Hyposmia and substantia nigra hyperechogenicity (SN+) are characteristic markers of Parkinson's disease (PD), although their diagnostic value in isolation may be limited. We evaluated the combined prevalence of both disorders in patients diagnosed with PD and assessed their diagnostic yield compared to a sample with essential tremor (ET) and another group of healthy subjects.
MethodsPatients diagnosed with PD and ET and treated in our outpatient clinic were enrolled. Olfaction was assessed using the “Sniffin Sticks” odour identification test (SS-12) and hyperechogenicity of the substantia nigra (SN+) was assessed by transcranial duplex ultrasound.
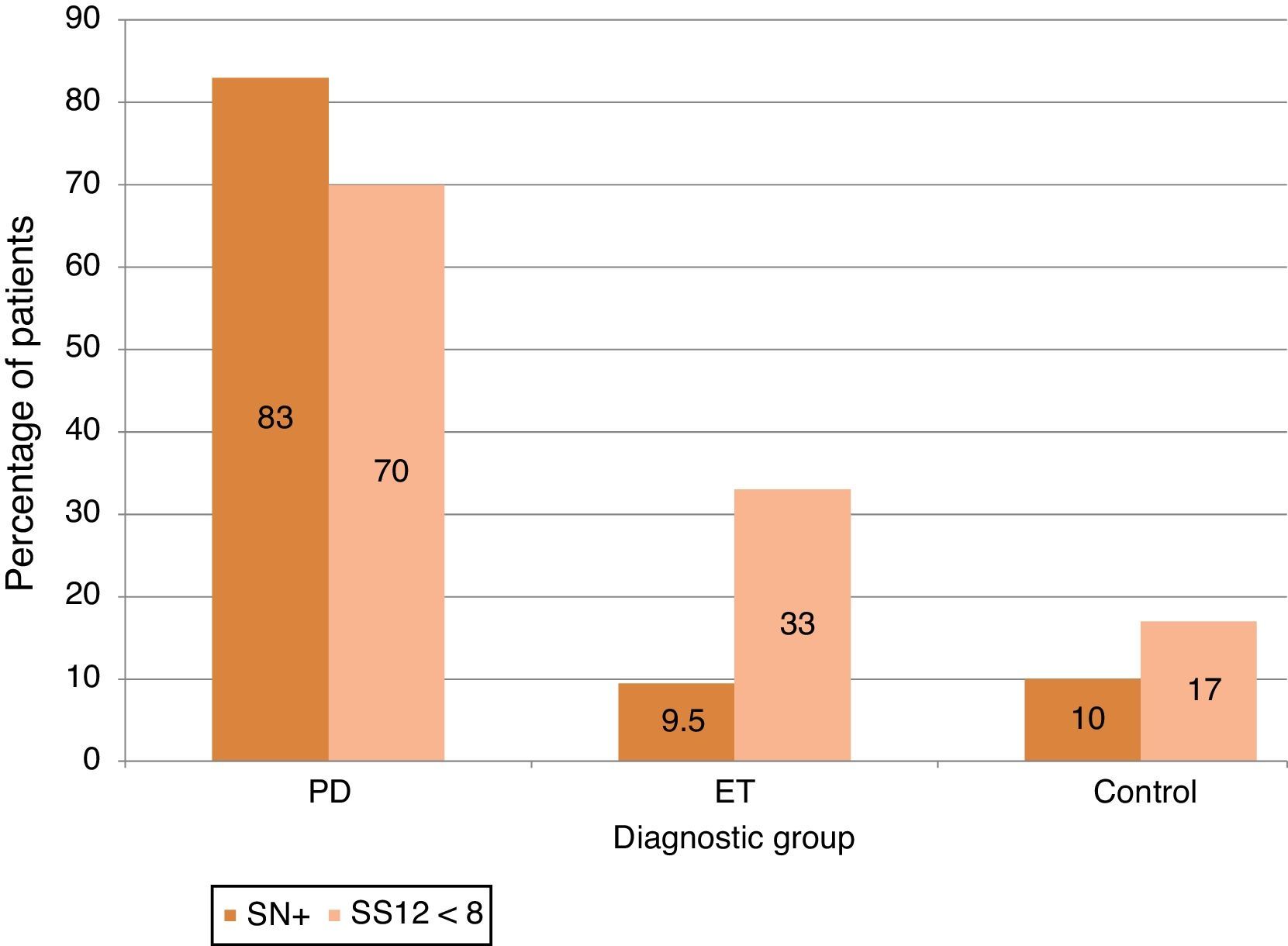
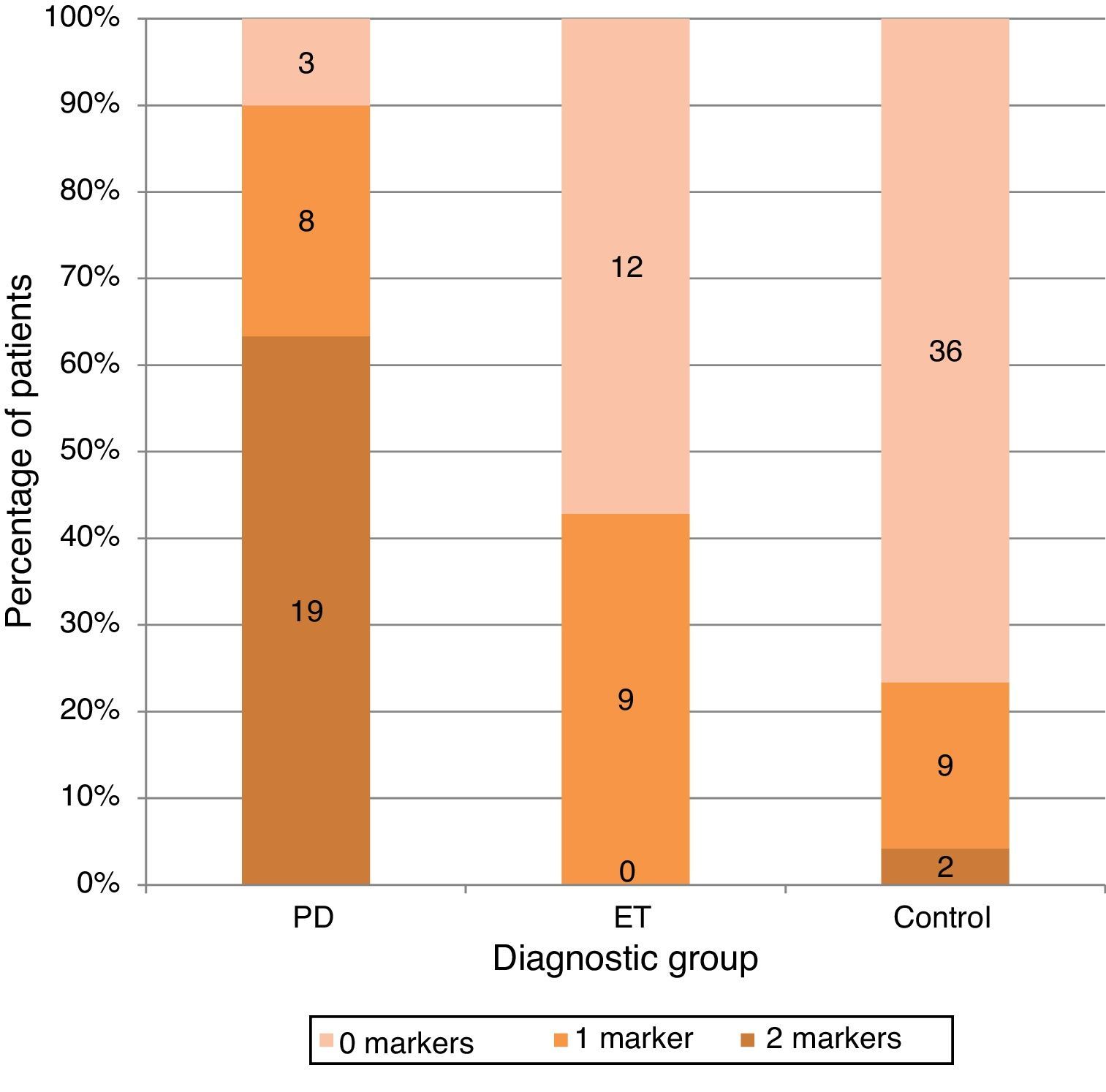
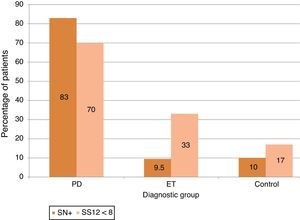
ResultsA total of 98 subjects were analysed, comprising 30 with PD, 21 with ET, and 47 controls. The respective prevalence rates of hyposmia (SS-12<8) and SN+ (area>0.24cm2) were 70% and 83.3% in PD, 33.3% and 9.5% in ET, and 17% and 10.6% in controls. Both markers were present in 63% of patients with PD, none of the patients with ET, and only 2 of the controls.
ConclusionsCombined use of substantia nigra sonography and olfactory testing with SS-12, two rapid, safe, and accessible tests, was more specific than each isolated marker for distinguishing patients with PD from patients with ET and control subjects. Since both markers have been described in very early phases of PD, combined use may be helpful in providing early diagnosis of PD.
La hiposmia y la hiperecogenicidad de la sustancia negra (SN+) son marcadores característicos de la enfermedad de Parkinson (EP), aunque su valour diagnóstico de forma aislada puede ser limitado. Se evalúa la prevalencia combinada de ambos marcadores en pacientes diagnosticados de enfermedad de Parkinson (EP) y su rentabilidad diagnóstica frente a una muestra con temblor esencial (TE) y otra de sujetos sanos.
MétodosSe incluyó a pacientes con diagnóstico de EP y TE procedentes de nuestra consulta externa. La olfación se evaluó con el test de identificación de olores Sniffin Sticks test (SS-12) y la evaluación de la sustancia negra mediante dúplex transcraneal.
ResultadosSe evaluó a 98 individuos, 30 con diagnóstico de EP, 21 con TE y 47 controles. Las prevalencias de hiposmia (SS-12 < 8) e hiperecogenicidad de SN (área > 0,24cm2) fueron del 70 y el 83,3% en EP, el 33,3 y el 9,5% en TE y el 17 y el 10,6% en los controles, respectivamente. La combinación de ambos marcadores estaba presente en el 63% de los pacientes con EP y en ninguno de los pacientes con TE y solo en 2 de los controles.
ConclusionesLa evaluación combinada de la evaluación olfativa mediante el SS-12 y de la sustancia negra mediante ecografía, 2 test rápidos, inocuos y accesibles, mejora la especificidad aislada que cada marcador tiene en el diagnóstico de la EP frente a pacientes con TE o controles. Dado que ambos marcadores se han descrito en fases muy precoces de la EP, su aplicación podría ayudarnos en su diagnóstico precoz.
Parkinson's disease (PD) is one of the most common neurodegenerative diseases and affects approximately 2% of all adults older than 60 years.1
Recent decades have witnessed significant advances in the process of diagnosing PD. Nevertheless, diagnosis of this disease is still based on clinical criteria except within the research setting. Especially when PD is in its early stages, it can be quite difficult to distinguish the disease from other processes, including essential tremor (ET), multisystem atrophy, progressive supranuclear paralysis, and vascular or drug-induced parkinsonism.2
Furthermore, results from such diagnostic procedures as the apomorphine test, radiotracer studies, and other neuroimaging techniques are not always conclusive, and findings may remain normal until the patient is in a more advanced stage of PD.3,4
For the above reasons, researchers express a growing interest in developing biological markers to improve the accuracy of PD diagnoses. Olfactory dysfunction and substantia nigra hyperechogenicity on the transcranial ultrasound (SN+) are markers that are frequently associated with PD. Not only are they present in early phase, they remain constant throughout the course of the disease,5–9 and the procedures for assessing them are simple and inexpensive, cause no discomfort, and can be performed by any neurologist.
Nevertheless, we should recall that these changes are not specific to PD, and they have only limited diagnostic value when they are present without other signs. A combined analysis of both markers may increase the diagnostic specificity.
With this in mind, our aim is to assess the sensitivity, specificity, and positive predictive value of the presence of SN+ detected with transcranial duplex ultrasound, and of olfactory alteration detected with the Sniffin Sticks test (SS-12), in a sample of patients with PD compared to a sample of patients with ET and another of healthy controls.
Patients and methodsThis observational study was carried out in the neurology department at Hospital IMED Levante (Alicante, Spain). It included patients clinically diagnosed with PD or ET one year previously or more, based on accepted diagnostic criteria.10,11
Healthy controls were recruited among patients’ companions and other patients seen in the neurology department who had no clinical signs or personal or family history of movement disorders.
Subjects were excluded from the analysis if they lacked a suitable acoustic window or had prior olfactory anomalies (rhinitis, nasal surgery) that might interfere with an olfactory study.
The study was conducted between May 2011 and May 2012. After obtaining informed consent from all participants, they underwent olfactory testing and a transcranial ultrasound study carried out by the same researcher.
Olfactory studyOlfaction was assessed using the SS-12 test (Burghart Messtechnik, Wedel, Germany). We chose this identification test with 12 odourants because it can be completed in the clinic in only a few minutes and it has already been used in PD patient populations. According to the manufacturer's recommendations, the test was administered in a well-ventilated room, and always by the same researcher. After removing the pen cap, the researcher held each odourant-containing pen under the patient's nostrils without touching the skin and for no more than 3 to 4seconds. Each correct answer is assigned one point and the test has a forced-choice answer format, meaning that the subject must select one of the 4 options provided before proceeding to the next pen. The approximate interval between presenting different odours was 20seconds. The total score for correct responses is given as a numeric value between 0 and 12.
Receiver operating characteristic (ROC) curves were used to establish the best cut-off point. We also evaluated the frequency of olfactory changes according to the normogram produced by German studies.12 Scores lower than the 10th percentile for the subject's age group and sex were considered pathological. The combined analysis employed the absolute criterion only.
Transcranial ultrasound studyTranscranial ultrasound was performed through the temporal window using a 1-4mHz ultrasound probe (Acuson Antares, Siemens, Erlangen, Germany) configured as follows: field depth 14-16cm, dynamic range 45-50dB, and G-mode post-processing.
The left and right SN were insonated separately. We located the mesencephalic axial plane with the hyperechogenic region corresponding to the SN. The image was then frozen, augmented 3 to 4 times, and recorded (image and video) for later use in planimetric measurements. These measurements were made by manually tracing the circumference of the hyperechogenic region, at which point the region's area was calculated automatically. All studies were performed by the same researcher (NL), who was not blinded to the subject's diagnostic group at the time of the study. Nevertheless, planimetric measurements were completed at another time after the patients’ personal data had been masked.
As recommended by international guidelines,13 we calculated our own reference value and classified findings as pathological (SN+) when SN hyperechogenicity on at least one side was above the 90th percentile for all laboratory values in control subjects.
Statistical analysisThis section presents the descriptive analysis for each diagnostic group in frequencies and means±standard deviation for sex and age.
Sensitivity, specificity, and positive predictive value were calculated for each of the diagnostic tests.
The chi-square test was used to investigate dependence of the qualitative diagnostic variable (PD/no PD) on the result (positive/negative) for each of the markers. Comparison of means was achieved by testing for a normal data distribution (using Kolmogorov-Smirnov and Shapiro–Wilk tests) and by applying ANOVA and the t-test. A P-value<.05 was considered statistically significant.
These tests were performed with SPSS 15 for Windows (IBM).
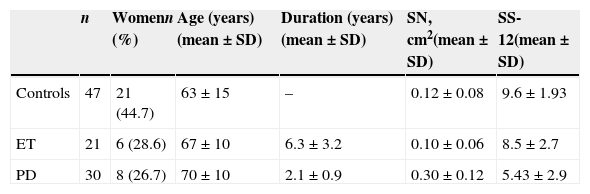
ResultsOf an initial sample of 107 individuals, we excluded a total of 9 with insufficient acoustic windows. The excluded subjects comprised 8 women (88.9%) with a mean (SD) age of 72.8 (6.4) years. The final study group contained 98 subjects (35 women, 35.7%) with a mean (SD) age of 66 (13.2). There were 30 patients with PD, 21 with ET, and 47 control subjects. Sample characteristics are described in Table 1.
Demographic characteristics of the population and results of ultrasound and olfactory studies by diagnostic group.
| n | Womenn (%) | Age (years)(mean±SD) | Duration (years)(mean±SD) | SN, cm2(mean±SD) | SS-12(mean±SD) | |
|---|---|---|---|---|---|---|
| Controls | 47 | 21 (44.7) | 63±15 | – | 0.12±0.08 | 9.6±1.93 |
| ET | 21 | 6 (28.6) | 67±10 | 6.3±3.2 | 0.10±0.06 | 8.5±2.7 |
| PD | 30 | 8 (26.7) | 70±10 | 2.1±0.9 | 0.30±0.12 | 5.43±2.9 |
SD: standard deviation; SN: substantia nigra; SS-12: Sniffin Sticks test.
Mean scores on the SS-12 per group are shown in Table 1.
ROC curve analysis yielded an area under the curve of 0.70. The cut-off value of less than 8 correct responses was the best for differentiating patients with PD from other subjects.
Olfactory impairment was more frequently detected using an absolute criterion (score<8) than when using normative values for age and sex: 70% in PD [vs 56.7%], 33.3% in ET [vs 19%] and 17% in control subjects [vs 8.5%] (see Fig. 1).
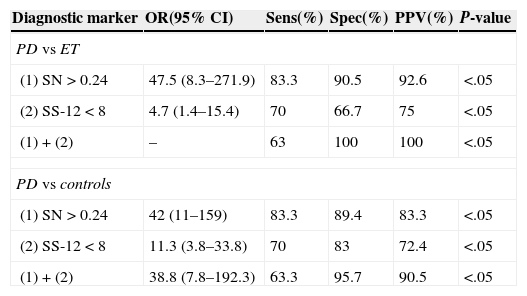
Subjects with an SS-12 score of less than 8 were considered to have hyposmia. The diagnostic utility of this finding for differentiating patients with PD from those with ET (and from control subjects) showed a sensitivity of 70% (70% for controls), 66.7% (83% for controls), and 75% (72.4% for controls) (Table 2).
Diagnostic markers differentiating PD patients from ET patients and controls.
| Diagnostic marker | OR(95% CI) | Sens(%) | Spec(%) | PPV(%) | P-value |
|---|---|---|---|---|---|
| PD vs ET | |||||
| (1) SN>0.24 | 47.5 (8.3–271.9) | 83.3 | 90.5 | 92.6 | <.05 |
| (2) SS-12<8 | 4.7 (1.4–15.4) | 70 | 66.7 | 75 | <.05 |
| (1)+(2) | – | 63 | 100 | 100 | <.05 |
| PD vs controls | |||||
| (1) SN>0.24 | 42 (11–159) | 83.3 | 89.4 | 83.3 | <.05 |
| (2) SS-12<8 | 11.3 (3.8–33.8) | 70 | 83 | 72.4 | <.05 |
| (1)+(2) | 38.8 (7.8–192.3) | 63.3 | 95.7 | 90.5 | <.05 |
Spec: specificity; PD: Parkinson's disease; CI: confidence interval; OR: odds ratio; Sens: sensitivity; ET: essential tremor; PPV: positive predictive value.
Values reflecting SN hyperechogenicity for each diagnostic group are shown in Table 1. The hyperechogenic area was significantly larger in the PD group (0.30±0.13cm2) than in either the ET group (0.10±0.07cm2) or the control group (0.12±0.08cm2).
Using values obtained from the control group, we calculated the 90th percentile level to define individuals with a large area of SN+. The cut-off point was 0.24cm2. This criterion naturally meant that 10% of the control subjects presented SN+, and the proportion of SN+ was similar in the ET group at 9.5%. In contrast, 93% of the patients with PD showed SN+ (Fig. 1).
The presence of this marker enabled us to differentiate patients with PD from ET patients (and controls). Sensitivity was 83.3% (83.3% for controls), specificity was 90.5% (89.4% for controls), and the positive predictive value was 92.6% (83.3% for controls).
Combined analysisAssessed alone, SN hyperechogenicity is a more sensitive and specific diagnostic marker than olfaction (Table 2).
Nevertheless, combining the 2 markers significantly increased diagnostic precision; diagnostic specificity for PD compared to controls reached 95.7% (OR=38.8), and for PD compared to ET, it reached 100% (OR not calculable).
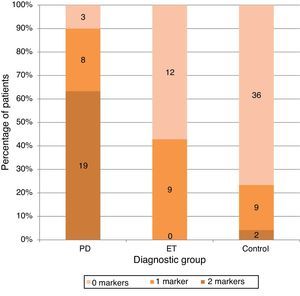
Only one of the 2 markers was present in 26.6% of the PD group (n=8), vs 42.9% in the ET group (n=9) and 19.1% in the control group. However, both markers were present in 63.3% of all patients in the PD group (n=19), in only 4.3% of the controls (n=2), and in none of the ET patients (Fig. 2).
DiscussionOur results show that the combined presence of SN hyperechogenicity and hyposmia is highly specific for differentiating patients with PD from both healthy controls and from patients with ET.
None of the patients diagnosed with ET and only 2 of the control subjects (4.3%) displayed this combination of markers. The above finding, the data now supporting the presence of these markers in the early stages of PD, and the ease with which both markers can be measured by any neurologist in an outpatient setting (quickly and without discomfort to the patient or costly procedures), all indicate that combined assessment is a promising strategy for diagnosing PD in its early stages.
While combined use of these markers does decrease the diagnostic sensitivity for PD, this approach significantly increases the diagnostic specificity and positive predictive value.
Our results are comparable with those previously published by Busse et al.14 Those researchers analysed the diagnostic value of the combination of SN hyperechogenicity (established as SN+>0.24cm2), olfactory impairment (<8 correct responses on SS-12), and motor asymmetry evaluated with the UPDRS in the differential diagnosis of PD and other causes of parkinsonism (including ET). The association of olfactory impairment and SN+ shows a sensitivity of 66%, compared to 75% for olfactory impairment alone and 90% for SN+ alone. Specificity for both markers is 89%, vs 70% for olfactory impairment alone and 63% for SN+ alone. The positive predictive value for both markers is 95%, vs 88% for olfactory impairment alone and 88% for SN+ alone. Presence of both markers delivers the best diagnostic yield.
Izawa et al.15 also analysed the combination of SN+ (defined as>0.16cm2), olfactory impairment identified by the OPSIT-J test (similar to SS-12 and adapted to the Japanese population), and MIBG myocardial scintigraphy in 33 patients with PD vs 32 control subjects. Despite these methodological differences (SN+ cut-off point of 0.16cm2 and OPSIT-J olfaction test), the researchers also found that the combination of SN+ and impaired olfaction showed a better sensitivity, specificity, and positive predictive value (61.8%, 100%, and 100%, respectively) compared to values for each marker assessed separately.
Olfactory impairment in PD is present even in the earliest phases of the disease,16,17 and it is compatible with neuropathology findings.18 It does not clearly correlate with the duration of the disease, or to its severity.8 Nevertheless, this finding is not specific; it has been described in patients with multisystem atrophy, Lewy body dementia, and Alzheimer disease, although it is less prevalent in these populations.19 Assessing olfactory deficiency alone may be limited as a diagnostic strategy.
Among patients with PD, the prevalence of olfactory impairment ranges from 45% to 96%.8
This wide range may be due to the age distribution, sample size, normative data, or the type of olfactory test administered. Our main reason for testing olfaction with the SS-12 was its short application time (about 4minutes).
There are also more complex olfactory tests that measure detection threshold and discrimination between odours. The SS-12 is actually a simplified version of a 16-item test. And whereas the mentioned tests are more complex, costly, and time-consuming, evidence also suggests that the odourant subset used in the SS-12 is the most sensitive for detecting olfactory loss in PD.8
There is no consensus regarding the cut-off point that defines olfactory impairment. A normative table distributed by age and sex does exist for the SS-12, but it was created using data from a Central European population and it has not been validated in Spain.
While some authors have applied these normative criteria to define hyposmia,8 others have employed absolute cut-off values: <9 correct responses in one study20 vs <8 in another.14 This seems to aid differentiation between the diagnostic groups.
Our study also adopted the cut-off value of <8 and the frequency of this finding in PD was 70%, which is similar to these authors’ findings.
On the other hand, and as other authors have reported,21,22 results on the olfaction test were slightly lower in patients with ET than in healthy controls, but these differences were not statistically significant.
SN hyperechogenicity, viewed with transcranial ultrasound, was identified in approximately 90% of the patients with PD and in 10% of the healthy subjects.
This marker appears in very early phases of PD and remains stable over the course of the disease,23 and it also seems to suggest a higher risk of developing PD in seemingly healthy subjects. Its prevalence is high in conditions with a demonstrated association with PD.24
A more recent longitudinal study spanning 3 years associates presence of SN hyperechogenicity in healthy subjects with a relative risk of 17 for developing PD.25
The specificity of this marker is also controversial; it may appear, although less frequently, in other neurodegenerative diseases, such as atypical parkinsonism (multisystem atrophy, progressive supranuclear paralysis) or spinocerebellar ataxia.26 Even subjects with no known neurological disease may display increased hyperechogenicity; this varies between studies but appears in approximately 10% of all healthy subjects. This marker seems to be positively associated with age.
Hyperechogenicity was present in 83.3% of the patients with PD in our study population; this prevalence is similar to figures cited in the literature. It was also detected in 9.5% of patients with ET and in 10% of the control subjects.
We should stress the limitations of our study. Firstly, the researcher performing the ultrasound was not blinded to the patient's diagnosis given that some of the patients came from his clinic and he would have been able to ascertain the diagnosis while evaluating the markers. While such a bias would have been of very little consequence for the olfactory assessment, we saved the ultrasound images so that they could be measured at another time, after having masked patient data, so as to minimise bias in the ultrasound studies.
Another weakness is the lack of normative data for SS-12 in the Spanish population. We used the normative data taken from a German population and published by Hummel et al.,12 mindful that cultural and racial factors might affect the results. Future studies, conducted in healthy subjects residing in our setting, will have to determine which are the optimal criteria for defining olfactory impairment.
Lastly, controls were selected among patient's companions and other patients from the clinic who lacked any personal or family history of neurodegenerative disease, movement disorders, or olfactory impairment. As a result, they may not constitute a representative sample of the general population, which would decrease the external validity of this study.
We conclude that these findings support the utility of using the combination of ultrasound evaluation of SN and olfactory assessment with SS-12 in the process of diagnosing PD. The high specificity and positive predictive value of this combination of markers, added to the ease and convenience of administering and taking these tests in any neurology clinic, point to a promising diagnostic tool that can help identify patients at high risk of presenting PD from among patients showing parkinsonian traits in general.
Further studies will have to clarify the role of these markers in the earliest stages of PD and even in asymptomatic subjects by surveying large samples of patients with other types of parkinsonism. This may help us diagnose the disease at even earlier stages, and it could also be helpful in selecting potential candidates for trials of disease-modifying therapies.
Conflicts of interestThe authors have no conflicts of interest to declare. No external funding was received for this study.
Please cite this article as: López Hernández N, García Escrivá A, Shalabi Benavent M. Valor de la evaluación combinada de olfación e hiperecogenicidad de sustancia negra en el diagnóstico de la enfermedad de Parkinson. Neurología. 2015;30:496–501.
This study was presented as an oral communication at the 64th Annual Meeting of the Spanish Society of Neurology, Barcelona, 2012.