualizado precozmente por sus dramáticas repercusiones individuales y colectivas.
DesarrolloRecientemente, la Liga Internacional Contra la Epilepsia ha definido la ERF como aquella en la que no se controlen las crisis tras haber tomado de forma adecuada dos fármacos antiepilépticos apropiados y bien tolerados, entendiendo como falta de control la aparición de crisis en un año o en un tiempo inferior a tres veces el intervalo entre crisis que mostraba antes de iniciar el tratamiento. Esta sociedad internacional recomienda en todo paciente con ERF una evaluación rápida y detallada en una unidad de epilepsia. Se entiende como Unidad Clínica de Epilepsia (UCE) el conjunto de profesionales que actuando en colaboración tienen como objetivo primario el diagnóstico y tratamiento del paciente con epilepsia. Las UCE en España pueden ser estratificadas en distintos niveles, dependiendo de la actividad que se desarrolle en cada una de ellas. La consulta específica de epilepsia se considera como el germen de toda UCE, siendo necesaria la figura del experto en epilepsia. En las UCE médicas se realiza la monitorización vídeo-EEG prolongada. En las UCE médico-quirúrgicas además se realiza cirugía de epilepsia de dificultad diversa.
ConclusionesTodas las UCE deben cooperar con protocolos consensuados, debiendo existir un flujo bidireccional entre ellas. La estratificación de las UCE permite una alta eficacia y eficiencia, debiendo existir el suficiente número que garantice el fácil acceso de todos los pacientes con epilepsia.
Conventional treatment for epilepsy is based on long-term, continuous administration of antiepileptic drugs (AEDs). The purpose of these drugs is to achieve total control over epileptic seizures without provoking side effects in order to improve the patient's quality of life. Even when provided with optimal AED treatment, approximately 25% of patients with epilepsy continue to experience seizures. These patients have what is known as intractable, refractory, or drug-resistant epilepsy (DRE).1
Need for a definition of drug-resistant epilepsySince the diagnosis of epilepsy is almost always based on data from the patient's medical history, errors in the initial diagnosis are common. This may be due to diagnosing the wrong type of epilepsy, or even to confusion with other paroxysmal events similar to epileptic seizures. Approximately 25% of all patients identified as having DRE are misdiagnosed. Video-EEG monitoring to view and sequence critical events is an essential diagnostic technique in these cases. Furthermore, some patients diagnosed with DRE have not received proper treatment or show poor treatment adherence. In many cases, therefore, simply correcting the treatment can result in epilepsy control.2
Surgery, on the other hand, has emerged in recent years as a safe and effective option that may and should be discussed with many DRE patients. Epilepsy surgery should not be used only as a last resort in certain types of epilepsy, specifically temporal lobe epilepsy, neocortical epilepsy with well-circumscribed lesions, and cases of unilateral hemispheric damage triggering DRE. In such cases, a high percentage of patients achieve complete seizure remission3 and their condition generally remains stable over time.4 Numerous international medical societies recommend suggesting surgical treatment to patients who are candidates for elective epilepsy surgery as soon as they are shown not to respond to AEDs within a reasonable amount of time.5,6 Other forms of treatment for DRE, such as vagus nerve stimulation, cerebral stimulation, and ketogenic diet, may be effective in selected patients.
Patients with DRE generally have a poor quality of life, various comorbidities, and a high mortality rate. It would take many years to treat an epileptic patient with the entire range of AEDs available today in both monotherapy and combination therapy. This would confirm the patient's epilepsy as completely intractable, but would also consume crucial time during which the patient could suffer irreparable harm. For this reason, DRE should be treated as an entity requiring personalised treatment as early as possible to provide the precise diagnosis, propose the best possible pharmacological treatment or consider alternative treatment, and recommend lifestyle changes that are important for the patient's social adaptation.7
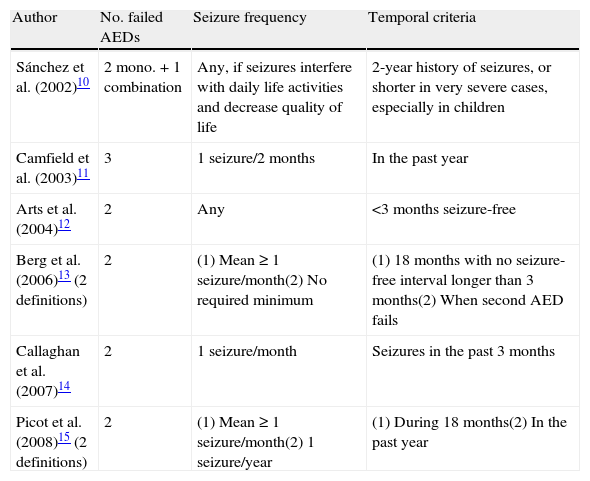
Need for a single definition of drug-resistant epilepsyUntil only quite recently, the concept of DRE varied according to the criteria of different authors, which resulted in the numerous definitions we find today.8 This was mainly caused by widespread use of the term “refractory”, applied equally to patients with mild, sporadic seizures that were partially drug-resistant and patients with severe, frequent seizures with no response whatsoever to AED. The purpose of the research, whether in an observational study, a clinical trial with an experimental AED, or a study for epilepsy surgery, also has an effect on the concept.9 The different definitions that have been used in recent years consider a number of different factors. The only point on which most authors agree is that 2 or 3 AEDs must be shown to be ineffective at controlling seizures in DRE. The rest of the factors vary considerably (Table 1).10–15
Characteristics of some definitions of drug-resistant epilepsy that have been used in recent years (AED: antiepileptic drug).
| Author | No. failed AEDs | Seizure frequency | Temporal criteria |
| Sánchez et al. (2002)10 | 2 mono.+1 combination | Any, if seizures interfere with daily life activities and decrease quality of life | 2-year history of seizures, or shorter in very severe cases, especially in children |
| Camfield et al. (2003)11 | 3 | 1 seizure/2 months | In the past year |
| Arts et al. (2004)12 | 2 | Any | <3 months seizure-free |
| Berg et al. (2006)13 (2 definitions) | 2 | (1) Mean≥1 seizure/month(2) No required minimum | (1) 18 months with no seizure-free interval longer than 3 months(2) When second AED fails |
| Callaghan et al. (2007)14 | 2 | 1 seizure/month | Seizures in the past 3 months |
| Picot et al. (2008)15 (2 definitions) | 2 | (1) Mean≥1 seizure/month(2) 1 seizure/year | (1) During 18 months(2) In the past year |
This variability is the reason why clinical results and findings from research are so dissimilar with regard to the epidemiology, diagnosis, and treatment of DRE. This is the case, for example, in an epidemiology study in adults in which DRE prevalence is calculated at 15.6% of all patients with active epilepsy, based on a definition of 2 failed AEDs and a mean seizure frequency of once monthly in the preceding 18 months. If by DRE we mean cases of 2 failed AEDs and 1 seizure in the past year, the prevalence rises to 22.5%.15 With this in mind, doctors need a single definition providing a valid and reliable method of determining DRE epidemiology. This will permit implementation of such healthcare resources as are necessary, facilitate research on the most appropriate treatment options for each patient type, and provide doctors with clear and useful guidelines for proper management of patients with DRE.7
Current definition of DRE according to the International League Against EpilepsyIn light of the need for unified DRE criteria, a working group from the International League Against Epilepsy (ILAE) with experts in different epilepsy-related fields has proposed a structured definition of DRE with the primary objective of improving care for epilepsy patients. In their conclusions, the authors mention a list of unknowns that should be investigated in the future using the new definition. To this end, they stress that their proposed definition will have to be validated by rigorous prospective studies and reworked if new evidence is obtained. Their definition is therefore a consensus definition backed by the ILAE, and we consider it to be the starting point for gaining better knowledge about DRE patients.16
Overall, the ILAE's definition is structured into 2 hierarchical levels. Level 1 defines the basic concepts of treatment success and failure for an AED. Level 2 establishes the definition for DRE, based on the criteria set forth in Level 1. In summary:
Level 1. Includes all data that must be known when administering an AED, with a conceptual analysis of the following aspects:
- •
AED appropriate for the disease it is intended to treat: The drug must have been proven effective against the specific type of epilepsy being treated.
- •
Proper use of the AED: Dosage, interval between doses, necessary adherence, and other aspects. However, dosage may have a wide margin of interindividual variability, especially as concerns age, sex, type of use (monotherapy, combination therapy), or comorbidities. For adults, we recommend adhering to the daily dose defined by the World Health Organization, that is, the daily maintenance dose of a drug used for its primary indication.17
- •
Adverse effects of an AED: Adverse effects are evaluated based on the medical history and clinical exploration.18 However, some effects, such as cognitive effects, may be subtle and present without the patient detecting them. In such cases, doctors must determine whether another AED or a different type of therapy is indicated, especially if high doses of the AED causing side effects are needed to achieve seizure control.
If the prescribed AED is not appropriate, has been taken improperly or discontinued due to intolerance, or if the patient was lost to follow-up, the result of that AED is unknown and, as such, the drug cannot be considered ineffective.16
- •
Seizure-free patient: A patient who experiences total absence of seizures, including auras and provoked seizures (those induced by sleep deprivation, fever, light stimulation, etc.).
- •
Seizure control duration for which an AED is considered effective: We applied the “rule of three”: for a 95% confidence level that an AED has had a positive effect on seizure control, the rule is that seizure-free interval has to be 3 times the longest seizure-free interval experienced in the year prior to beginning treatment.
Example: If the patient's maximum seizure-free period in the year immediately prior to treatment onset was 6 months, the AED cannot be considered effective unless the seizure-free interval extends to 18 months.
Obviously, this rule cannot be applied in cases in which treatment is started following the first crisis, or when the frequency of seizures before starting treatment exceeded 1 year. In such a case, the patient would have to achieve an extremely long seizure-free interval, which would not be practical. The working group also establishes that, in order for an AED to be considered effective, the seizure-free interval must be at least 1 year, which is the minimum time period for the patient to be able to detect a significant change in his or her quality of life.
Treatment success is, therefore, considered to be the total absence of seizures for either 3 times the longest inter-seizure interval prior to treatment with the AED or a full year after starting treatment, whichever is longer. If a patient remains seizure-free during more than 3 times his longest seizure-free period prior to treatment, but that period is less than 12 months, the outcome is considered to be undetermined. However, if the patient suffers an additional seizure before completing 1 year of treatment, the AED is considered to be a treatment failure even though seizure frequency has dropped below its pre-treatment level.16
Level 2. This is the central part of the definition of DRE: failure of 2 AED schedules to achieve sustained seizure freedom. This measurement comes from studies analysing control over seizures using different AED schedules. These studies showed that when 2 AEDs fail, seizure freedom is rarely achieved by using additional AEDs, whether in monotherapy or in combination, in both children12 and adults.19 We will not include the definitions of seizure frequency, treatment duration, or observation time in this analysis of treatment failure as they are listed in Level 1 (Table 2).
Current definition of drug-resistant epilepsy according to the International League Against Epilepsy (see text for additional information). AED: antiepileptic drug.
| AED-resistant epilepsy is defined as “failure of adequate trials of two tolerated and appropriately chosen and used AED schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom” where drugs are not discontinued due to intolerance; in addition: |
| (1) All data about how these two AEDs were taken are known. |
| (2) Treatment success is considered to be the total absence of seizures, including auras, during either 3 times the longest inter-seizure interval prior to treatment with the AED or the full year following onset of treatment with that drug, whichever is longer. |
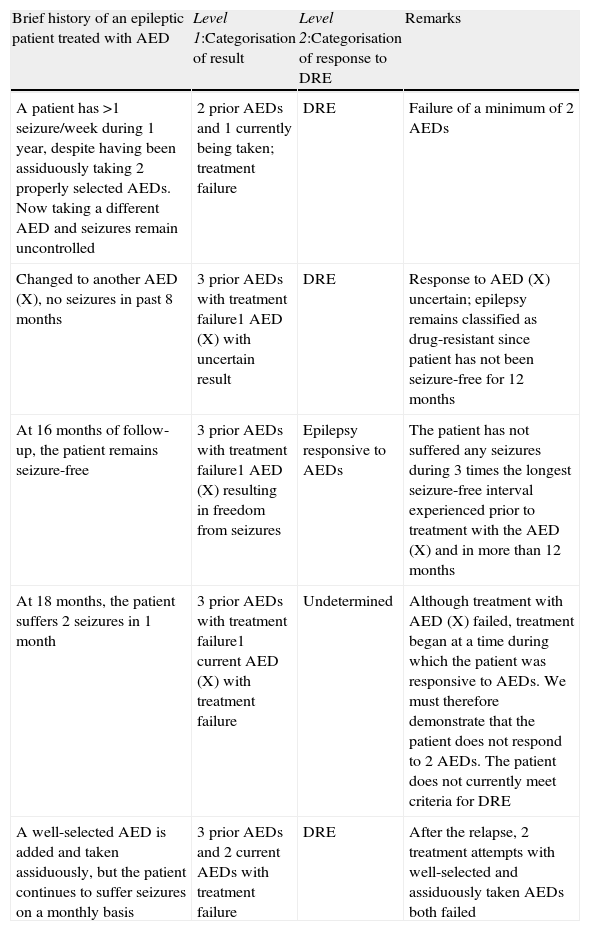
Epilepsy is a dynamic entity, and for that reason, diagnosing DRE at a particular moment is only valid at that time. The situation may not be permanent, as the condition may resolve as a result of different treatments, whether or not they are pharmacological. During the course of the disease, a patient's epilepsy may fluctuate between drug-resistant and drug-responsive states. At certain points during the course of the disease, it is not possible to determine whether epilepsy is drug-resistant or drug-responsive. This is true in the case of newly diagnosed patients who have not yet been treated long enough to establish treatment resistance. In such cases, the outcome of the AED is undetermined (Table 3).16
Example of how the designation of a patient's epilepsy may change over time according to the response to antiepileptic drugs (AED: antiepileptic drug. DRE: drug-resistant epilepsy).
| Brief history of an epileptic patient treated with AED | Level 1:Categorisation of result | Level 2:Categorisation of response to DRE | Remarks |
| A patient has >1 seizure/week during 1 year, despite having been assiduously taking 2 properly selected AEDs. Now taking a different AED and seizures remain uncontrolled | 2 prior AEDs and 1 currently being taken; treatment failure | DRE | Failure of a minimum of 2 AEDs |
| Changed to another AED (X), no seizures in past 8 months | 3 prior AEDs with treatment failure1 AED (X) with uncertain result | DRE | Response to AED (X) uncertain; epilepsy remains classified as drug-resistant since patient has not been seizure-free for 12 months |
| At 16 months of follow-up, the patient remains seizure-free | 3 prior AEDs with treatment failure1 AED (X) resulting in freedom from seizures | Epilepsy responsive to AEDs | The patient has not suffered any seizures during 3 times the longest seizure-free interval experienced prior to treatment with the AED (X) and in more than 12 months |
| At 18 months, the patient suffers 2 seizures in 1 month | 3 prior AEDs with treatment failure1 current AED (X) with treatment failure | Undetermined | Although treatment with AED (X) failed, treatment began at a time during which the patient was responsive to AEDs. We must therefore demonstrate that the patient does not respond to 2 AEDs. The patient does not currently meet criteria for DRE |
| A well-selected AED is added and taken assiduously, but the patient continues to suffer seizures on a monthly basis | 3 prior AEDs and 2 current AEDs with treatment failure | DRE | After the relapse, 2 treatment attempts with well-selected and assiduously taken AEDs both failed |
The ILAE's definition of DRE contains a number of positive points. It is easily to use and helpful to doctors involved in caring for patients with epilepsy, especially for professionals who are not experts in DRE management. It provides a personalised method for assessing epilepsy patients, considering that the frequency of a patient's seizures and his response to AEDs are the factors indicating whether or not he has DRE. Using this approach, the patient receives prompt, personalised treatment for DRE. If a patient suffers a seizure every 2 months, and seizure frequency remains unchanged after treatment with 2 tolerated, appropriately chosen and properly used AEDs, he will be diagnosed with DRE in just a few months. However, the most noteworthy thing about the new definition is that the ILAE has decided to publish its opinion, a move which promotes the study and proper treatment of this patient subgroup. As the ILAE consensus rightly indicates, all patients meeting criteria for DRE should have past diagnoses and treatments assessed promptly in addition to planning for the future, preferably in an epilepsy clinic or unit.16
This definition can be applied to support a diagnostic evaluation in an epilepsy unit, consider surgery as a treatment option, design randomised trials with AEDs, or fulfil other research purposes. In any case, as opting for a pre-surgical evaluation, surgery, or any other treatment attempt entails certain risks, an individual risk-benefit evaluation must always be performed, taking the patient's characteristics into account. Where surgery is a possibility, attempting treatment with an additional drug may be more than justified in some cases. Many studies have shown that the substitution or addition of a new AED in DRE patients who have tried several AEDs on prior occasions may result in seizure remission.14,20 In other studies, however, seizure remission due to an AED following diagnosis with DRE was temporary in children21 and in adults.22,23
Other authors who have studied the number of AEDs that may provide some benefit in the form of additional response calculate that 6 drugs are sufficient to demonstrate complete drug resistance.24 For this reason, some have argued that resistance to AEDs should be stratified according to the number of failed AED treatment attempts, and suggest that criteria for surgical treatment should consider the number of AEDs previously taken.25 However, all patients with DRE should undergo thorough study in an epilepsy unit to determine whether or not the patient is really resistant to AEDs and evaluate the appropriate treatment options to be explored in the future. Our goal must be to avoid treatment scepticism with DRE patients and attempt to achieve seizure control as early as possible. This will grant the patient the best possible health-related quality of life and ensure his or her participation in society and the workplace.
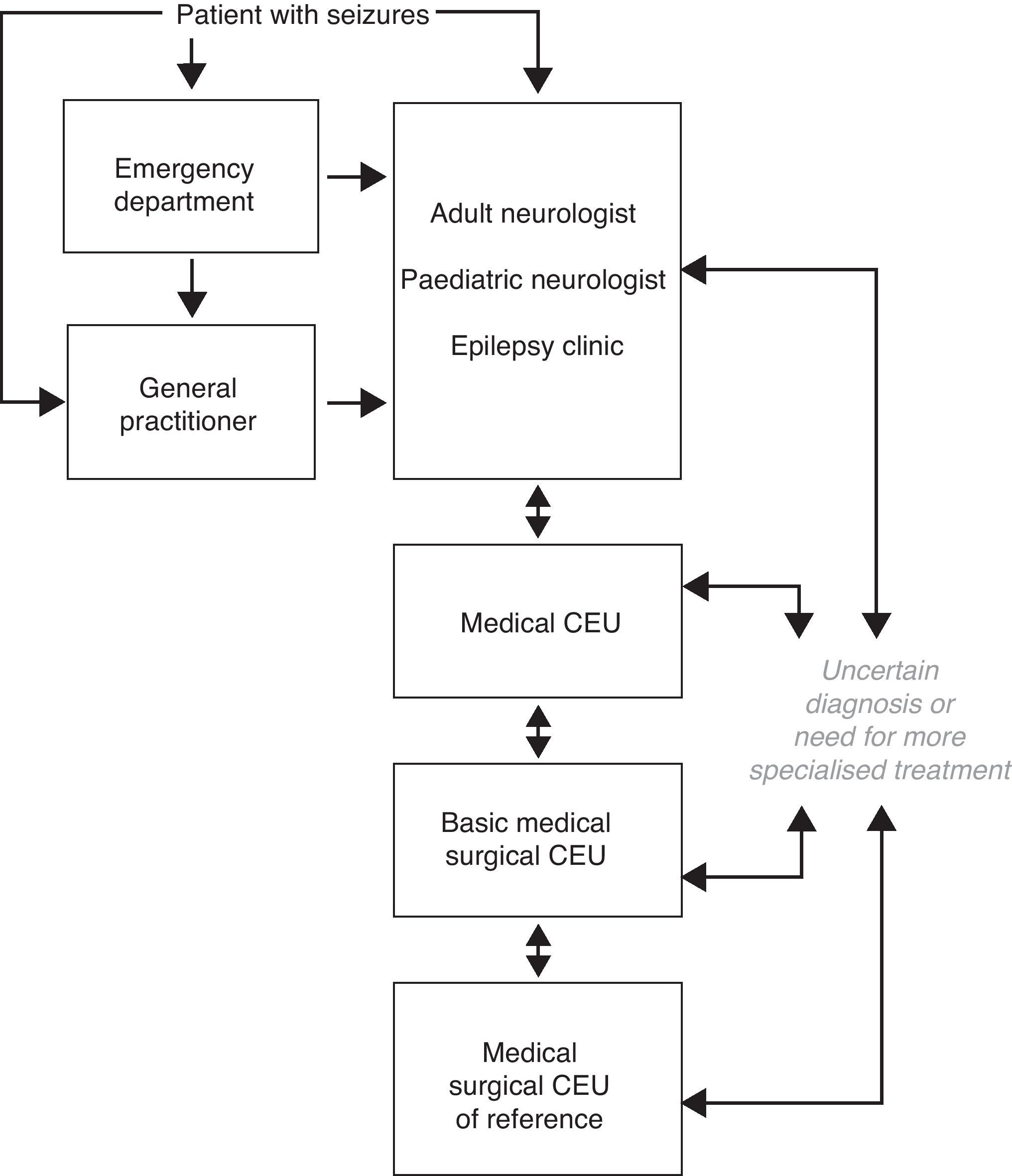
Care levels for epilepsy. Clinical epilepsy unitsIn countries such as Spain that enjoy good healthcare systems, patients with epilepsy are normally first seen by emergency department personnel or general practitioners. As a general rule, these professionals refer the patient to a general adult or paediatric neurologist who will assign a precise diagnosis, start the patient on the most appropriate treatment, and monitor the patient over the long-term with the help of the general practitioner. Some of these patients may have their seizures properly diagnosed and controlled and therefore require no further levels of care. However, another group of patients constituting a variable percentage of the total may face diagnostic uncertainty, further seizures, and the need for specific procedures. These patients require more in-depth, specialised assessment.
A Clinical Epilepsy Unit (CEU) has been defined as a group of doctors and other health professionals with special training and experience with epilepsy. The main purpose of this group is to work together to diagnose and treat DRE patients.26 The unit either has its own diagnostic and therapeutic resources and equipment or is provided with access to the same. The concept of a CEU was recently redefined as the network of healthcare professionals dedicated to integral epilepsy management plus the equipment they use. The definition specified the essential services, personnel, and infrastructure that CEUs must have at different levels of care.27 Although the primary objective of a CEU is managing DRE patients, it can also provide diagnostic and therapeutic support to patients with controlled epilepsy in its early stages or during certain stages in the course of the disease.
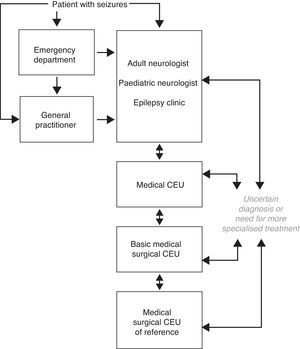
In our social and economic setting, CEUs may be stratified in different levels according to the specific activity that each one carries out. We are currently observing increased activity among specialised epilepsy clinics in adult or paediatric neurology departments, medical CEUs, basic medical/surgical CEUs, and medical/surgical CEUs of reference. These care levels listed above should be in close contact and connected to one another (Fig. 1). As stated in earlier reviews of this subject in Spain, specialised epilepsy clinics already constitute a care level. At present, the next level of care should be a medical CEU that performs video-EEG monitoring to record seizures since this has come to be such a widespread and well-developed technique. In addition, basic medical/surgical CEUs are now clearly differentiated from medical/surgical CEUs of reference since so many centres currently offer basic surgical treatments for epilepsy.28,29
First level of care. Specialised epilepsy clinicThe first level of care for epilepsy is constituted by the adult or paediatric neurologist, in close cooperation with the patient's general practitioner. The neurologist provides the patient's initial evaluation based on clinical data, neurophysiology, and basic cerebral imaging. Starting from that first level, patients can then consult with epilepsy experts or be referred to the specialised epilepsy clinic, in centres in which this step is feasible, so that patients can benefit from a more in-depth diagnosis and a larger treatment selection. The specialised epilepsy clinic is an entity falling roughly between the first and second care levels. Centres lacking a specialised epilepsy clinic must be able to refer patients who need more advanced levels of care (medical or medical/surgical CEUs). Approximately two-thirds of all epileptic patients will receive appropriate care at this level, with the help of continuity of care and an action protocol.
The specialised epilepsy clinic should be coordinated by an adult or paediatric neurologist with at least 2 years of experience in epilepsy. This doctor must be able to interpret complementary diagnostic methods and use the most appropriate AEDs and AED schedules for each type of epilepsy. This clinic should see patients at least once weekly and evaluate a minimum of 40 epileptic patients per year. The functions of such clinics are listed in Table 4.
Functions of the specialised epilepsy clinic.
| • Provide precise diagnostic and therapeutic evaluations to patients referred by emergency responders, primary care, or other specialised care services, including the same adult or paediatric neurology department. |
| • Refer patients to more specialised levels of care when necessary (medical or medical/surgical CEUs) for prolonged video-EEG monitoring. This is done for patients suspected of having been diagnosed erroneously or patients who may be candidates for surgical evaluation. |
| • Provide treatment follow-up and optimisation for patients with a confirmed diagnosis of epilepsy and for DRE patients for whom other more specialised treatment methods have been ruled out (surgical treatment, vagal nerve stimulation). |
These units constitute the level immediately above the specialised epilepsy clinic. The main feature distinguishing them from epilepsy clinics is their use of prolonged video-EEG monitoring with seizure recording, a method which provides a sure diagnosis of epilepsy and its subtype. Medical epilepsy units also have other diagnostic tools employing structural or functional brain imaging. A large body of evidence informs us that prolonged video-EEG monitoring is of vital importance for documenting the electroclinical correlation in epileptic patients in order to differentiate their seizures from other non-epileptic paroxysmal events; to establish the type of epilepsy the patient has; and to study patients who may be candidates for epilepsy surgery. Furthermore, there are standards stipulating the necessary equipment, protocols for data acquisition and transfer, safety protocols for patients, and guidelines for efficient and effective use that should be implemented in all centres offering prolonged video-EEG monitoring to record epileptic seizures.30
The primary care level should refer to the medical CEU those patients whose diagnosis with epilepsy is uncertain, those whose epileptic seizures cannot be controlled within approximately a year, those who experience acute early-onset symptomatic seizures for any reason, recurrent late-onset seizures, or those experiencing any adverse effects from the AEDs that are employed.
It is also recommended that medical CEUs dispose of their own equipment, or have access to other centres, for the following procedures:
- •
Pharmacokinetic studies for AEDs.
- •
Structural and functional brain imaging with specific protocols for epilepsy.
- •
Performing genetic studies.
- •
Neuropsychological and cognitive assessments.
- •
Assessing and treating specific populations (pregnant or elderly patients, those with medical or psychiatric comorbidities).
- •
Evaluation and treatment of non-epileptic psychogenic attacks or other non-epileptic paroxysmal events.
- •
Prescription of ketogenic diet for candidate patients.
- •
The medical CEU should set out clear protocols for referring any candidates for surgical evaluation to a medical-surgical CEU.
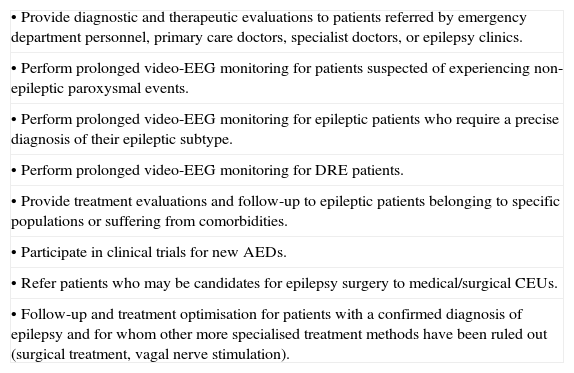
Medical CEUs must be coordinated by neurologists specialising in epilepsy. An epilepsy specialist or epileptologist is an adult or paediatric neurologist with sufficient experience (at least 3 years) in diagnosing, treating, and caring for epilepsy patients. Medical CEUs should examine a minimum of 80 new epileptic patients and perform at least 40 prolonged video-EEG monitoring studies per year. The functions of medical CEUs are listed in Table 5.
Functions of the medical CEU.
| • Provide diagnostic and therapeutic evaluations to patients referred by emergency department personnel, primary care doctors, specialist doctors, or epilepsy clinics. |
| • Perform prolonged video-EEG monitoring for patients suspected of experiencing non-epileptic paroxysmal events. |
| • Perform prolonged video-EEG monitoring for epileptic patients who require a precise diagnosis of their epileptic subtype. |
| • Perform prolonged video-EEG monitoring for DRE patients. |
| • Provide treatment evaluations and follow-up to epileptic patients belonging to specific populations or suffering from comorbidities. |
| • Participate in clinical trials for new AEDs. |
| • Refer patients who may be candidates for epilepsy surgery to medical/surgical CEUs. |
| • Follow-up and treatment optimisation for patients with a confirmed diagnosis of epilepsy and for whom other more specialised treatment methods have been ruled out (surgical treatment, vagal nerve stimulation). |
This care level must provide all types of medical and psychological treatment and social support to patients with DRE. Where indicated, it must also provide basic surgical procedures for epilepsy, such as antero-medial temporal lobectomy and temporal/extratemporal lesionectomy in areas far removed from functional eloquent areas, certain basic disconnection techniques, and vagus nerve stimulation. These CEUs will complete the studies carried out at other levels of care, as necessary, and work closely with medical/surgical CEUs of reference in order to refer patients requiring study for invasive or more sophisticated surgery. Its patient referral protocols must be clear. It is also recommended that basic medical/surgical CEUs have the same equipment as medical CEUs and possess all the necessary resources for performing basic surgical techniques and completing the appropriate preoperative studies.
Staff at a CEU should include at least 1 adult or paediatric neurologist specialising in epilepsy and acting as a coordinator, a neuroradiologist with experience in epilepsy, and a neurosurgeon with experience in epilepsy surgery. These CEUs should study at least 100 new epilepsy patients and perform a minimum of 60 prolonged video-EEG monitoring tests and 12 epilepsy surgeries per year. The functions of basic medical/surgical CEUs are listed in Table 6.
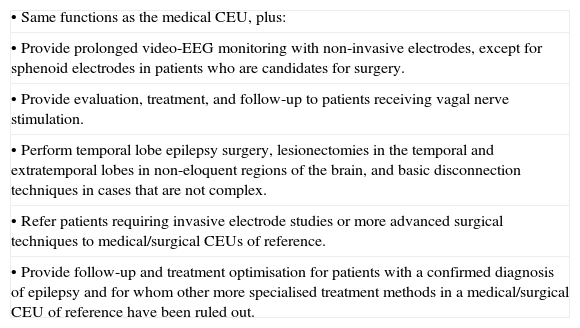
Functions of the basic medical/surgical CEU.
| • Same functions as the medical CEU, plus: |
| • Provide prolonged video-EEG monitoring with non-invasive electrodes, except for sphenoid electrodes in patients who are candidates for surgery. |
| • Provide evaluation, treatment, and follow-up to patients receiving vagal nerve stimulation. |
| • Perform temporal lobe epilepsy surgery, lesionectomies in the temporal and extratemporal lobes in non-eloquent regions of the brain, and basic disconnection techniques in cases that are not complex. |
| • Refer patients requiring invasive electrode studies or more advanced surgical techniques to medical/surgical CEUs of reference. |
| • Provide follow-up and treatment optimisation for patients with a confirmed diagnosis of epilepsy and for whom other more specialised treatment methods in a medical/surgical CEU of reference have been ruled out. |
These units should provide epilepsy patients with all types of medical, psychological, or psychosocial treatment. In addition to basic surgical techniques, these units perform surgeries requiring an invasive preoperative study (subdural, deep, and epidural electrodes; cortical electrical stimulation), temporal and extra-temporal resections with lesions adjacent to or included in eloquent functional areas, cortical resections of lesions that cannot be detected by neuroimaging, all types of disconnection techniques, and paediatric epilepsy surgery. Given that the underlying processes of paediatric surgical patients differ from those of adults and require a different type of management, these patients should be treated only in specific paediatric CEUs.
The accreditation process for medical/surgical CEUs of reference was recently revised by the Inter-regional Council of Spain's National Health System. The accreditation document revises the requirements for necessary specific equipment and indicators for results. It establishes that these units should perform a minimum of 15 to 20 surgeries yearly, and that specialists at the medical/surgical CEU of reference should provide continuous daily appointments with neurologists and neurosurgeons and include at least 2 neurologists, 2 neurosurgeons, 2 neurophysiologists. Specialists must have logged at least 3 years of experience in the medical/surgical evaluation and treatment of adult or paediatric DRE patients.31
This level of care must be able to offer complete and multidisciplinary care to patients with DRE. CEUs must have safety protocols for patient referral to and coordination with other levels of care so as to provide easy access to any DRE patients with additional care needs. They must also have the same equipment as basic medical/surgical CEUs and possess all the resources needed to perform any type of surgical procedure for epilepsy and its accompanying preoperative study, including invasive studies.
We believe that a medical-surgical CEU of reference should, at the very least, be staffed by a team of 2 adult or paediatric neurologists specialising in epilepsy, 1 to 2 neurosurgeons experienced in epilepsy surgery, and a neuroradiologist with experience in epilepsy. The CEU must be coordinated by an adult or paediatric neurologist with at least 5 years of experience in epilepsy. These CEUs should study at least 100 new epilepsy patients and perform a minimum of 60 prolonged video-EEG monitoring tests and 15 to 20 epilepsy surgeries per year; this last figure includes all types of procedures. The functions of medical/surgical CEUs of reference are listed in Table 7.
Functions of the medical/surgical CEU of reference.
| • Same functions as the basic medical/surgical CEU, plus: |
| • Perform prolonged video-EEG monitoring with invasive (intracranial) electrodes in patients who are candidates for surgery. |
| • Perform cortical electrical stimulation for the study of possible surgical procedures in eloquent regions of the brain |
| • Perform all types of epilepsy resection surgery in the temporal and extra-temporal lobes. |
| • Perform all types of cerebral disconnection techniques (hemispherectomy or variants, inter-hemispheric disconnection techniques, and subpial sections). |
| • Provide preoperative evaluations and surgery to paediatric patients. |
All patients with DRE require in-depth study in order to provide the most correct diagnosis and optimal treatment. This should be carried out in CEUs with video-EEG monitoring, which is considered the “gold-standard” method for proper management of these patients. Additionally, a significant number of DRE patients may benefit from elective epilepsy surgery. Launching CEUs will reduce the number of errors in the process of managing epilepsy patients. These include diagnostic studies that are used incorrectly, wrong diagnoses of epilepsy and epileptic subtype, use of inappropriate AEDs and combination therapy, and unnecessary delays in implementing non-pharmacological treatments for epilepsy, primarily surgery.
Stratifying CEUs according to their degree of complexity and the studies performed there will make them more streamlined and allow us to develop them with a view to providing service to DRE patients as efficiently and effectively as possible. Different CEUs must foster close contact with one another and cooperate using protocols adopted by mutual consensus in order to avoid unnecessary or redundant studies and treatments. Patient referrals should flow in both directions, depending on whether more complex or less complex procedures are required. Although the exact number of CEUs in our setting remains undefined, we should have enough units to guarantee fast and easy access to all epileptic patients, whether or not they have DRE, so as to avoid unnecessary and potentially dangerous delays. All professionals providing care to epilepsy patients should have the support of CEUs and be able to use their resources quickly, properly, and according to protocol, thereby guaranteeing good management of their patients.
Please cite this article as: Sánchez-Álvarez JC, et al. Epilepsia resistente a fármacos antiepilépticos: recomendaciones de actuación diagnóstica y terapéutica en España. Neurología. 2012;27:575–84.